Identification of Placental Aspartic Proteinase in the Eurasian Beaver (Castor fiber L.)
Abstract
1. Introduction
2. Results
2.1. Identification of the AP Sequence in Placental Transcriptome of the Beaver
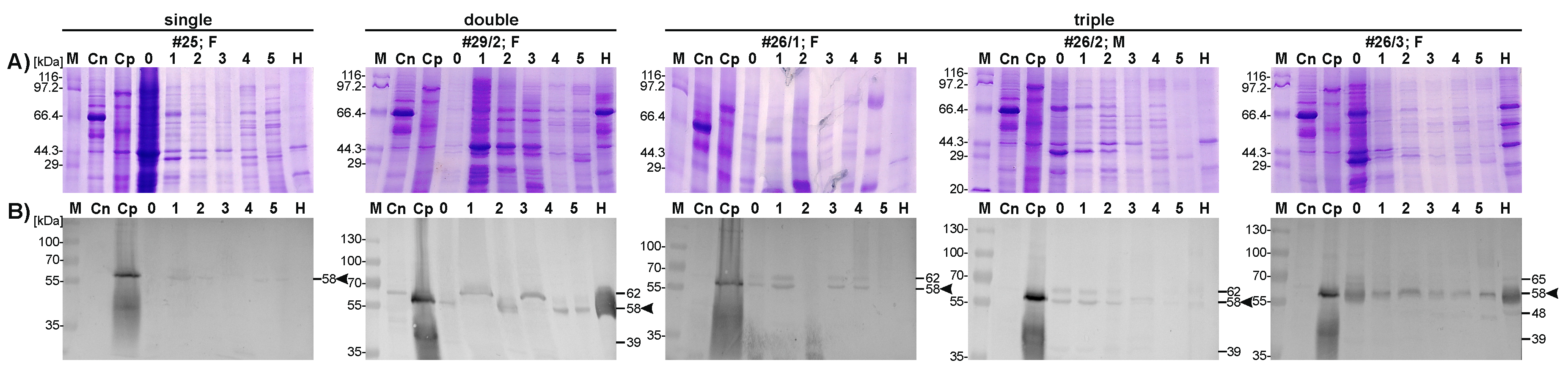
2.2. Identification of Placental pep/PAG-L Isoforms
2.3. Placental Localization of the pep/PAG-L Cellular Expression
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Tissue Harvesting
4.3. RNA-seq (cDNA Library Construction, Paired-End Sequencing and Bioinformatics)
4.4. Capillary Sequencing
4.5. Cross-Species Specificity of Anti-PAG Polyclonals
4.6. In Vitro Cultures and Western Blotting
4.7. Heterologous Double Fluorescent Immunohistochemistry (htdF-IHC)
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AP | Aspartic proteinases |
| cDNA | complementary DNA |
| Cf | Castor fiber |
| PAG-L | Pregnancy-Associated Glycoprotein-Like family |
| PAGs | Pregnancy-Associated Glycoproteins |
| pep/PAG-L | AP identified in the Eurasian beaver placenta |
References
- Hsu, T.C.; Benirschke, K. Atlas of Mammalian Chromosomes; Folio 59; Springer: New York, NY, USA, 1968; Volume 2, ISBN 0387041982. [Google Scholar]
- Lavrov, L.S.; Orlov, V.N. Karyotypes and taxonomy of modern beavers (Castor, Castoridae, Mammalia). Zool. Zh. 1973, 52, 734–742. (In Russian) [Google Scholar]
- Montgelard, C.; Forty, E.; Arnal, V.; Matthee, C.A. Suprafamilial relationships among Rodentia and the phylogenetic effect of removing fast-evolving nucleotides in mitochondrial, exon and intron fragments. BMC Evol. Biol. 2008, 8, 321. [Google Scholar] [CrossRef] [PubMed]
- Blanga-Kanfi, S.; Miranda, H.; Penn, O.; Pupko, T.; DeBry, R.W.; Huchon, D. Rodent phylogeny revised: Analysis of six nuclear genes from all major rodent clades. BMC Evol. Biol. 2009, 9, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Horn, S.; Durka, W.; Wolf, R.; Ermala, A.; Stubbe, A.; Stubbe, M.; Hofreiter, M. Mitochondrial genomes reveal slow rates of molecular evolution and the timing of speciation in beavers (Castor), one of the largest rodent species. PLoS ONE 2011, 1, e14622. [Google Scholar] [CrossRef] [PubMed]
- Doronina, L.; Matzke, A.; Churakov, G.; Stoll, M.; Huge, A.; Schmitz, J. The Beaver’s Phylogenetic Lineage Illuminated by Retroposon Reads. Sci. Rep. 2017, 7, 43562. [Google Scholar] [CrossRef] [PubMed]
- Batbold, J.; Batsaikhan, N.; Shar, S.; Hutterer, R.; Kryštufek, B.; Yigit, N.; Mitsain, G.; Palomo, L. Castor Fiber (Errata Version Published in 2017); e.T4007A115067136; The IUCN Red List of Threatened Species: Cambridge, UK, 2016. [Google Scholar]
- Kaufmann, P. Vergleichend-anatomische und funktionelle Aspekte des Placenta-Baues. Funkt. Biol. Med. 1983, 2, 71–79. [Google Scholar]
- Szafranska, B.; Panasiewicz, G.; Majewska, M. Biodiversity of multiple pregnancy-associated glycoprotein (PAG) family: Gene cloning and chorionic protein purification in domestic and wild eutherians (Placentalia)—A review. Reprod. Nutr. Dev. 2006, 5, 481–502. [Google Scholar] [CrossRef] [PubMed]
