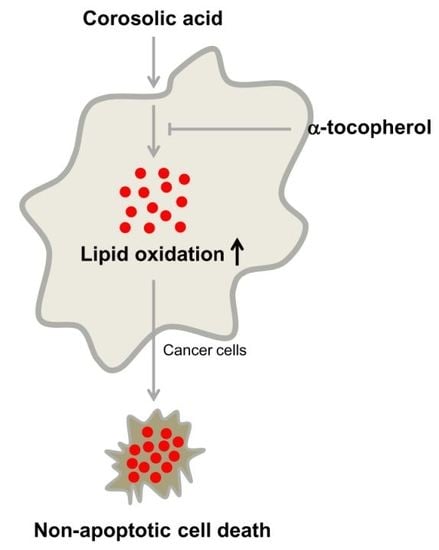
Corosolic Acid Induces Non-Apoptotic Cell Death through Generation of Lipid Reactive Oxygen Species Production in Human Renal Carcinoma Caki Cells
Abstract
:1. Introduction
2. Results
2.1. Corosolic Acid Induces Caspase-Independent Cell Death in Renal Carcinoma Caki Cells
2.2. Corosolic Acid-Induced Cell Death Is not Associated with Necroptosis
2.3. The Generation of ROS Is not Involved in Corosolic Acid-Induced Cell Death
2.4. Corosolic Acid-Mediated Cell Death Is Independent of Ferroptosis
2.5. α-Tocopherol Protects Against Corosolic Acid-Induced ROS Generation, Lipid Peroxidation, and Cell Death
2.6. Effect of Corosolic Acid on Cell Death in Other Cancer and Normal Cells
3. Discussion
4. Materials and Methods
4.1. Cell Cultures and Materials
4.2. Cell Viability Assay
4.3. Lactate Dehydrogenase (LDH) Release Assay
4.4. Asp–Glu–Val–Asp–ase (DEVDase) Activity Assay
4.5. Western Blot Analysis
4.6. Annexin V and 7-AAD Staining and Propidium Iodide (PI) Uptake
4.7. Small Interfering RNA (siRNA)
4.8. Measurement of ROS
4.9. Detection of Lipid Peroxidation
4.10. Statistical Analysis
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| ROS | Reactive oxygen species |
| STAT | Signal transducer and activator of transcription |
| CHX | Cycloheximide |
| PARP | Poly (ADP-ribose) polymerase |
| 7-AAD | 7-Aminoactinomycin D |
| PI | Propidium iodide |
| RIP1 | Receptor-interacting protein kinase1 |
| AIF | Apoptosis-inducing factor |
| NAC | N-acetyl-l-cysteine |
| DFO | Deferoxamine |
References
- Devasagayam, T.P.; Tilak, J.C.; Boloor, K.K.; Sane, K.S.; Ghaskadbi, S.S.; Lele, R.D. Free radicals and antioxidants in human health: Current status and future prospects. J. Assoc. Physicians India 2004, 52, 794–804. [Google Scholar] [PubMed]
- Boonstra, J.; Post, J.A. Molecular events associated with reactive oxygen species and cell cycle progression in mammalian cells. Gene 2004, 337, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Toyokuni, S.; Okamoto, K.; Yodoi, J.; Hiai, H. Persistent oxidative stress in cancer. FEBS Lett. 1995, 358, 1–3. [Google Scholar] [CrossRef]
- Bielski, B.H.; Arudi, R.L.; Sutherland, M.W. A study of the reactivity of HO2/O2- with unsaturated fatty acids. J. Biol. Chem. 1983, 258, 4759–4761. [Google Scholar] [PubMed]
- Gaschler, M.M.; Stockwell, B.R. Lipid peroxidation in cell death. Biochem. Biophys. Res. Commun. 2017, 482, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Ayala, A.; Munoz, M.F.; Arguelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef] [PubMed]
- Barrera, G. Oxidative stress and lipid peroxidation products in cancer progression and therapy. ISRN Oncol. 2012, 2012, 137289. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Li, Y.; Zhang, Q.; Wei, X.; Peng, A.; Chen, L.; Wei, Y. Triterpene acids isolated from Lagerstroemia speciosa leaves as α-glucosidase inhibitors. Phytother. Res. 2009, 23, 614–618. [Google Scholar] [CrossRef] [PubMed]
- Liby, K.T.; Yore, M.M.; Sporn, M.B. Triterpenoids and rexinoids as multifunctional agents for the prevention and treatment of cancer. Nat. Rev. Cancer 2007, 7, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, M.; Matsuyama, F.; Ueda, N.; Egawa, K.; Takemoto, J.; Kajimoto, Y.; Yonaha, N.; Miura, T.; Kaneko, T.; Nishi, Y.; et al. Effect of corosolic acid on postchallenge plasma glucose levels. Diabetes Res. Clin. Pract. 2006, 73, 174–177. