2.1. Study of the Aldehyde-Dextran Reactivity
The aldehyde-dextran-Toyopearl (ADT) support was prepared to demonstrate that the aldehydes of an oxidized dextran polymer present the same chemical reactivity as the aldehyde groups of a glyoxyl-agarose support. Glyoxyl groups are short aliphatic aldehyde groups obtained from the oxidation of glyceryl groups. These aldehydes are stable at pH 10, which allows for immobilization onto the support through ε-amino groups from Lys residues [
28]. This methodology, which achieved very high stabilization factors, has been applied for the immobilization-stabilization of many enzymes [
29,
30,
31,
32,
33].
An epoxy-Toyopearl support was activated with cysteine, which has two nucleophilic groups (amino and thiol). This support is activated at pH 7.0 to leave the amino groups of the cysteine-Toyopearl support free. Thus, the support activation occurs only through the reaction between the thiol groups of the cysteine and the epoxy groups of the epoxy-Toyopearl support [
21]. In this manner, the support containing only primary amino groups would react with the aldehyde groups from the 20% oxidized dextran polymer using the Schiff′s base mechanism in the next step of the activation. After reduction with borohydride, Schiff´s bases between amino and aldehyde groups are converted into secondary amino bonds. At this point, all the aldehyde groups of the support are converted to inert hydroxyl groups. To activate the support, a second oxidation would be necessary to obtain reactive aldehyde groups (ADT support).
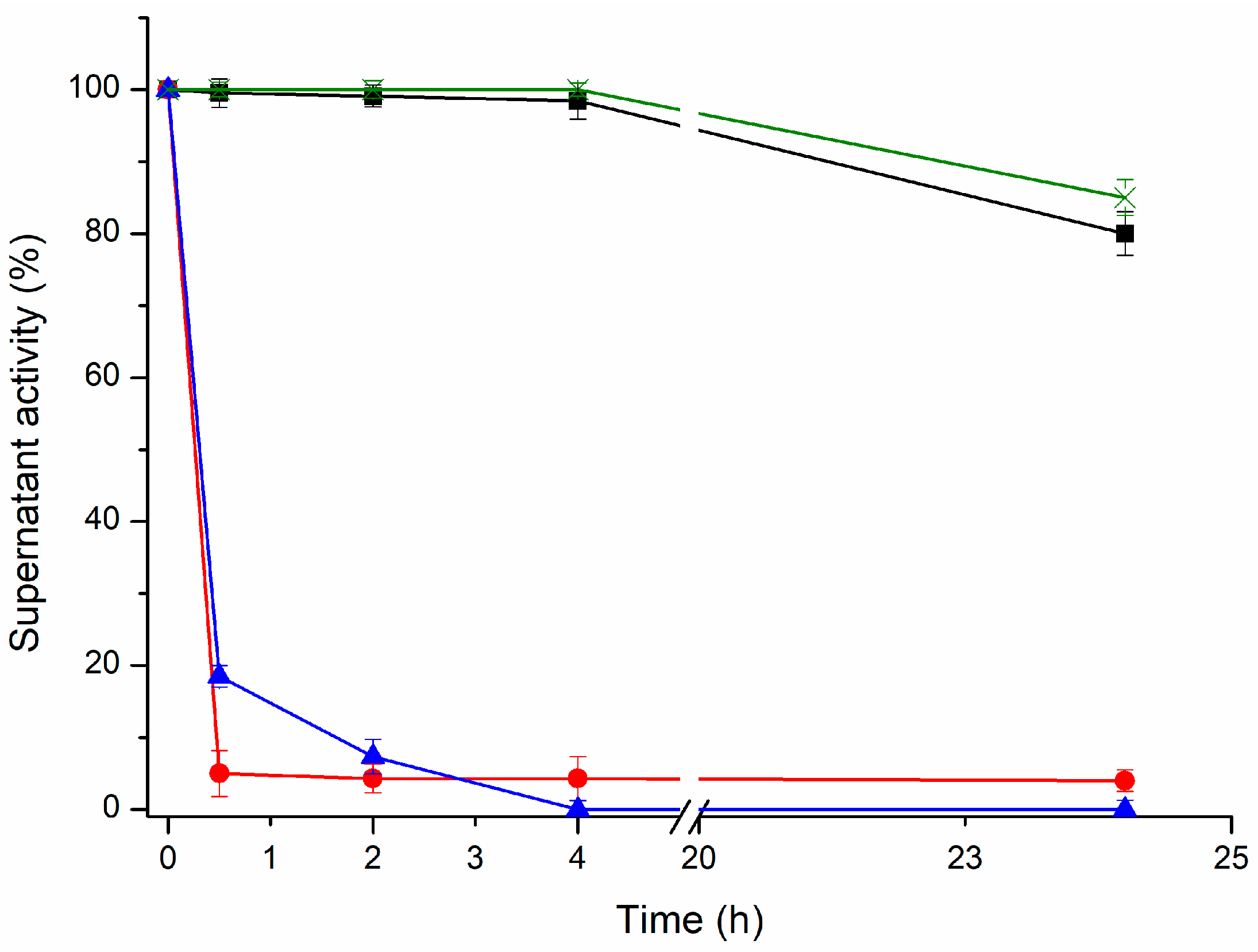
PGA was selected as the model enzyme. It was immobilized on ADT support under different pH and buffer conditions (
Figure 2). At pH 8.5 where only the amino terminal is non-protonated, only 2.5% of the PGA was immobilized on a freshly prepared ADT support after 4 h. A number of isolated aldehyde groups should compose the polymer and their reaction with single amino groups on the enzyme surface occurs via the formation of very unstable Schiff´s bases. However, in the presence of dithiothreitol (DTT), the one-point amino-aldehyde attachments become irreversible. This stabilizing effect of DTT on single Schiff’s bases has been previously reported [
34]. This means that the amino terminus of the PGA is exposed to the medium but that it is unable to react by one-point attachment with aldehyde groups in the absence of stabilizers.
