Hierarchical ZIF-8 toward Immobilizing Burkholderia cepacia Lipase for Application in Biodiesel Preparation
Abstract
:1. Introduction
2. Results and Discussion
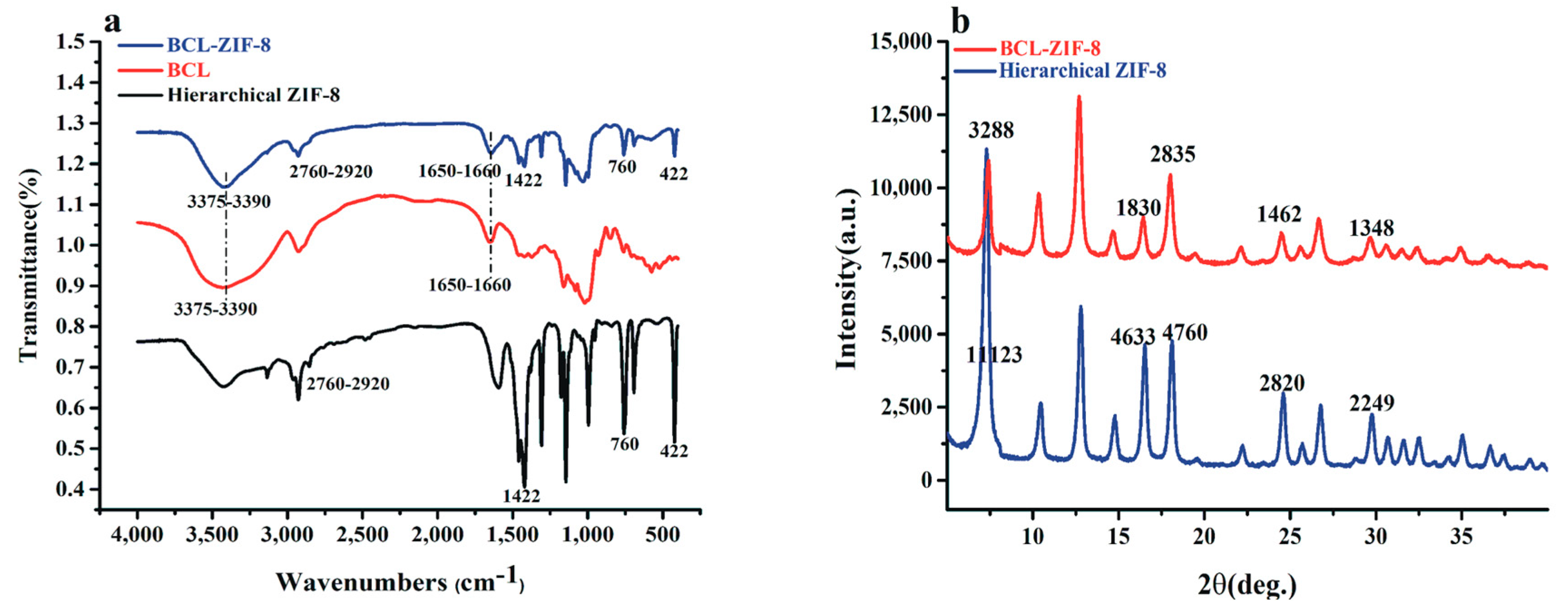
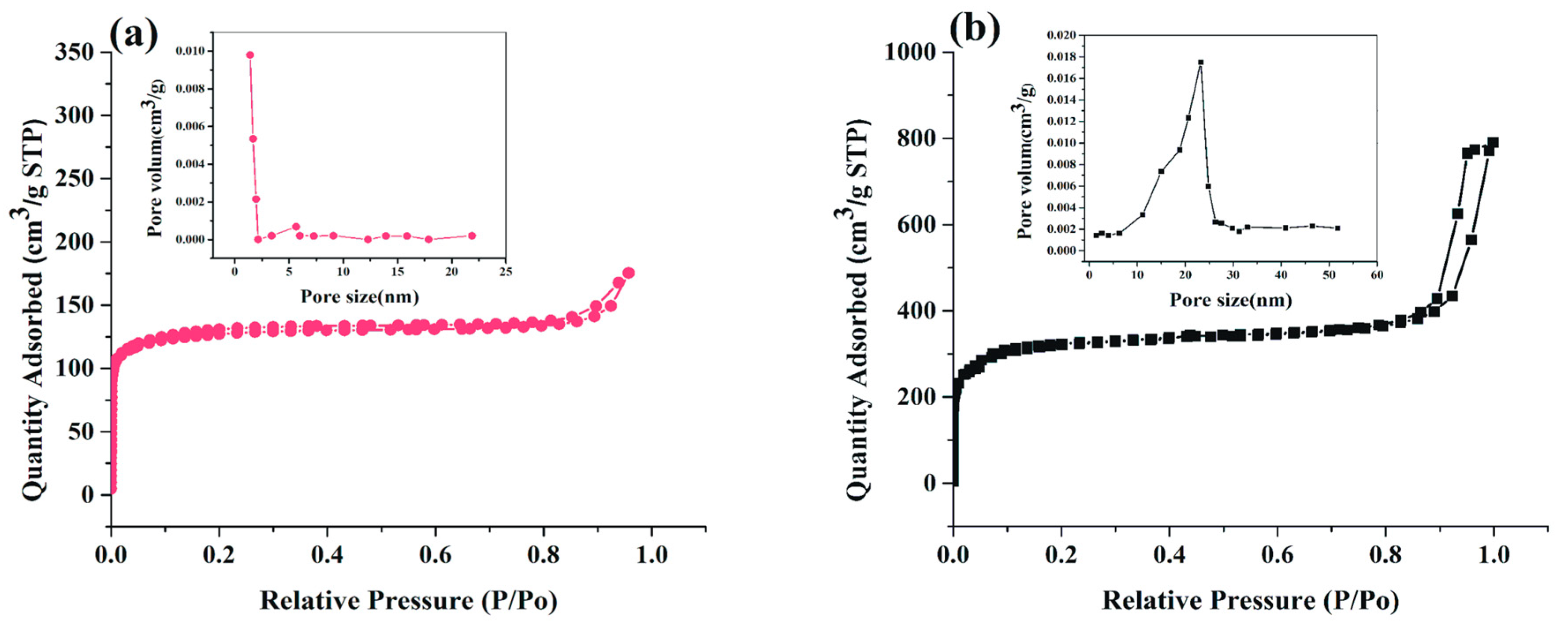
2.1. Characterization and Synthesis of Hexahedral, Hierarchical ZIF-8, and Adsorbed Lipase into Mesoporous ZIF-8 (BCL-ZIF-8)
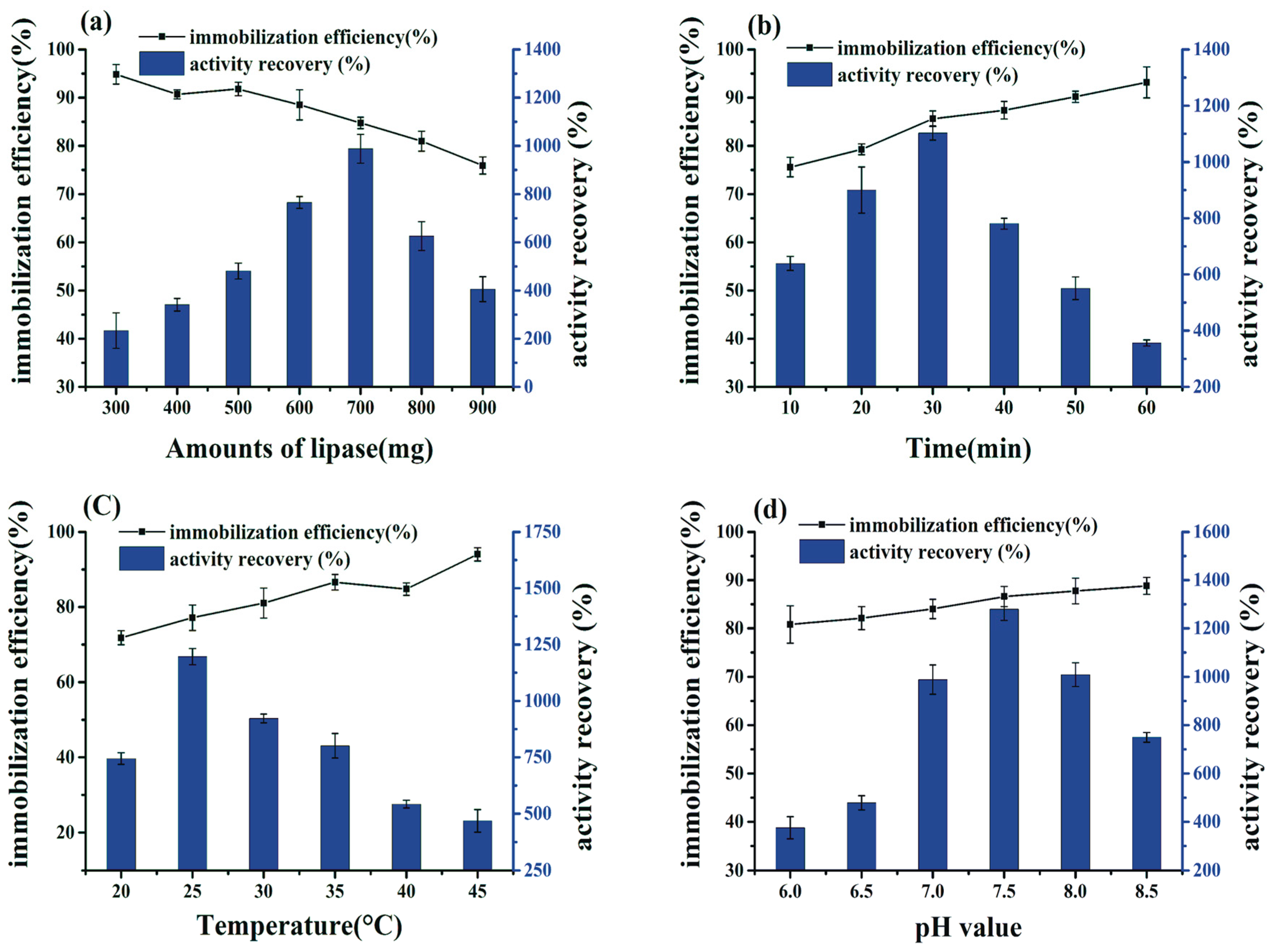
2.2. Effects of the Conditions of Preparation of BCL-ZIF-8 on the Enzyme Activity
2.3. Effect of Transesterification Condition on Biodiesel Production
2.4. Reusability of Immobilized BCL-ZIF-8
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Microporous Hexahedral ZIF-8
3.3. Synthesis of Mesoporous Hierarchical ZIF-8
3.4. BCL Immobilization into Mesoporous ZIF-8
3.5. Enzyme Activity Assays
3.6. Characterization
3.7. Biodiesel Production via Enzymatic Catalysis
3.8. Measurement of Biodiesel Yield by GC
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Guldhe, A.; Singh, B.; Mutanda, T.; Permaul, K.; Bux, F. Advances in synthesis of biodiesel via enzyme catalysis: Novel and sustainable approaches. Renew. Sustain. Energy Rev. 2015, 41, 1447–1464. [Google Scholar] [CrossRef]
- Atabani, A.E.; Silitonga, A.S.; Badruddin, I.A.; Mahlia, T.M.I.; Masjuki, H.H.; Mekhilef, S. A comprehensive review on biodiesel as an alternative energy resource and its characteristics. Renew. Sustain. Energy Rev. 2012, 16, 2070–2093. [Google Scholar] [CrossRef]
- Tan, T.; Lu, J.; Nie, K.; Deng, L.; Wang, F. Biodiesel production with immobilized lipase: A review. Biotechnol. Adv. 2010, 28, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Sankaran, R.; Show, P.L.; Chang, J.-S. Biodiesel production using immobilized lipase: Feasibility and challenges. Biofuels Bioprod. Bio. 2016, 10, 896–916. [Google Scholar] [CrossRef]
- Abdulla, R.; Ravindra, P. Cross-Linked Lipase in Hybrid Matrix for Biodiesel Production from Crude Jatropha Curcas Oil; Springer: Boston, MA, USA, 2013; pp. 197–202. [Google Scholar]
- Adnan, M.; Li, K.; Xu, L.; Yan, Y. X-shaped zif-8 for immobilization rhizomucor miehei lipase via encapsulation and its application toward biodiesel production. Catalysts 2018, 8, 96. [Google Scholar] [CrossRef]
