Microfabrication-Based Three-Dimensional (3-D) Extracellular Matrix Microenvironments for Cancer and Other Diseases
Abstract
:1. Introduction
2. Microfabrication Techniques in the Effective Reconstruction of 3-D ECM Microenvironments In Vitro
2.1. Microfabrication Techniques Contribute to the Construction of Special Structures, Such as Interface, Regions of Oriented Collagen Fibers, and Vascular Structures in the ECM
2.2. Advanced Microfabrication Techniques Can Realize a Systematic and Quantitative Regulation of Cell Geometry, Such as Density, Volume, Size, Shape, and Location, in a Complex 3-D ECM Setting for Long Periods of Culture
2.3. Modern Microfabrication Techniques Can Help to Control Precisely the Concentration Gradient of the Medium, with Respect to Growth Factosr, Nutrients, and Drugs, in the ECM
3. New Discoveries about Cancer and Other Diseases, Based on 3-D ECM Systems In Vitro
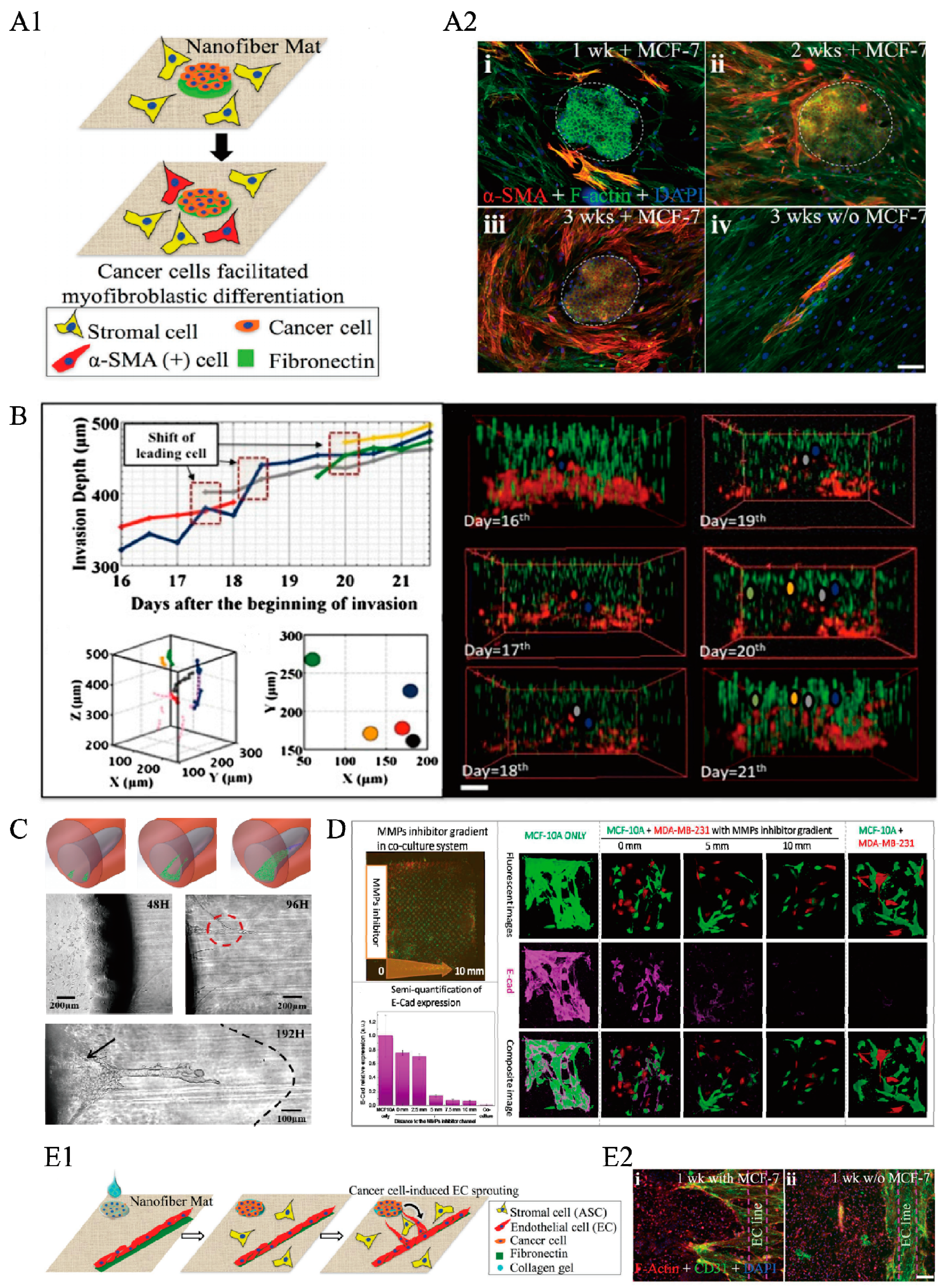
3.1. The Impact of Tumor Biotic Factors and Secretion on Normal Tissues and Cells via 3-D ECM Microenvironments
3.2. The Collective Invasion of Cancer Cells into a Homogeneous ECM System Driven by Medium, Nutrients, and Growth Factor Gradients
3.3. Intravasation of Cancer Cells into a Heterogeneous ECM Structure with the Help of Gradients, Stress, or Aligned Collagen Fibers
3.4. Biophysical and Mechanical Properties of the ECM, such as Geometry, Density, Stiffness, Contractility, and Crosslinking of Collagen Fibers, Influence Tumor Angiogenesis Both Directly and Indirectly
3.5. 3-D Microenvironments for Drug Screening
3.6. Other Diseases (Wound Healing, Clonal Acinar Development, Differentiation of Embryoid Bodies, Neurodevelopmental Processes, Cartilage Defects) Related to 3-D Cells–ECM Microenvironments
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| 3-D | Three dimensional |
| 2-D | Two dimensional |
| ECM | Extracellular matrix |
| BSA | Bovine serum albumin |
| TGF | Transforming growth factor |
| HUVEC | Human umbilical vein endothelial cells |
| MMPs | Matrix metalloproteinases |
| EGF | Epidermal growth factor |
| DIGME | Diskoid In Geometrically Micropatterned ECM |
| EMT | Epithelial-mesenchymal transition |
| PEG | Polyethylene glycol |
| PEGDA | Poly(ethylene glycol) diacrylate |
| DMD-PP | Digital micromirror device-based projection printing |
| FLDW | Femtosecond laser direct writing |
| MMPs | Matrix Metalloproteinases |
| PVA | Poly(vinyl alcohol) |
References
- Sporn, M.B. The war on cancer. Lancet 1996, 347, 1377. [Google Scholar] [CrossRef]
- Sleeman, J.P.; Steeg, P.S. Cancer metastasis as a therapeutic target. Eur. J. Cancer 2010, 46, 1177–1180. [Google Scholar] [CrossRef] [PubMed]
- Mowers, E.E.; Sharifi, M.N.; Macleod, K.F. Autophagy in cancer metastasis. Oncogene 2017, 36, 1619–1630. [Google Scholar] [CrossRef] [PubMed]