At pH 10, 100% of the PGA was rapidly (4 h) and irreversibly immobilized. At this pH, the ε-NH
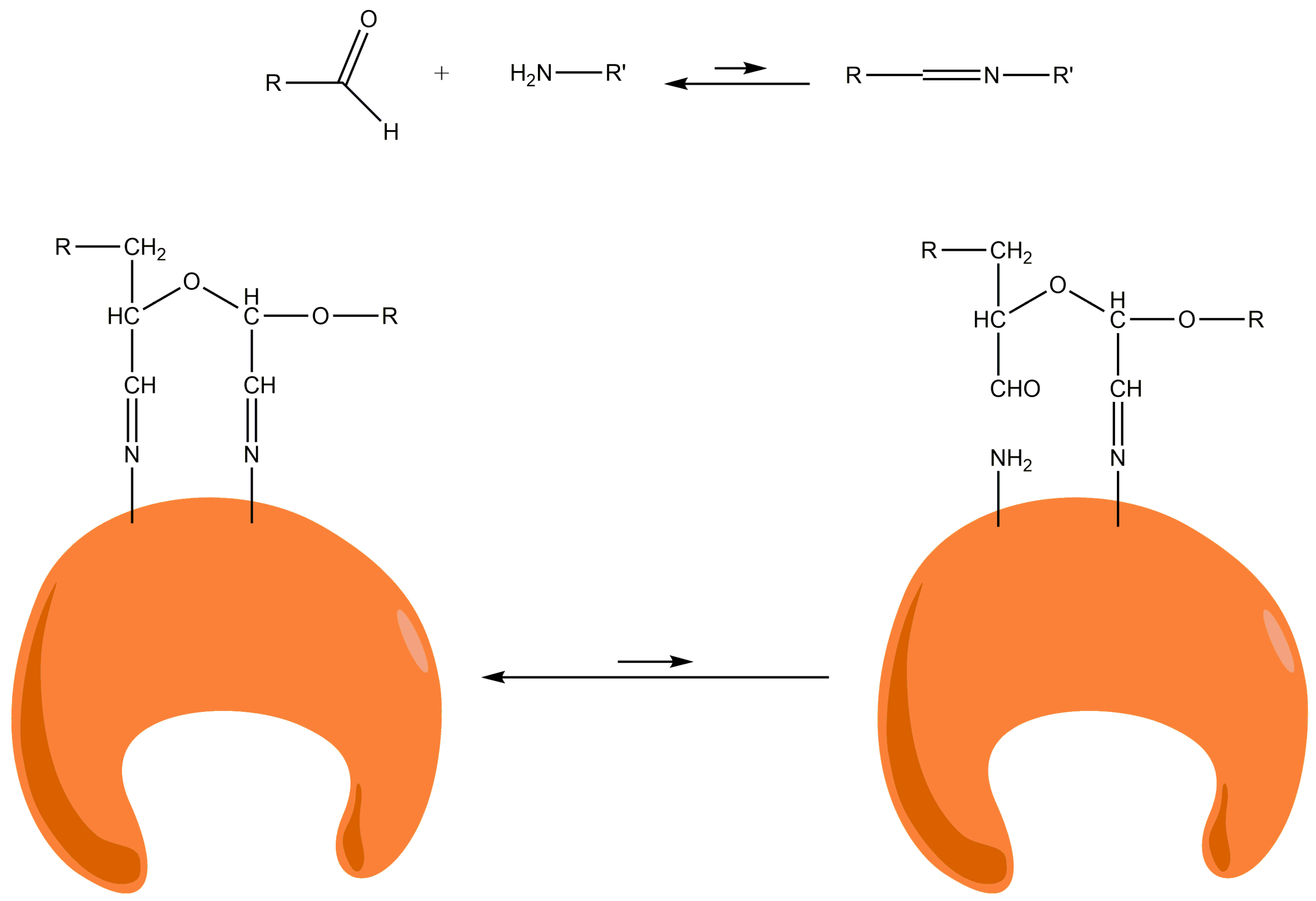
2 groups of the Lys residues on the enzyme surface become partially non-protonated. Such immobilization seems to be due to the formation of stable two-point amino-aldehyde attachments. When the two-point attachment occurs between two amino groups of vicinal Lys and two vicinal aldehyde groups (e.g., produced on the same glucose monomer), there is an intramolecular stabilization of unstable bonds. When one attachment is broken, both the amino and aldehyde group remain very close and the local concentrations of both reagents are extremely high. In this way, the attachment is instantaneously restored and the two-point attachment becomes irreversible (
Figure 3).