- Xie, W.; Wang, J. Enzymatic production of biodiesel from soybean oil by using immobilized lipase on fe3o4/poly(styrene-methacrylic acid) magnetic microsphere as a biocatalyst. Energy Fuels 2014, 28, 2624–2631. [Google Scholar] [CrossRef]
- Labus, K.; Szymańska, K.; Bryjak, J.; Jarzębski, A.B. Immobilisation of tyrosinase on siliceous cellular foams affording highly effective and stable biocatalysts. Chem. Pap. 2015, 69. [Google Scholar] [CrossRef]
- Datta, S.; Christena, L.R.; Rajaram, Y.R. Enzyme immobilization: An overview on techniques and support materials. 3 Biotech 2013, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kuan, I.C.; Lee, C.-C.; Tsai, B.-H.; Lee, S.-L.; Lee, W.-T.; Yu, C.-Y. Optimizing the production of biodiesel using lipase entrapped in biomimetic silica. Energies 2013, 6, 2052–2064. [Google Scholar] [CrossRef]
- Yan, X.; Komarneni, S.; Zhang, Z.; Yan, Z. Extremely enhanced CO2 uptake by HKUST-1 metal–organic framework via a simple chemical treatment. Microporous Mesoporous Mater. 2014, 183, 69–73. [Google Scholar] [CrossRef]
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; Van Duyne, R.P.; Hupp, J.T. Metal-organic framework materials as chemical sensors. Chem. Rev. 2012, 112, 1105–1125. [Google Scholar] [CrossRef] [PubMed]
- Horcajada, P.; Gref, R.; Baati, T.; Allan, P.K.; Maurin, G.; Couvreur, P.; Ferey, G.; Morris, R.E.; Serre, C. Metal-organic frameworks in biomedicine. Chem. Rev. 2012, 112, 1232–1268. [Google Scholar] [CrossRef] [PubMed]
- Stock, N.; Biswas, S. Synthesis of metal-organic frameworks (MOFS): Routes to various MOF topologies, morphologies, and composites. Chem. Rev. 2011, 112, 933–969. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.N.; Zhou, M.; Zhang, B.; Wu, B.; Li, J.; Qiao, J.; Guan, X.; Li, F. Amino acid assisted templating synthesis of hierarchical zeolitic imidazolate framework-8 for efficient arsenate removal. Nanoscale 2014, 6, 1105–1112. [Google Scholar] [CrossRef] [PubMed]