- Seyfried, T.N.; Huysentruyt, L.C. On the Origin of Cancer Metastasis. Crit. Rev. Oncog. 2013, 18, 43–73. [Google Scholar] [CrossRef] [PubMed]
- Wells, A.; Grahovac, J.; Wheeler, S.; Ma, B.; Lauffenburger, D.A. Targeting tumor cell motility as a strategy against invasion and metastasis. Trends Pharmacol. Sci. 2013, 34, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Butcher, D.T.; Alliston, T.; Weaver, V.M. A tense situation: Forcing tumour progression. Nat. Rev. Cancer 2009, 9, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Sinkus, R.; Lorenzen, J.; Schrader, D.; Lorenzen, M.; Dargatz, M.; Holz, D.J. High-resolution tensor MR elastography for breast tumour detection. Phys. Med. Biol. 2000, 45, 1649–1664. [Google Scholar] [CrossRef] [PubMed]
- Paszek, M.J.; Zahir, N.; Johnson, K.R.; Lakins, J.N.; Rozenberg, G.I.; Gefen, A.; Reinhartking, C.A.; Margulies, S.S.; Dembo, M.; Boettiger, D. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005, 3, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Broekelmann, T.J.; Limper, A.H.; Colby, T.V.; Mcdonald, J.A. Transforming growth factor beta 1 is present at sites of extracellular matrix gene expression in human pulmonary fibrosis. Proc. Natl. Acad. Sci. USA 1991, 88, 6642–6646. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Atsuchi, N.; Ooshima, A.; Takeshita, A.; Ueno, H. Blockade of type β transforming growth factor signaling prevents liver fibrosis and dysfunction in the rat. Proc. Natl. Acad. Sci. USA 1999, 96, 2345–2349. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Lee, E.K.; Choo, J.; Yuh, J.; Hong, J.W. Micro 3D cell culture systems for cellular behavior studies: Culture matrices, devices, substrates, and in-situ sensing methods. Biotech. J. 2015, 10, 1682–1688. [Google Scholar] [CrossRef] [PubMed]
- Kolacna, L.; Bakesova, J.; Varga, F.; Kosťakova, E.; Planka, L.; Necas, A.; Lukas, D.; Amler, E.; Pelouch, V. Biochemical and Biophysical Aspects of Collagen Nanostructure in the Extracellular Matrix. Physiol. Res. 2007, 56, S51–S60. [Google Scholar] [PubMed]
- Gupta, G.P.; Massague, J. Cancer Metastasis: Building a Framework. Cell 2006, 127, 679–695. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Chen, S.; Yuan, W.; Fan, Q.; Tian, J.; Wang, X.; Chen, L.; Zhang, X.; Wei, W.; Liu, R.; et al. Oriented collagen fibers direct tumor cell intravasation. Proc. Natl. Acad. Sci. USA 2016, 113, 11208–11213. [Google Scholar] [CrossRef] [PubMed]
- Genovese, L.; Zawada, L.; Tosoni, A.; Ferri, A.; Zerbi, P.; Allevi, R.; Nebuloni, L.; Alfano, M. Cellular Localization, Invasion, and Turnover Are Differently Influenced by Healthy and Tumor-Derived Extracellular Matrix. Tissue Eng. Part A 2014, 20, 2005. [Google Scholar] [CrossRef] [PubMed]
- Alfano, M.; Nebuloni, M.; Allevi, R.; Zerbi, P.; Longhi, E.; Luciano, R.; Locatelli, I.; Pecoraro, A.; Indrieri, M.; Speziali, C.; et al. Linearized texture of three-dimensional extracellular matrix is mandatory for bladder cancer cell invasion. Sci. Rep. 2016, 6, 36128. [Google Scholar] [CrossRef] [PubMed]