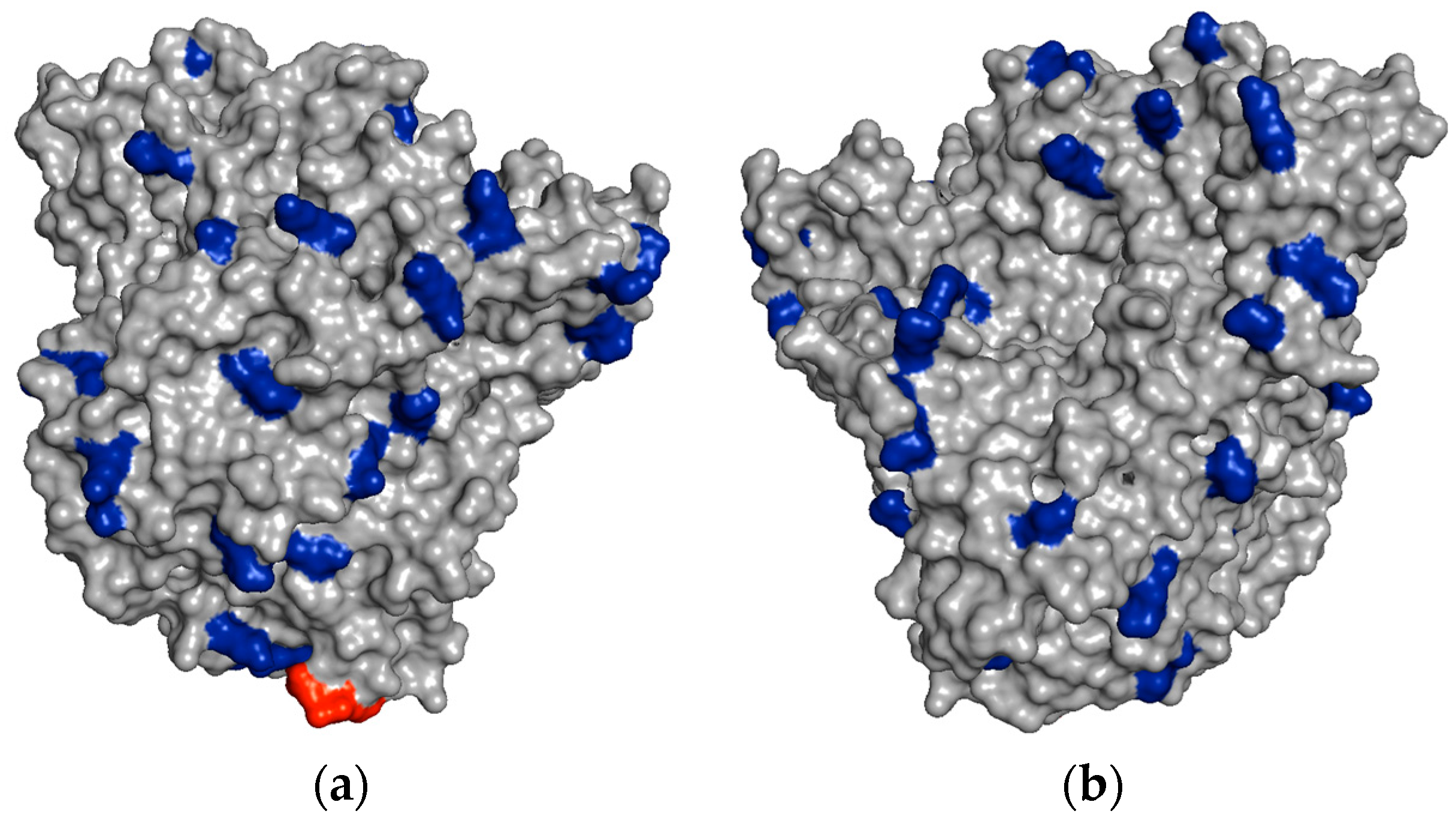
In
Figure 4, it is possible to see some regions of the PGA surface containing several vicinal Lys residues, which could explain the very rapid immobilization of PGA on aldehyde-dextran at pH 10.