- Cravillon, J.; Nayuk, R.; Springer, S.; Feldhoff, A.; Huber, K.; Wiebcke, M. Controlling zeolitic imidazolate framework nano-and microcrystal formation: Insight into crystal growth by time-resolved in situ static light scattering. Chem. Mater. 2011, 23, 2130–2141. [Google Scholar] [CrossRef]
- Qiu, L.G.; Xu, T.; Li, Z.Q.; Wang, W.; Wu, Y.; Jiang, X.; Tian, X.Y.; Zhang, L.D. Hierarchically micro-and mesoporous metal-organic frameworks with tunable porosity. Angew. Chem. Int. Ed. 2008, 47, 9487–9491. [Google Scholar] [CrossRef] [PubMed]
- Md Nordin, N.A.H.; Racha, S.M.; Matsuura, T.; Misdan, N.; Abdullah Sani, N.A.; Ismail, A.F.; Mustafa, A. Facile modification of ZIF-8 mixed matrix membrane for CO2/CH4 separation: Synthesis and preparation. RSC Adv. 2015, 5, 43110–43120. [Google Scholar] [CrossRef]
- Cheong, L.-Z.; Wei, Y.; Wang, H.; Wang, Z.; Su, X.; Shen, C. Facile fabrication of a stable and recyclable lipase@amine-functionalized ZIF-8 nanoparticles for esters hydrolysis and transesterification. J. Nanopart. Res. 2017, 19. [Google Scholar] [CrossRef]
- Amedi, H.R.; Aghajani, M. Aminosilane-functionalized zif-8/peba mixed matrix membrane for gas separation application. Microporous Mesoporous Mater. 2017, 247, 124–135. [Google Scholar] [CrossRef]
- Hu, X.; Yan, X.; Zhou, M.; Komarneni, S. One-step synthesis of nanostructured mesoporous zif-8/silica composites. Microporous Mesoporous Mater. 2016, 219, 311–316. [Google Scholar] [CrossRef]
- Li, D.; Teoh, W.Y.; Gooding, J.J.; Selomulya, C.; Amal, R. Functionalization strategies for protease immobilization on magnetic nanoparticles. Adv. Funct. Mater. 2010, 20, 1767–1777. [Google Scholar] [CrossRef]
- Fan, Y.; Su, F.; Li, K.; Ke, C.; Yan, Y. Carbon nanotube filled with magnetic iron oxide and modified with polyamidoamine dendrimers for immobilizing lipase toward application in biodiesel production. Sci. Rep. 2017, 7, 45643. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Fan, Y.; He, Y.; Zeng, L.; Han, X.; Yan, Y. Burkholderia cepacia lipase immobilized on heterofunctional magnetic nanoparticles and its application in biodiesel synthesis. Sci. Rep. 2017, 7, 16473. [Google Scholar] [CrossRef] [PubMed]