- Nebuloni, M.; Albarello, L.; Andolfo, A.; Magagnotti, C.; Genovese, L.; Locatelli, I.; Tonon, G.; Longhi, E.; Zerbi, P.; Allevi, R.; et al. Insight on Colorectal Carcinoma Infiltration by Studying Perilesional Extracellular Matrix. Sci Rep. 2016, 6, 22522. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.; Liu, R.; Jiao, Y.; Tian, C.; Farrell, J.D.; Diao, W.; Wang, X.; Zhang, F.; Yuan, W.; Han, H. A novel 3-D bio-microfluidic system mimicking in vivo heterogeneous tumour microstructures reveals complex tumour-stroma interactions. Lab Chip 2017. [Google Scholar] [CrossRef] [PubMed]
- Sung, K.E.; Beebe, D.J. Microfluidic 3D models of cancer. Adv. Drug Deliv. Rev. 2014, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Muthuswamy, S.K. 3D culture reveals a signaling network. Breast Cancer Res. 2011, 13, 103. [Google Scholar] [CrossRef] [PubMed]
- Patil, P.U.; Dambrosio, J.; Inge, L.J.; Mason, R.W.; Rajasekaran, A.K. Carcinoma cells induce lumen filling and EMT in epithelial cells through soluble E-cadherin-mediated activation of EGFR. J. Cell Sci. 2015, 128, 4366–4379. [Google Scholar] [CrossRef] [PubMed]
- Tanner, K.; Gottesman, M.M. Beyond 3D culture models of cancer. Sci. Transl. Med. 2015, 7. [Google Scholar] [CrossRef] [PubMed]
- Bao, M.; Xie, J.; Piruska, A.; Wts, H. 3D microniches reveal the importance of cell size and shape. Nature Commun. 2017. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimkhani, M.R.; Neiman, J.A.; Raredon, M.S.; Hughes, D.J.; Griffith, L.G. Bioreactor Technologies to Support Liver Function in vitro. Adv. Drug Deliv. Rev. 2014, 132–157. [Google Scholar] [CrossRef] [PubMed]
- Esch, M.B.; King, T.L.; Shuler, M.L. The Role of Body-on-a-Chip Devices in Drug and Toxicity Studies. Annu. Rev. Biomed. Eng. 2011, 13, 55–72. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.P.; Perezcastillejos, R.; Love, J.C.; Whitesides, G.M. Partitioning microfluidic channels with hydrogel to construct tunable 3-D cellular microenvironments. Biomaterials 2008, 29, 1853–1861. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Ishii, K.S.; Fan, Q.; Ohta, A.T. Hydrogel microrobots actuated by optically generated vapour bubbles. Lab Chip 2012, 12, 3821–3826. [Google Scholar] [CrossRef] [PubMed]
- Hahan, N.P.; Dolega, M.E.; Liguori, L.; Marquette, C.; Gac, S.L.; Gidrol, X.; Martin, D.K. A 3D Toolbox to Enhance Physiological Relevance of Human Tissue Models. Trends Biotechnol. 2016, 34, 757–769. [Google Scholar]
- Jia, C.; Luo, B.; Wang, H.; Bian, Y.; Li, X.; Li, S.; Wang, H. Precise and Arbitrary Deposition of Biomolecules onto Biomimetic Fibrous Matrices for Spatially Controlled Cell Distribution and Functions. Adv. Mater. 2017, 1701154. [Google Scholar] [CrossRef] [PubMed]