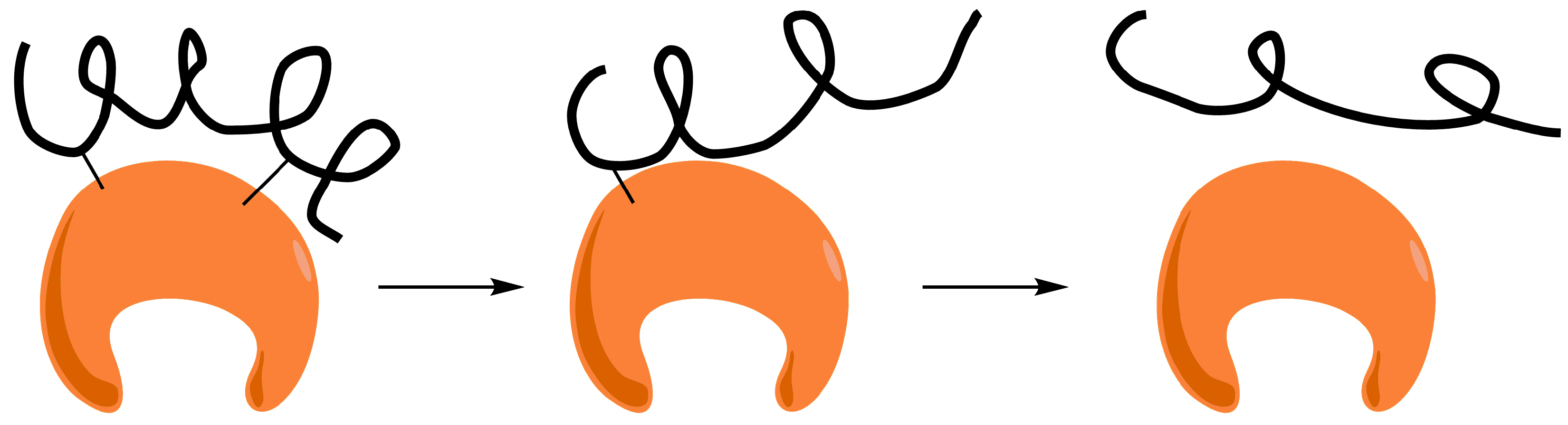
However, the stabilization of unstable Schiff′s bases by two-point attachment is not possible when two amino groups of non-vicinal Lys react with two non-vicinal aldehyde groups placed at a medium or long distance. Dextran is a very flexible polymer and there is no intramolecular stabilization due to two-point attachment. When one attachment is broken, the released amino and aldehyde groups separate very quickly to a distance where neither can interact (the aldehyde in the oxidized dextran moves very fast) and the attachment remains broken. Following this, the second attachment is broken again and irreversible immobilization is impossible (
Figure 5).
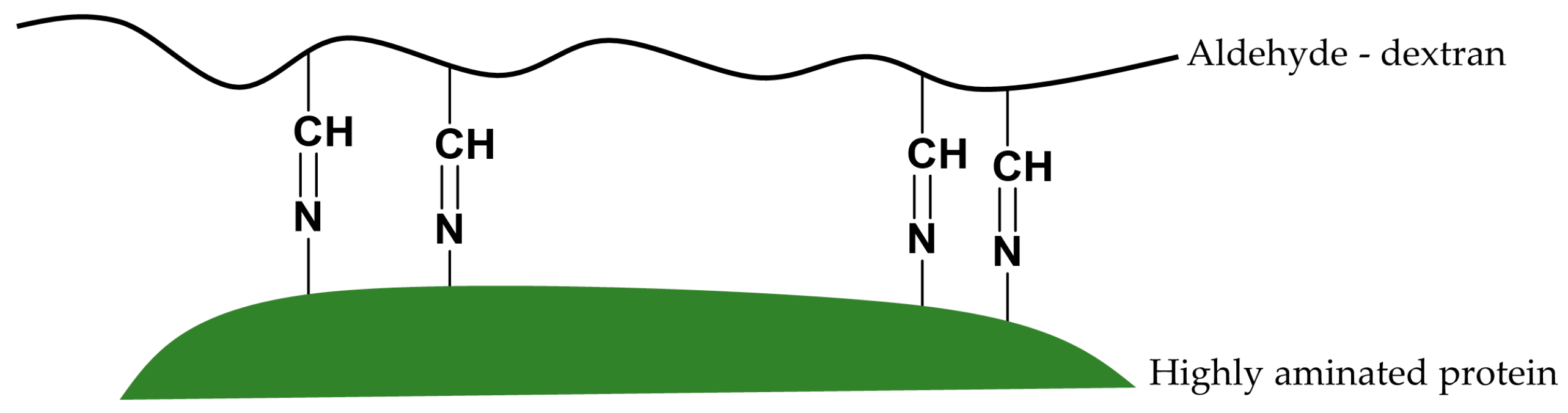
The possible reaction mechanism between vicinal Lys and vicinal aldehyde groups could promote the interesting stabilization of enzymes via intramolecular cross-linking between highly aminated enzyme surfaces and freshly prepared aldehyde-dextran polymers.