- Su, F.; Li, G.; Zhang, H.; Yan, Y. Enhanced performance of rhizopus oryzae lipase immobilized on hydrophobic carriers and its application in biorefinery of rapeseed oil deodorizer distillate. BioEnergy Res. 2014, 7, 935–945. [Google Scholar] [CrossRef]
- Liu, T.; Liu, Y.; Wang, X.; Li, Q.; Wang, J.; Yan, Y. Improving catalytic performance of burkholderia cepacia lipase immobilized on macroporous resin NKA. J. Mol. Catal. B Enzym. 2011, 71, 45–50. [Google Scholar] [CrossRef]
- Panzavolta, F.; Soro, S.; D’Amato, R.; Palocci, C.; Cernia, E.; Russo, M.V. Acetylenic polymers as new immobilization matrices for lipolytic enzymes. J. Mol. Catal. B Enzym. 2005, 32, 67–76. [Google Scholar] [CrossRef]
- Jesionowski, T.; Zdarta, J.; Krajewska, B. Enzyme immobilization by adsorption: A review. Adsorption 2014, 20, 801–821. [Google Scholar] [CrossRef]
- Hwang, E.T.; Gu, M.B. Enzyme stabilization by nano/microsized hybrid materials. Eng. Life Sci. 2013, 13, 49–61. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, X.; Hou, M.; Li, X.; Wu, X.; Ge, J. Immobilization on metal-organic framework engenders high sensitivity for enzymatic electrochemical detection. ACS Appl. Mater. Interfaces 2017, 9, 13831–13836. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.; Coghlan, C.J.; Bell, S.G.; Doonan, C.; Falcaro, P. Enzyme encapsulation in zeolitic imidazolate frameworks: A comparison between controlled co-precipitation and biomimetic mineralisation. Chem. Commun. 2016, 52, 473–476. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.I.; Lan, D.; Durrani, R.; Huan, W.; Zhao, Z.; Wang, Y. The lid domain in lipases: Structural and functional determinant of enzymatic properties. Front. Bioeng. Biotechnol. 2017, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Ke, C.; Fan, Y.; Chen, Y.; Xu, L.; Yan, Y. A new lipase–inorganic hybrid nanoflower with enhanced enzyme activity. RSC Adv. 2016, 6, 19413–19416. [Google Scholar] [CrossRef]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, A.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Strategies for the one-step immobilization-purification of enzymes as industrial biocatalysts. Biotechnol. Adv. 2015, 33, 435–456. [Google Scholar] [CrossRef] [PubMed]