- Loo, Y.; Hauser, C.A. Bioprinting synthetic self-assembling peptide hydrogels for biomedical applications. Biomed. Mater. 2015, 11. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Ozbolat, I.T. Bioprinting scale-up tissue and organ constructs for transplantation. Trends Biotechnol. 2015, 33, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Boghaert, E.; Gleghorn, J.P.; Lee, K.; Gjorevski, N.; Radisky, D.C.; Nelson, C.M. Host epithelial geometry regulates breast cancer cell invasiveness. Proc. Natl. Acad. Sci. USA 2012, 109, 19632–19637. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Liang, L.; Jiao, Y.; Liu, L. Enhanced Invasion of Metastatic Cancer Cells via Extracellular Matrix Interface. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Romero, R.; Han, Y.M.; Kim, H.C.; Kim, C.J.; Hong, J.S.; Huh, D. Placenta-on-a-chip: A novel platform to study the biology of the human placenta. J. Matern. Fetal Neonatal Med. 2016, 29, 1046–1054. [Google Scholar] [CrossRef] [PubMed]
- Kraning-Rush, C.M.; Carey, S.P.; Lampi, M.C.; Reinhart-King, C.A. Microfabricated collagen tracks facilitate single cell metastatic invasion in 3D. Integr. Biol. 2013, 5, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Soman, P.; Kelber, J.A.; Lee, J.W.; Wright, T.N.; Vecchio, K.S.; Klemke, R.L.; Chen, S. Cancer cell migration within 3D layer-by-layer microfabricated photocrosslinked PEG scaffolds with tunable stiffness. Biomaterials 2012, 3, 7064–7070. [Google Scholar] [CrossRef] [PubMed]
- Sie, Y.D.; Li, Y.C.; Chang, N.S.; Campagnola, P.J.; Chen, S.J. Fabrication of three-dimensional multi-protein microstructures for cell migration and adhesion enhancement. Biomed. Opt. Express 2015, 6, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Markovic, M.; Van Hoorick, J.; Holzl, K.; Tromayer, M.; Gruber, P.M.; Nurnberger, S.; Dubruel, P.; Vlierberghe, S.V.; Liska, R.; Ovsianikov, A. Hybrid Tissue Engineering Scaffolds by Combination of Three-Dimensional Printing and Cell Photoencapsulation. J. Nanotechnol. Eng. Med. 2015, 6, 210011–210017. [Google Scholar] [CrossRef] [PubMed]
- Casey, J.; Yue, X.; Nguyen, T.D.; Acun, A.; Zellmer, V.R.; Zhang, S.; Zorlutuna, P. 3D hydrogel-based microwell arrays as a tumor microenvironment model to study breast cancer growth. Biomed. Mater. 2017, 12, 025009. [Google Scholar] [CrossRef] [PubMed]
- Alobaidi, A.A.; Sun, B. Probing three-dimensional collective cancer invasion with DIGME. Cancer Converg. 2017, 1. [Google Scholar] [CrossRef]
- Alobaidi, A.A.; Xu, Y.; Chen, S.; Jiao, Y.; Sun, B. Probing cooperative force generation in collective cancer invasion. Phys. Biol. 2017, 14, 045005. [Google Scholar] [CrossRef] [PubMed]