In this way, polyaldehyde-dextran has three different reactivities:
Multipoint reaction with poly-aminated structures;
One-point attachment with single amino groups in the presence of Schiff’s base stabilizers;
One-point attachment between single amino groups and modified cyclic structures.
Bearing in mind all the properties of aldehyde-dextran, this polymer is almost ideal for the modification of protein surfaces:
The density of aldehyde groups in the polymer is very high;
There are no steric hindrances for the amino-aldehyde reaction;
The reaction with a poly-aminated structure involves a number of multipoint covalent attachments in the absence of stabilizers.
Multipoint covalent attachments should occur between very close aldehyde groups and very close amino groups. That is, a number of stabilizing cross-links along the enzyme surface may be formed.
2.2. Cross-Linking of Lipases Adsorbed on Octyl-Sepharose Support
The main hypothesis of this work is that aldehyde-dextran is not only an excellent cross-linker agent but also a very good agent for the promotion of intense intramolecular cross-linking, leading to an increment in the stability of the proteins. To demonstrate this fact, the intramolecular cross-linking of three different lipases were studied as model enzymes. The three lipases were immobilized on a hydrophobic OA support that was not expected to suffer any modifications during the chemical amination of enzymes. Initially, two different variables were selected in order to reach different cross-linking intensities: the amination degree of the enzyme surface and the oxidation degree of a 6 kDa dextran-aldehyde.
On the one hand, in order to obtain different amination degrees of the enzyme surface, the amount of 1-ethyl-3-[3-dimethylaminopropil]carbodiimide (EDAC) was gradually increased from 1 mM to 10 mM (for a modification of 50% to 100% of superficial carboxylic acids) [
35].
Table 1 shows the number of Asp, Glu, and Lys from the different lipases used in this study. After the chemical modification of the carboxylic groups of Asp and Glu, the number of reactive amino groups available was increased depending on the amination degree. The exposed amino acid residues are more likely to react with aldehyde groups from the modified dextran.
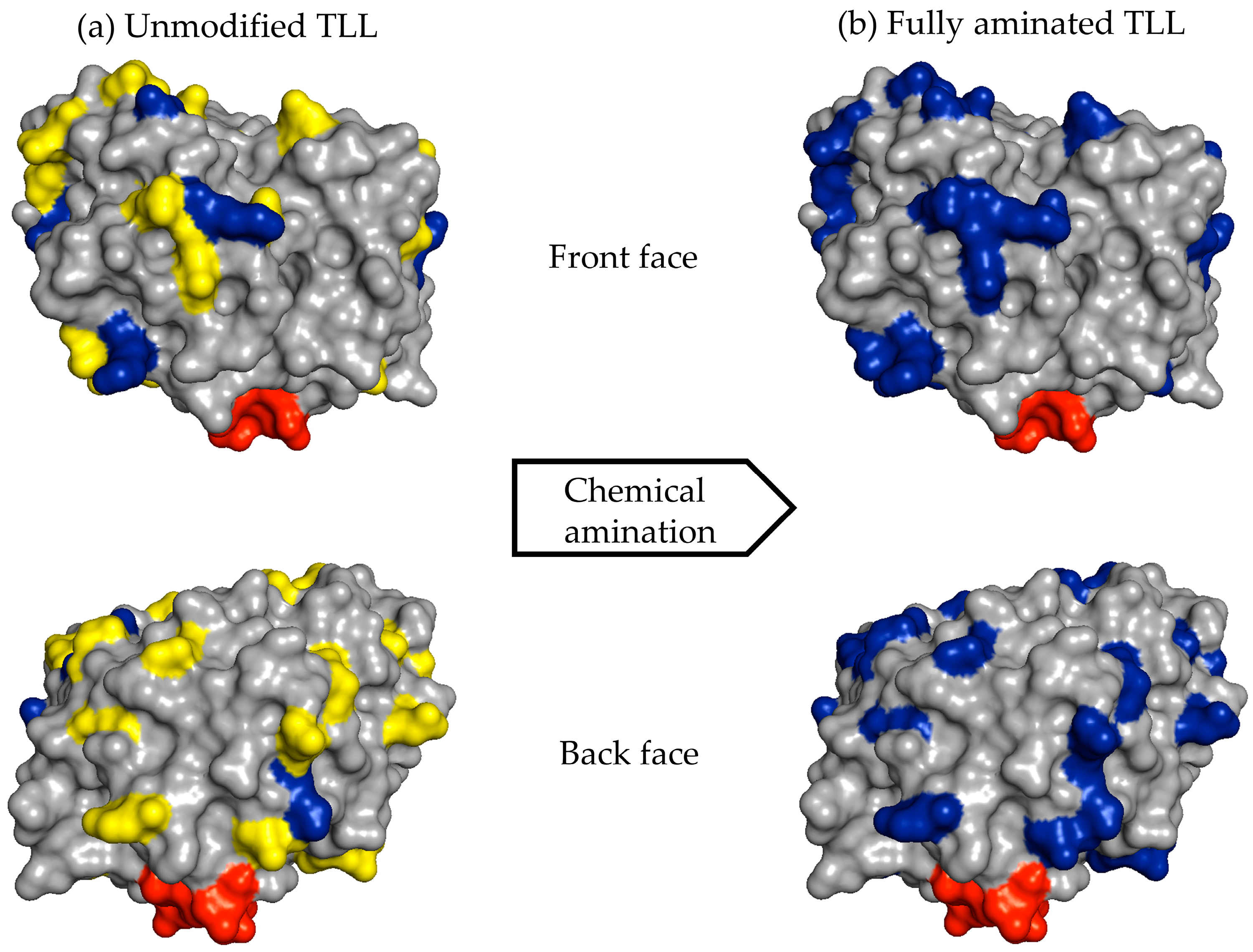
For example, the unmodified TLL has six exposed Lys residues and one N-terminal that can react with the aldehyde groups of the modified dextran (
Figure 6a). After full amination of the TLL, the number of exposed residues that had reactive amino groups could be increased to 30 (
Figure 6b). In the other cases, the exposed amino groups were increased in CALB from 8 to 20 and in RML from 6 to 26 (
Table 1).
On the other hand, the required percentage of aldehyde groups can be obtained by adding a certain amount of sodium periodate for the oxidation of the dextran polymer [