- Andrade, T.A.; Errico, M.; Christensen, K.V. Influence of the reaction conditions on the enzyme catalyzed transesterification of castor oil: A possible step in biodiesel production. Bioresour. Technol. 2017, 243, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Ke, C.; Su, F.; Li, K.; Yan, Y. Various types of lipases immobilized on dendrimer-functionalized magnetic nanocomposite and application in biodiesel preparation. Energy Fuels 2017, 31, 4372–4381. [Google Scholar] [CrossRef]
- Yan, Y.; Li, X.; Wang, G.; Gui, X.; Li, G.; Su, F.; Wang, X.; Liu, T. Biotechnological preparation of biodiesel and its high-valued derivatives: A review. Appl. Energy 2014, 113, 1614–1631. [Google Scholar] [CrossRef]
- Rafiei, S.; Tangestaninejad, S.; Horcajada, P.; Moghadam, M.; Mirkhani, V.; Mohammadpoor-Baltork, I.; Kardanpour, R.; Zadehahmadi, F. Efficient biodiesel production using a lipase@zif-67 nanobioreactor. Chem. Eng. J. 2018, 334, 1233–1241. [Google Scholar] [CrossRef]
- Li, Q.; Yan, Y. Production of biodiesel catalyzed by immobilized Pseudomonas cepacia lipase from sapium sebiferum oil in micro-aqueous phase. Appl. Energy 2010, 87, 3148–3154. [Google Scholar] [CrossRef]
- Kaieda, M.; Samukawa, T.; Matsumoto, T.; Ban, K.; Kondo, A.; Shimada, Y.; Noda, H.; Nomoto, F.; Ohtsuka, K.; Izumoto, E.; et al. Biodiesel fuel production from plant oil catalyzed by rhizopus oryzae lipase in a water-containing system without an organic solvent. J. Biosci. Bioeng. 1999, 88, 627–631. [Google Scholar] [CrossRef]
- Fan, Y.; Wu, G.; Su, F.; Li, K.; Xu, L.; Han, X.; Yan, Y. Lipase oriented-immobilized on dendrimer-coated magnetic multi-walled carbon nanotubes toward catalyzing biodiesel production from waste vegetable oil. Fuel 2016, 178, 172–178. [Google Scholar] [CrossRef]
- Royon, D.; Daz, M.; Ellenrieder, G.; Locatelli, S. Enzymatic production of biodiesel from cotton seed oil using t-butanol as a solvent. Bioresour. Technol. 2007, 98, 648–653. [Google Scholar] [CrossRef] [PubMed]
- Szczęsna Antczak, M.; Kubiak, A.; Antczak, T.; Bielecki, S. Enzymatic biodiesel synthesis—Key factors affecting efficiency of the process. Renew. Energy 2009, 34, 1185–1194. [Google Scholar] [CrossRef]