- Bissell, M.J.; Radisky, D.C. Putting tumours in context. Nat. Rev. Cancer 2001, 1, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Nelson, C.M.; VanDuijn, M.M.; Inman, J.L.; Fletcher, D.A.; Bissell, M.J. Tissue Geometry Determines Sites of Mammary Branching Morphogenesis in Organotypic Cultures. Science 2006, 314, 298–300. [Google Scholar] [CrossRef] [PubMed]
- Khademhosseini, A.; Langer, R. Microengineered hydrogels for tissue engineering. Biomaterials 2007, 28, 5087–5092. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Hydrogels for Tissue Engineering. Chem. Rev. 2001, 101, 1869–1879. [Google Scholar] [CrossRef] [PubMed]
- Willerth, S.M.; Arendas, K.J.; Gottlieb, D.I.; Sakiyama-Elbert, S.E. Optimization of Fibrin Scaffolds for Differentiation of Murine Embryonic Stem Cells into Neural Lineage Cells. Biomaterials 2006, 27, 5990–6003. [Google Scholar] [CrossRef] [PubMed]
- Raeber, G.P.; Lutolf, M.P.; Hubbell, J.A. Molecularly engineered PEG hydrogels: A novel model system for proteolytically mediated cell migration. Biophys. J. 2005, 89, 1374–1388. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.X.; Choi, J. Biodegradable Polymer Scaffolds with Well-Defined Interconnected Spherical Pore Network. Tissue Eng. 2001, 7, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Kuntz, R.M.; Saltzman, W.M. Neutrophil motility in extracellular matrix gels: Mesh size and adhesion affect speed of migration. Biophys. J. 1997, 72, 1472–1480. [Google Scholar] [CrossRef]
- Fedorovich, N.E.; Wijnberg, H.M.; Dhert, W.J.; Alblas, J. Distinct Tissue Formation by Heterogeneous Printing of Osteo- and Endothelial Progenitor Cells. Tissue Eng. Part A 2011, 17, 2113–2121. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.C.; Nair, K.; Sun, W. Three dimensional multi-scale modelling and analysis of cell damage in cell-encapsulated alginate constructs. J. Biomech. 2010, 43, 1031–1038. [Google Scholar] [PubMed]
- Cheng, J.; Lin, F.; Liu, H.; Yan, Y.; Wang, X.; Zhang, R.; Xiong, Z. Rheological Properties of Cell-Hydrogel Composites Extruding Through Small-Diameter Tips. J. Manuf. Sci. Eng. 2008, 130, 021014. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, X.; Pan, Y.; Liu, H.; Cheng, J.; Xiong, Z.; Lin, F.; Wu, R.; Zhang, R.; Lu, Q. Fabrication of viable tissue-engineered constructs with 3D cell-assembly technique. Biomaterials 2005, 26, 5864–5871. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wang, X.; Yan, Y.; Zheng, W.; Xiong, Z.; Lin, F.; Wu, R.; Zhang, R. Rapid Prototyping Three-Dimensional Cell/Gelatin/Fibrinogen Constructs for Medical Regeneration. J. Bioact. Compat. Polym. 2007, 22, 363–377. [Google Scholar] [CrossRef]
- Jaeger, A.A.; Das, C.K.; Morgan, N.Y.; Pursley, R.; Mcqueen, P.G.; Hall, M.D.; Pohida, T.J.; Gottesman, M.M. Microfabricated polymeric vessel mimetics for 3-D cancer cell culture. Biomaterials 2013, 34, 8301–8313. [Google Scholar] [CrossRef] [PubMed]