- Wallace, R.M.; Pohler, K.G.; Smith, M.F.; Green, J.A. Placental PAGs: Gene origins, expression patterns, and use as markers of pregnancy. Reproduction 2015, 149, R115–R126. [Google Scholar] [CrossRef] [PubMed]
- Majewska, M.; Panasiewicz, G.; Dabrowski, M.; Gizejewski, Z.; Beckers, J.F.; Szafranska, B. Multiple forms of pregnancy-associated glycoproteins released in vitro by porcine chorion or placentomal and interplacentomal explants of wild and domestic ruminants. Reprod. Biol. 2005, 5, 185–203. [Google Scholar] [PubMed]
- Szafranska, B.; Panasiewicz, G.; Dabrowski, M.; Majewska, M.; Gizejewski, Z.; Beckers, J.F. Chorionic mRNA expression and N-glycodiversity of pregnancy-associated glycoprotein family (PAG) of the European bison (Bison bonasus). Anim. Reprod. Sci. 2005, 88, 225–243. [Google Scholar] [CrossRef] [PubMed]
- Szafranska, B.; Panasiewicz, G.; Majewska, M.; Romanowska, A.; Dajnowiec, J. Pregnancy-associated glycoprotein family (PAG)—As chorionic signaling ligands for gonadotropin receptors of cyclic animals. Anim. Reprod. Sci. 2007, 99, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Panasiewicz, G.; Majewska, M.; Romanowska, A.; Dajnowiec, J.; Szafranska, B. Radiocompetition of secretory pregnancy-associated glycoproteins as chorionic ligands with luteal and uterine gonadotrophin receptors of pregnant pigs. Anim. Reprod. Sci. 2007, 99, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Lopes-da-Costa, L.; e Silva, J.C.; Deloche, M.C.; Jeanguyot, N.; Humblot, P.; Horta, A.E. Effects of embryo size at transfer (whole versus demi) and early pregnancy progesterone supplementation on embryo growth and pregnancy-specific protein bovine concentrations in recipient dairy heifers. Theriogenology 2011, 76, 522–531. [Google Scholar] [CrossRef] [PubMed]
- García-Ispierto, I.; Almería, S.; Serrano, B.; de Sousa, N.M.; Beckers, J.F.; López-Gatius, F. Plasma concentrations of pregnancy-associated glycoproteins measured using anti-bovine PAG-2 antibodies on day 120 of gestation predict abortion in dairy cows naturally infected with Neospora caninum. Reprod. Domest. Anim. 2013, 4, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Breukelman, S.P.; Perényi, Z.; Taverne, M.A.; Jonker, H.; van der Weijden, G.C.; Vos, P.L.; de Ruigh, L.; Dieleman, S.J.; Beckers, J.F.; Szenci, O. Characterisation of pregnancy losses after embryo transfer by measuring plasma progesterone and bovine pregnancy-associated glycoprotein-1 concentrations. Vet. J. 2012, 11, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, T.; Tanabe, K.; Koiwai, O. Structure and development of rabbit pepsinogens Stage-specific zymogens nucleotide sequences of cDNAs molecular evolution and gene expression during development. J. Biol. Chem. 1990, 28, 17031–17038. [Google Scholar]
- Kageyama, T. Pepsinogens progastricsins and prochymosins: Structure function evolution and development. Cell. Mol. Life. Sci. 2002, 59, 288–306. [Google Scholar] [CrossRef] [PubMed]