- Nielsen, P.M.; Brask, J.; Fjerbaek, L. Enzymatic biodiesel production: Technical and economical considerations. Eur. J. Lipid Sci. Technol. 2008, 110, 692–700. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, T.; Wang, X.; Xu, L.; Yan, Y. Biodiesel synthesis catalyzed by Burkholderia cenocepacia lipase supported on macroporous resin NKA in solvent-free and isooctane systems. Energy Fuels 2011, 25, 1206–1212. [Google Scholar] [CrossRef]
- Gumel, A.M.; Annuar, M.S.M. Thermomyces lanuginosus lipase-catalyzed synthesis of natural flavor esters in a continuous flow microreactor. 3 Biotech 2016, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- You, Q.; Yin, X.; Zhao, Y.; Zhang, Y. Biodiesel production from jatropha oil catalyzed by immobilized burkholderia cepacia lipase on modified attapulgite. Bioresour. Technol. 2013, 148, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Du, W.; Liu, D.; Wang, L.; Li, Z. Lipase-catalyzed transesterification of rapeseed oils for biodiesel production with a novel organic solvent as the reaction medium. J. Mol. Catal. B Enzym. 2006, 43, 58–62. [Google Scholar] [CrossRef]
- Mittelbach, M. Lipase catalyzed alcoholysis of sunflower oil. J. Am. Oil Chem. Soc. 1990, 67, 168–170. [Google Scholar] [CrossRef]
- Kaieda, M.; Samukawa, T.; Kondo, A.; Fukuda, H. Effect of methanol and water contents on production of biodiesel fuel from plant oil catalyzed by various lipases in a solvent-free system. J. Biosci. Bioeng. 2001, 91, 12–15. [Google Scholar] [CrossRef]
- Oliveira, A.; Rosa, M. Enzymatic transesterification of sunflower oil in an aqueous-oil biphasic system. J. Am. Oil Chem. Soc. 2006, 83, 21–25. [Google Scholar] [CrossRef]
- Zhang, K.-P.; Lai, J.-Q.; Huang, Z.-L.; Yang, Z. Penicillium expansum lipase-catalyzed production of biodiesel in ionic liquids. Bioresour. Technol. 2011, 102, 2767–2772. [Google Scholar] [CrossRef] [PubMed]
- Nelson, L.A.; Foglia, T.A.; Marmer, W.N. Lipase-catalyzed production of biodiesel. J. Am. Oil Chem. Soc. 1996, 73, 1191–1195. [Google Scholar] [CrossRef]