- Baker, B.M.; Trappmann, B.; Stapleton, S.C.; Toro, E.; Chen, C.S. Microfluidics embedded within extracellular matrix to define vascular architectures and pattern diffusive gradients. Lab Chip 2013, 13, 3246–3252. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Chi, M.; Yi, B.; Kim, S.H.; Oh, S.; Kim, Y.; Park, S.; Sung, J.H. Three-dimensional intestinal villi epithelium enhances protection of human intestinal cells from bacterial infection by inducing mucin expression. Integr. Biol. 2014, 6, 1122–1131. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.H.; Yu, J.; Luo, D.; Shuler, M.L.; March, J.C. Microscale 3-D hydrogel scaffold for biomimetic gastrointestinal (GI) tract model. Lab Chip 2011, 11, 389–392. [Google Scholar] [CrossRef] [PubMed]
- Pavithra, V.; Sowmya, S.V.; Rao, R.S.; Patil, S.; Augustine, D.; Haragannavar, V.C.; Nambiar, S.K. Tumor-associated Collagen Signatures: An Insight. World J. Dent. 2017, 8, 224–230. [Google Scholar] [CrossRef]
- Gillette, B.M.; Jensen, J.A.; Tang, B.; Yang, G.J.; Bazarganlari, A.; Zhong, M.; Sia, S.K. In situ collagen assembly for integrating microfabricated three-dimensional cell-seeded matrices. Nat. Mater. 2008, 7, 636–640. [Google Scholar] [CrossRef] [PubMed]
- Alexandra, S.P.; Nerger, B.A.; Wolf, A.E.; Sundaresan, S.; Nelson, C.M. Dynamics of Tissue-Induced Alignment of Fibrous Extracellular Matrix. Biophys. J. 2017, 113, 702–713. [Google Scholar]
- Lee, V.K.; Lanzi, A.M.; Ngo, H.; Yoo, S.; Vincent, P.A.; Dai, G. Generation of Multi-Scale Vascular Network System within 3D Hydrogel using 3D Bio-Printing Technology. Cell. Mol. Bioeng. 2014, 7, 460–472. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Duclos, G.; Sun, B.; Lee, J.; Wu, A.; Kam, Y.; Sontag, E.D.; Stone, H.A.; Sturm, J.C.; Gatenby, R.A.; Austin, R.H. Minimization of thermodynamic costs in cancer cell invasion. Proc. Natl. Acad. Sci. USA 2013, 110, 1686–1691. [Google Scholar] [CrossRef] [PubMed]
- Scadden, D.T. The stem-cell niche as an entity of action. Nature 2006, 441, 1075–1079. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lilly, G.D.; Doty, R.C.; Podsiadlo, P.; Kotov, N.A. In vitro toxicity testing of nanoparticles in 3D cell culture. Small 2009, 5, 1213–1221. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Wood, D.K.; Hsu, C.M.; Bhatia, S.N. DNA-templated assembly of droplet-derived PEG microtissues. Lab Chip 2011, 11, 2967–2975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vazquez, A.; Liu, J.; Zhou, Y.; Oltvai, Z.N. Catabolic efficiency of aerobic glycolysis: The Warburg effect revisited. BMC Syst. Biol. 2010, 4, 58. [Google Scholar] [CrossRef] [PubMed]
- Fong, E.L.; Lamhamedicherradi, S.E.; Burdett, E.; Ramamoorthy, V.; Lazar, A.J.; Kasper, F.K.; Farachcarson, M.C.; Vishwamitra, D.; Demicco, E.G.; Menegaz, B.A.; et al. Modeling Ewing sarcoma tumors in vitro with 3D scaffolds. Proc. Natl. Acad. Sci. USA 2013, 110, 6500–6505. [Google Scholar] [CrossRef] [PubMed]