20]. By controlling both parameters, different lipase preparations with different bond amounts between the enzyme and the dextran-aldehyde were obtained.
In all cases, the recovered activities after the cross-linking process were over 70% of their initial catalytic activity (unmodified lipase-OA). The thermal stability of all the lipase preparations was studied by measuring the retained enzyme activity after 24 h using the unmodified lipases as a control. In the TLL preparations, the thermal stability was measured at pH 7.0 and 70 °C, while in the CALB and RML preparations the thermal stability was measured at pH 7.0 and 60 °C (
Table 2). The use of soluble lipases as control was ignored since they were immediately denatured under those conditions.
A quick overview of the results shows a higher retained activity for most of the cross-linked preparations than the unmodified preparations. This means a higher thermal stability of the cross-linked conjugates than of the unmodified ones. Nevertheless, for TLL and RML, the modification with a full amination of the enzyme surface and an intramolecular cross-linking with a fully oxidized dextran led to the highest retained activity after the thermal inactivation process, from 7.5% to 48% and from 6.7% to 40% of retained activity after 24 h, respectively. Therefore, for these two lipases, a higher number of bonds between the enzyme and the dextran-aldehyde promotes an improvement in the thermal stability. For both enzymes, the other preparations with a smaller number of interactions between polymer and enzyme retained lower activities. Nonetheless, the results for CALB showed a different behavior. A fully aminated CALB surface and a fully oxidized dextran did not lead to an increment in the retained activity; this preparation retained 10.6% of its initial activity, whereas the unmodified CALB retained 10% after 24 h at 60 °C and pH 7.0. The highest increment in the retained activity for CALB was the preparation aminated with 1 mM of EDAC and cross-linked with a fully oxidized dextran, which showed a retained activity of 36.3% after the thermal inactivation process. In light of these results, the critical role of the cross-linking intensity is evident and reveals that it is necessary to study all the combinations to optimize the increment in the thermal stability of the enzymes.
2.3. Optimizing the Lipase Cross-Linking
Two more variables were studied to obtain an optimized coating of the surface of the lipases: dextran-aldehyde size and cross-linking time. The half-life of different preparations of lipases with different dextran sizes (from 1.5 kDa to 25 kDa) or cross-linking times (3 or 24 h) were studied under the optimal conditions of surface amination and oxidation degree of the dextran for RML and TLL.