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Ge, R.; Du, J.; Xin, H.; Yi, T.; Sheng, J.; Wang, Y.; Ling, C. Corosolic acid induces apoptosis through mitochondrial pathway and caspase activation in human cervix adenocarcinoma HeLa cells. Cancer Lett. 2009, 284, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Sung, B.; Kang, Y.J.; Kim, D.H.; Hwang, S.Y.; Lee, Y.; Kim, M.; Yoon, J.H.; Kim, C.M.; Chung, H.Y.; Kim, N.D. Corosolic acid induces apoptotic cell death in HCT116 human colon cancer cells through a caspase-dependent pathway. Int. J. Mol. Med. 2014, 33, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Uto, T.; Sakamoto, A.; Tung, N.H.; Fujiki, T.; Kishihara, K.; Oiso, S.; Kariyazono, H.; Morinaga, O.; Shoyama, Y. Anti-proliferative activities and apoptosis induction by triterpenes derived from Eriobotrya japonica in Human Leukemia Cell Lines. Int. J. Mol. Sci. 2013, 14, 4106–4120. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Zhang, H.; Tong, D.; Tan, Z.; Han, D.; Ji, F.; Hu, W. Corosolic acid triggers mitochondria and caspase-dependent apoptotic cell death in osteosarcoma MG-63 cells. Phytother. Res. 2011, 25, 1354–1361. [Google Scholar] [CrossRef] [PubMed]
- Nho, K.J.; Chun, J.M.; Kim, H.K. Corosolic acid induces apoptotic cell death in human lung adenocarcinoma A549 cells in vitro. Food Chem. Toxicol. 2013, 56, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Cha, E.Y.; Thuong, P.T.; Kim, J.Y.; Ahn, M.S.; Sul, J.Y. Down-regulation of human epidermal growth factor receptor 2/neu oncogene by corosolic acid induces cell cycle arrest and apoptosis in NCI-N87 human gastric cancer cells. Biol. Pharm. Bull. 2010, 33, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, Y.; Komohara, Y.; Ikeda, T.; Takeya, M. Corosolic acid inhibits glioblastoma cell proliferation by suppressing the activation of signal transducer and activator of transcription-3 and nuclear factor-κ B in tumor cells and tumor-associated macrophages. Cancer Sci. 2011, 102, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Yuan, H.; Shan, S.; Xu, G.; Yu, J.; Zhao, C.; Mou, X. Corosolic acid inhibits the proliferation of osteosarcoma cells by inducing apoptosis. Oncol. Lett. 2016, 12, 4187–4194. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Mayo, M.W.; Baldwin, A.S., Jr. TNF- and cancer therapy-induced apoptosis: Potentiation by inhibition of NF-κB. Science 1996, 274, 784–787. [Google Scholar] [CrossRef] [PubMed]
- Darzynkiewicz, Z.; Juan, G.; Li, X.; Gorczyca, W.; Murakami, T.; Traganos, F. Cytometry in cell necrobiology: Analysis of apoptosis and accidental cell death (necrosis). Cytometry 1997, 27, 1–20. [Google Scholar] [CrossRef]
- Zhang, D.; Lin, J.; Han, J. Receptor-interacting protein (RIP) kinase family. Cell. Mol. Immunol. 2010, 7, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Delavallee, L.; Cabon, L.; Galan-Malo, P.; Lorenzo, H.K.; Susin, S.A. AIF-mediated caspase-independent necroptosis: A new chance for targeted therapeutics. IUBMB Life 2011, 63, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Cabon, L.; Galan-Malo, P.; Bouharrour, A.; Delavallee, L.; Brunelle-Navas, M.N.; Lorenzo, H.K.; Gross, A.; Susin, S.A. BID regulates AIF-mediated caspase-independent necroptosis by promoting BAX activation. Cell Death Differ. 2012, 19, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Simon, H.U.; Haj-Yehia, A.; Levi-Schaffer, F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis 2000, 5, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yi, J. Cancer cell killing via ROS: To increase or decrease, that is the question. Cancer Biol. Ther. 2008, 7, 1875–1884. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Hou, W.; Song, X.; Yu, Y.; Huang, J.; Sun, X.; Kang, R.; Tang, D. Ferroptosis: Process and function. Cell Death Differ. 2016, 23, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; Stockwell, B.R. Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem. Biol. 2008, 15, 234–245. [Google Scholar] [CrossRef] [PubMed]