- Zervantonakis, I.K.; Hughesalford, S.K.; Charest, J.L.; Condeelis, J.; Gertler, F.B.; Kamm, R.D. Three-dimensional microfluidic model for tumor cell intravasation and endothelial barrier function. Proc. Natl. Acad. Sci. USA 2012, 109, 13515–13520. [Google Scholar] [CrossRef] [PubMed]
- Bryony, S.W.; Werb, Z. Stromal Effects on Mammary Gland Development and Breast Cancer. Science 2002, 296, 1046–1049. [Google Scholar]
- Saha, S.; Duan, X.; Wu, L.; Lo, P.; Chen, H.; Wang, Q. Electrospun fibrous scaffolds promote breast cancer cell alignment and epithelial-mesenchymal transition. Langmuir 2012, 28, 2028–2034. [Google Scholar] [CrossRef] [PubMed]
- Marsha, C.L.; Cynthia, A. Reinhart-King, Targeting extracellular matrix stiffness to attenuate disease: From molecular mechanisms to clinical trials. Sci Transl. Med. 2018, 10, eaao0475. [Google Scholar] [CrossRef]
- Potente, M.; Gerhardt, H.; Carmeliet, P. Basic and Therapeutic Aspects of Angiogenesis. Cell 2011, 146, 873–887. [Google Scholar] [CrossRef] [PubMed]
- Lagory, E.L.; Giaccia, A.J. The ever-expanding role of HIF in tumour and stromal biology. Nat. Cell Biol. 2016, 18, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Michele, D.P.; Biziato, D.; Petrova, T.V. Microenvironmental regulation of tumour angiogenesis. Nat. Rev. Cancer 2017, 17. [Google Scholar] [CrossRef]
- Mason, B.N.; Starchenko, A.; Williams, R.M.; Bonassar, L.J.; Reinhartking, C.A. Tuning three-dimensional collagen matrix stiffness independently of collagen concentration modulates endothelial cell behavior. Acta Biomater. 2013, 9, 4635–4644. [Google Scholar] [CrossRef] [PubMed]
- Skardal, A.; Mack, D.L.; Kapetanovic, E.; Atala, A.; Jackson, J.D.; Yoo, J.J.; Soker, S. Bioprinted Amniotic Fluid-Derived Stem Cells Accelerate Healing of Large Skin Wounds. Stem Cell. Transl. Med. 2012, 1, 792–802. [Google Scholar] [CrossRef] [PubMed]
- Dolega, M.E.; Abeille, F.; Picolletdhahan, N.; Gidrol, X. Controlled 3D culture in Matrigel microbeads to analyze clonal acinar development. Biomaterials 2015, 52, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Fung, W.; Beyzavi, A.; Abgrall, P.; Nguyen, N.; Li, H. Microfluidic platform for controlling the differentiation of embryoid bodies. Lab Chip 2009, 9, 2591–2595. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Mei, C.; Lin, C.; Yang, K.; Yu, J. The influence of bubble size on chondrogenic differentiation of adipose-derived stem cells in gelatin microbubble scaffolds. J. Mater. Chem. B 2018, 6, 125–132. [Google Scholar] [CrossRef]