For TLL, the optimal preparation presented a half-life of 36.7 h with a 24 h incubation with a dextran size of 25 kDa (
Table 3). This optimal cross-linking reached a stabilization factor of 42- and 33.3-fold regarding the unmodified or aminated preparation, respectively (
Table 3). In general, all the preparations increased the thermal stability of the immobilized and fully aminated TLL-OA by at least 15-fold. Moreover, no significant differences were found between the half-life times of the preparations, even when the preparations with the same dextran size but different cross-linking times were compared. This result is very interesting since in previous work reported by Rueda and co-workers, TLL immobilized on an agarose support and activated with octyl and glyoxyl groups showed a half-life of only 0.5 h, and the same biocatalyst when fully aminated showed a half-life of 2.25 h at 70 °C and pH 7.0 [
37,
38]. On the other hand, in a previous study conducted by our research group, PEGylation of aminated TLL adsorbed on an OA support led to obtaining a biocatalyst with a half-life of 20 h [
39]. Here, our best biocatalyst had a half-life of 36.7 h under the same inactivation conditions (70 °C and pH 7.0). Therefore, the strategy applied in this work allows a greater improvement in the thermal stability of the TLL, keeping a significant percentage of its catalytic activity intact.
The optimal RML preparation was obtained with the same conditions of cross-linking time and dextran size (24 h and 25 kDa, respectively). This preparation achieved a half-life of 20.5 h, which is 256- and 128-fold more stable than the unmodified RML–OA and the fully aminated RML–OA, respectively (
Table 3). As observed with the TLL preparations, which presented similar half-life times, RML preparations continued with the same tendency. Previous results reported by Rueda and co-workers showed a stabilization factor of the heterofunctional glyoxyl-octyl agarose that was 130-fold more stable than RML-OA at 50 °C and pH 7 [
37]. Again, the applied strategy improved by 2-fold compared with the previous work.
All these results suggest that intramolecular cross-linking between aldehyde-dextran polymers and highly aminated enzyme surfaces is mainly responsible for the significant improvement in enzyme stability. The relative reaction rates of intermolecular (between lipase molecules) and intramolecular (only one lipase molecule) cross-linking are determined by the polymer concentration and chain length. Accordingly, at very low polymer concentrations or when using short-chain length polymers, intramolecular interactions dominate, yielding intense cross-linking of enzyme molecules [
40]. Conversely, the use of long-chain dextran polymers promotes intermolecular cross-linking due to the covalent cross-linking of two or more of the enzyme’s molecules.
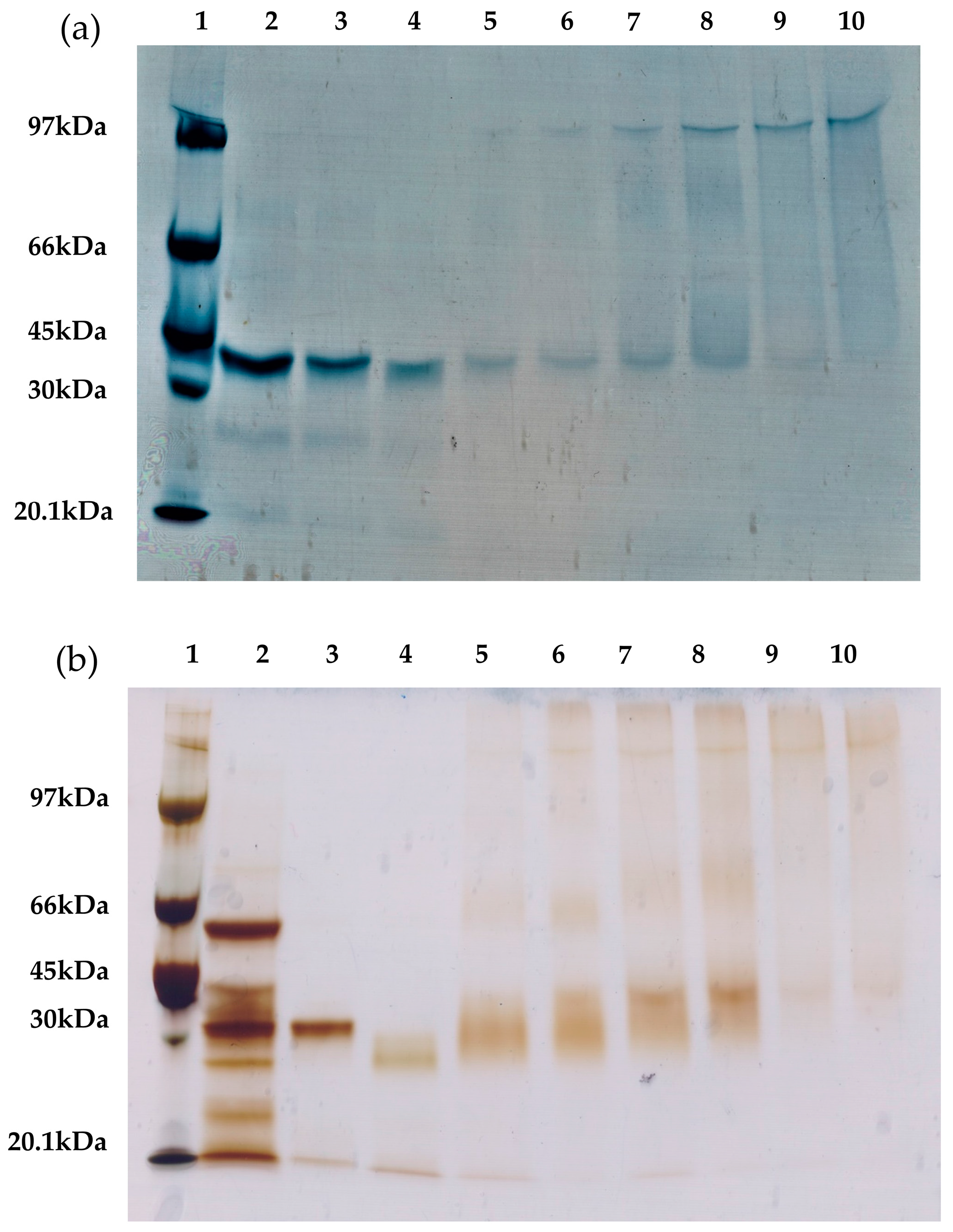
To confirm that the intramolecular cross-linking is primarily responsible for the improved thermal stability of the lipases, different RML and TLL preparations were analyzed via sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) (