- Talukder, M.M.R.; Wu, J.C.; Van Nguyen, T.B.; Fen, N.M.; Melissa, Y.L.S. Novozym 435 for production of biodiesel from unrefined palm oil: Comparison of methanolysis methods. J. Mol. Catal. B Enzym. 2009, 60, 106–112. [Google Scholar] [CrossRef]
- Karimi, M. Immobilization of lipase onto mesoporous magnetic nanoparticles for enzymatic synthesis of biodiesel. Biocatal. Agric. Biotechnol. 2016, 8, 182–188. [Google Scholar] [CrossRef]
- Tran, D.-T.; Chen, C.-L.; Chang, J.-S. Immobilization of Burkholderia sp. Lipase on a ferric silica nanocomposite for biodiesel production. J. Biotechnol. 2012, 158, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, K.; Oda, Y.; Takahashi, R. Application of a Burkholderia cepacia lipase-immobilized silica monolith to batch and continuous biodiesel production with a stoichiometric mixture of methanol and crude jatropha oil. Biotechnol. Biofuels 2011, 4, 42. [Google Scholar] [CrossRef] [PubMed]
- Jegannathan, K.R.; Leong, J.-Y.; Chan, E.-S.; Ravindra, P. Production of biodiesel from palm oil using liquid core lipase encapsulated in κ-carrageenan. Fuel 2010, 89, 2272–2277. [Google Scholar] [CrossRef]
- Zou, Z.; Wang, S.; Jia, J.; Xu, F.; Long, Z.; Hou, X. Ultrasensitive determination of inorganic arsenic by hydride generation-atomic fluorescence spectrometry using Fe3O4@ZIF-8 nanoparticles for preconcentration. Microchem. J. 2016, 124, 578–583. [Google Scholar] [CrossRef]
- Pan, S.; Liu, X.; Xie, Y.; Yi, Y.; Li, C.; Yan, Y.; Liu, Y. Esterification activity and conformation studies of burkholderia cepacia lipase in conventional organic solvents, ionic liquids and their co-solvent mixture media. Bioresour. Technol. 2010, 101, 9822–9824. [Google Scholar] [CrossRef] [PubMed]
- Su, F.; Li, G.-L.; Fan, Y.-L.; Yan, Y.-J. Enhancing biodiesel production via a synergic effect between immobilized rhizopus oryzae lipase and novozym 435. Fuel Process. Technol. 2015, 137, 298–304. [Google Scholar] [CrossRef]





| Enzyme | Substrate | Operating Conditions | System | Acyl Acceptor | Yield (%) | Reusability and Last Yield (%) | References |
|---|---|---|---|---|---|---|---|
| Pseudomonas lipase | Sunflower oil | 45 °C; 5 h | Petroleum ether | Methanol | 79.0 | Non | [49] |