- Mahadik, B.P.; Wheeler, T.D.; Skertich, L.J.; Kenis, P.J.; Harley, B.A. Microfluidic generation of gradient hydrogels to modulate hematopoietic stem cell culture environment. Adv. Healthc. Mater. 2014, 3, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Hou, Y.; Zhong, L.; Huang, D.; Qian, H.; Karperien, M.; Chen, W. Promoted Chondrogenesis of Cocultured Chondrocytes and Mesenchymal Stem Cells under Hypoxia Using In-situ Forming Degradable Hydrogel Scaffolds. Biomacromolecules 2018, 19, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, M.A.; Renner, M.; Martin, C.; Wenzel, D.; Bicknell, L.S.; Hurles, M.E.; Homfray, T.; Penninger, J.M.; Jackson, A.P.; Knoblich, J.A. Cerebral organoids model human brain development and microcephaly. Nature 2013, 501, 373–379. [Google Scholar] [CrossRef] [PubMed]

| Technique | Method | Material |
|---|---|---|
| 3-D bioprinting | BIP (bioink printing) [29] Extrusion printing [51] Laser-assisted bioprinting [37,38] Microvalve printing [52,53,54,55] | Hydrogel Biomolecular ink [29] Matrigel alginate [51] Chitosan [52] Gelatin [52] Alginate [54] Fibrinogen [55] |
| microfluidics | Soft lithography [33,35,36,56,57,58,59] | Matrigel [58] Collagen [18,33,35,57] Gelatin [57] Calcium alginate [58,59] |
| photochemistry | FLDW (femtosecond laser direct writing) [38] DMD-PP process(digital micromirror device-based projection printing) [37] UV-light exporsure [39] | BSA [38] Fibronectin [38] Polyethylene glycol (PEG) [37] Poly(ethylene glycol) diacrylate (PEGDA) [37] Methacrylamide-modified gelatin or hydrogel [39] |
| Technique | Strength | Limitation |
|---|---|---|
| 3-D bioprinting | Flexible | Unable to achieve accuracy at less than 50 μm (the highest accuracy is inkjet printing in publishing). The temperature is not easy to control precisely. High cost. |
| microfluidics | High precision Stable | Structures are limited, e.g., multilayers, lumen. |
| photochemistry | Curing fast High precision | Limited by optical characteristics. Must be combined with other technologies, e.g., 3-D printing, to achieve high accuracy and flexible structure. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, K.; Wang, Z.; Liu, R.; Chen, G.; Liu, L. Microfabrication-Based Three-Dimensional (3-D) Extracellular Matrix Microenvironments for Cancer and Other Diseases. Int. J. Mol. Sci. 2018, 19, 935. https://doi.org/10.3390/ijms19040935
Song K, Wang Z, Liu R, Chen G, Liu L. Microfabrication-Based Three-Dimensional (3-D) Extracellular Matrix Microenvironments for Cancer and Other Diseases. International Journal of Molecular Sciences. 2018; 19(4):935. https://doi.org/10.3390/ijms19040935
Chicago/Turabian StyleSong, Kena, Zirui Wang, Ruchuan Liu, Guo Chen, and Liyu Liu. 2018. "Microfabrication-Based Three-Dimensional (3-D) Extracellular Matrix Microenvironments for Cancer and Other Diseases" International Journal of Molecular Sciences 19, no. 4: 935. https://doi.org/10.3390/ijms19040935
APA StyleSong, K., Wang, Z., Liu, R., Chen, G., & Liu, L. (2018). Microfabrication-Based Three-Dimensional (3-D) Extracellular Matrix Microenvironments for Cancer and Other Diseases. International Journal of Molecular Sciences, 19(4), 935. https://doi.org/10.3390/ijms19040935