Figure 7). The presence of a protein band of high molecular weight indicates intermolecular cross-linking (two or more molecules of the same enzyme are covalently attached). Conversely, the presence of protein bands with a similar size as that of the unmodified lipases indicate intramolecular cross-linking (only the dextran molecules are covalently attached to the enzyme molecules).
Comparing the different cross-linked preparations (
Figure 7), the use of smaller dextran showed a higher amount of intramolecular cross-linked lipases. In contrast, the cross-linked preparations with the 25 kDa dextran polymer showed an increased presence of intermolecular cross-linking. On the one hand, this result confirms our previous assumption: the dextran polymers of smaller molecular weight promote intramolecular cross-links. On the other hand, cross-linking of RML or TLL preparations with a small aldehyde-dextran polymer (1.5 kDa) promotes very relevant stabilization, approximately 20-fold more stable than unmodified conjugates for both lipases. These stabilization factors are similar or significantly lower than the optimal cross-linked preparations with the 25 kDa dextran polymer. This fact proves the major contribution of the intramolecular cross-linking to the increment on the thermal stability of both immobilized lipases. Notwithstanding this, the cross-linking with a 25 kDa dextran polymer revealed that the intermolecular cross-linking also plays an important role in the stabilization of immobilized lipases. Likewise, it has been reported for RML that an increase in the dextran-aldehyde size (≥70 kDa) is the main factor responsible for reducing the desorption of RML molecules from the OA support since this molecular weight favored intense intermolecular and intramolecular cross-linking [
12].
Finally, for both RML and TLL lipases, the optimal cross-linking was obtained after 24 h of cross-linking using the 25 kDa dextran polymer; the CALB was directly cross-linked under the same conditions. In this case, the cross-linking of the partially aminated CALB (1 mM EDAC) and the fully aminated CALB (10 mM EDAC) was tested. In
Table 4 the results of the optimization of the cross-linking for the CALB-OA are summarized.
After the cross-linking with 25 kDa, both preparations (partial and fully aminated) presented higher thermal stability than the unmodified CALB-OA. What was more important was the increase in the thermal stability of the fully aminated and cross-linked preparation in view of the fully aminated CALB-OA, which was 14.3-fold more stable. On the contrary, the total amination of the unmodified CALB-OA destabilized the catalyst 3.4-fold (
Table 4). Siddiqui and Cavicchioli modified the soluble CALB with oxidized 40 kDa dextran polymer [
41]. The half-life of the modified CALB was only 1.4 h at 65 °C and pH 7.0. Our results are also an improvement from the previous ones published by Rueda and co-workers, who obtained an increase of 2.5-fold on the thermal stability using octyl-glyoxyl agarose as a support compared to CALB-OA at 70 °C and pH 7 [
37].