- Probst, L.; Dachert, J.; Schenk, B.; Fulda, S. Lipoxygenase inhibitors protect acute lymphoblastic leukemia cells from ferroptotic cell death. Biochem. Pharmacol. 2017, 140, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, K.L.; Kirkpatrick, P.J.; Weissberg, P.L.; Challis, I.R.; Dennis, I.F.; Freeman, M.A.; Mitchinson, M.J. Oral α-tocopherol supplementation inhibits lipid oxidation in established human atherosclerotic lesions. Free Radic. Res. 2003, 37, 1235–1244. [Google Scholar] [CrossRef] [PubMed]
- Vandenabeele, P.; Galluzzi, L.; Vanden Berghe, T.; Kroemer, G. Molecular mechanisms of necroptosis: An ordered cellular explosion. Nat. Rev. Mol. Cell Biol. 2010, 11, 700–714. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Deng, Q.; Bode, A.M.; Dong, Z.; Cao, Y. The role of necroptosis, an alternative form of cell death, in cancer therapy. Expert Rev. Anticancer Ther. 2013, 13, 883–893. [Google Scholar] [CrossRef] [PubMed]
- Evans, H.M.; Bishop, K.S. On the existence of a hitherto unrecognized dietary factor essential for reproduction. Science 1922, 56, 650–651. [Google Scholar] [CrossRef] [PubMed]
- Ricciarelli, R.; Zingg, J.M.; Azzi, A. Vitamin E: Protective role of a Janus molecule. FASEB J. 2001, 15, 2314–2325. [Google Scholar] [CrossRef] [PubMed]
- Traber, M.G.; Atkinson, J. Vitamin E, antioxidant and nothing more. Free Radic. Biol. Med. 2007, 43, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Mileva, M.; Bakalova, R.; Tancheva, L.; Galabov, A.; Ribarov, S. Effect of vitamin E supplementation on lipid peroxidation in blood and lung of influenza virus infected mice. Comp. Immunol. Microbiol. Infect. Dis. 2002, 25, 1–11. [Google Scholar] [CrossRef]
- Rao, M.V.; Sharma, P.S. Protective effect of vitamin E against mercuric chloride reproductive toxicity in male mice. Reprod. Toxicol. 2001, 15, 705–712. [Google Scholar] [CrossRef]
- Sigounas, G.; Anagnostou, A.; Steiner, M. dl-α-tocopherol induces apoptosis in erythroleukemia, prostate, and breast cancer cells. Nutr. Cancer 1997, 28, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Pratico, D.; Tangirala, R.K.; Rader, D.J.; Rokach, J.; FitzGerald, G.A. Vitamin E suppresses isoprostane generation in vivo and reduces atherosclerosis in ApoE-deficient mice. Nat. Med. 1998, 4, 1189–1192. [Google Scholar] [CrossRef] [PubMed]
- Pedeboscq, S.; Rey, C.; Petit, M.; Harpey, C.; De Giorgi, F.; Ichas, F.; Lartigue, L. Non-antioxidant properties of α-tocopherol reduce the anticancer activity of several protein kinase inhibitors in vitro. PLoS ONE 2012, 7, e36811. [Google Scholar] [CrossRef] [PubMed]






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woo, S.M.; Seo, S.U.; Min, K.-j.; Im, S.-S.; Nam, J.-O.; Chang, J.-S.; Kim, S.; Park, J.-W.; Kwon, T.K. Corosolic Acid Induces Non-Apoptotic Cell Death through Generation of Lipid Reactive Oxygen Species Production in Human Renal Carcinoma Caki Cells. Int. J. Mol. Sci. 2018, 19, 1309. https://doi.org/10.3390/ijms19051309
Woo SM, Seo SU, Min K-j, Im S-S, Nam J-O, Chang J-S, Kim S, Park J-W, Kwon TK. Corosolic Acid Induces Non-Apoptotic Cell Death through Generation of Lipid Reactive Oxygen Species Production in Human Renal Carcinoma Caki Cells. International Journal of Molecular Sciences. 2018; 19(5):1309. https://doi.org/10.3390/ijms19051309
Chicago/Turabian StyleWoo, Seon Min, Seung Un Seo, Kyoung-jin Min, Seung-Soon Im, Ju-Ock Nam, Jong-Soo Chang, Shin Kim, Jong-Wook Park, and Taeg Kyu Kwon. 2018. "Corosolic Acid Induces Non-Apoptotic Cell Death through Generation of Lipid Reactive Oxygen Species Production in Human Renal Carcinoma Caki Cells" International Journal of Molecular Sciences 19, no. 5: 1309. https://doi.org/10.3390/ijms19051309