| P. fluorescens lipase | Soybean oil | 35 °C; 90 h | Solvent-free | Methanol | 80.0 | Non | [50] |
| Rhizomucor miehei lipase | Sunflower oil | 40 °C; 48 h | Not specified | Methanol | 91.2 | 4 cycle; 67 | [51] |
| P. expansum lipase | Corn oil | 40 °C; 24 h | Ionic liquids | Methanol | 86.0 | Non | [52] |
| Candida antarctica | Tallow | 45 °C; 36 h | Isopropanol | Methanol | 90.0 | Non | [53] |
| Candida rugosa | Soybean oil | 45 °C; 60 h | Solvent-free | Methanol | 78.5 | 6 cycle; 56 | [38] |
| Novozym 435 | Refined palm oil | 40 °C; 30 h | Solvent-free | Ethanol | 85.0 | Non | [54] |
| B. cepacia lipase | Waste cooking oil | 40 °C; 35 h | N-hexane | Methanol | 91.0 | 5 cycle; 54 | [55] |
| Lipase from Burkholderia sp. C20 | Olive oil | 40 °C; 30 h | Solvent-free | Methanol | 92.0 | Not specified | [56] |
| B. cepacia lipase | Soybean oil | 45 °C;12 h | tert-Butanol | Methanol | 96.8 | 15 cycle; 65 | [24] |
| B. cepacia lipase | Jatropha oil | 40 °C;12 h | Solvent-free | Methanol | 90% | Not specified | [57] |
| B. cepacia lipase | Palm oil | 30 °C; 72 h | Solvent-free | Methanol | 100.0 | 10 cycle; 40 | [58] |
| B. cepacia lipase | Jatropha oil | 35 °C; 24 h | Solvent-free | Methanol | 94.0 | 10 cycle; 89 | [47] |
| B. cepacia lipase | Soybean oil | 40 °C; 12 h | Solvent-free | Ethanol | 93.4 | 8 cycle; 71.3 | This study |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adnan, M.; Li, K.; Wang, J.; Xu, L.; Yan, Y. Hierarchical ZIF-8 toward Immobilizing Burkholderia cepacia Lipase for Application in Biodiesel Preparation. Int. J. Mol. Sci. 2018, 19, 1424. https://doi.org/10.3390/ijms19051424
Adnan M, Li K, Wang J, Xu L, Yan Y. Hierarchical ZIF-8 toward Immobilizing Burkholderia cepacia Lipase for Application in Biodiesel Preparation. International Journal of Molecular Sciences. 2018; 19(5):1424. https://doi.org/10.3390/ijms19051424
Chicago/Turabian StyleAdnan, Miaad, Kai Li, Jianhua Wang, Li Xu, and Yunjun Yan. 2018. "Hierarchical ZIF-8 toward Immobilizing Burkholderia cepacia Lipase for Application in Biodiesel Preparation" International Journal of Molecular Sciences 19, no. 5: 1424. https://doi.org/10.3390/ijms19051424