- Carginale, V.; Trinchella, F.; Capasso, C.; Scudiero, R.; Riggio, M.; Parisi, E. Adaptive evolution and functional divergence of pepsin gene family. Gene 2004, 333, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Green, J.; Bixby, J.B.; Szafranska, B.; DeMartini, J.C.; Hecht, S.; Roberts, R.M. The diversity and evolutionary relationships of the pregnancy-associated glycoproteins an aspartic proteinase subfamily consisting of many trophoblast-expressed genes. Proc. Natl. Acad. Sci. USA 1997, 94, 12809–12816. [Google Scholar] [CrossRef] [PubMed]
- Bobe, J.; Goetz, F.W. An ovarian progastricsin is present in the trout coelomic fluid after ovulation. Biol. Reprod. 2001, 64, 1048–1055. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kurokawa, T.; Uji, S.; Suzuki, T. Identification of pepsinogen gene in the genome of stomachless fish, Takifugu rubripes. Comp. Biochem. Physiol. B. Biochem. Mol. Biol. 2005, 140, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Green, J.; Xie, S.; Szafranska, B.; Gan, X.; Newman, A.G.; McDowell, K.; Roberts, R.M. Identification of a new aspartic proteinase expressed by the outer chorionic cell layer of the equine placenta. Biol. Reprod. 1999, 60, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.L.; Green, J.A.; Garbayo, J.M.; Roberts, R.M. Adaptive diversification within a large family of recently duplicated, placentally expressed genes. Proc. Natl. Acad. Sci. USA 2000, 7, 3319–3323. [Google Scholar] [CrossRef]
- Chen, X.; Rosenfeld, C.S.; Roberts, R.M.; Green, J.A. An aspartic proteinase expressed in the yolk sac and neonatal stomach of the mouse. Biol. Reprod. 2001, 65, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.S.P.; Buus, O.T.; Wollenweber, B. Evolutionary Pattern of N-Glycosylation Sequon Numbers in Eukaryotic ABC Protein Superfamilies. Bioinform. Biol. Insights 2010, 4, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Szafranska, B.; Xie, S.; Green, J.; Roberts, R.M. Porcine pregnancy-associated glycoproteins: New members of the aspartic proteinase gene family expressed in trophectoderm. Biol. Reprod. 1995, 53, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Garbayo, J.M.; Green, J.; Manikkam, M.; Beckers, J.F.; Kiesling, D.O.; Ealy, A.D.; Roberts, R.M. Caprine Pregnancy-Associated Glycoproteins (PAGs): Their cloning expression and evolutionary relationship to other PAG. Mol. Reprod. Dev. 2000, 57, 311–322. [Google Scholar] [CrossRef]
- Kageyama, T.; Takahashi, K. Monkey pepsinogens and pepsins III Carbohydrate moiety of Japanese monkey pepsinogens and the amino acid sequence around the site of its attachment to protein. J. Biochem. 1978, 84, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.; Trieu-Cuot, P.; Collin, J.C.; Ribadeau-Dumas, B. Purification and characterization of bovine gastricsin. Eur. J. Biochem. 1982, 122, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Panasiewicz, G.; Majewska, M.; Szafranska, B. Trophoblastic cDNA cloning of porcine pregnancy-associated glycoprotein genes (pPAG) and in silico analysis of coded polypeptide precursors. Reprod. Biol. 2004, 4, 131–141. [Google Scholar] [PubMed]
- Brandt, G.A.; Parks, T.E.; Killian, G.; Ealy, A.D.; Green, J.A. A cloning and expression analysis of pregnancy-associated glycoproteins expressed in trophoblasts of the white-tail deer placenta. Mol. Reprod. Dev. 2007, 74, 1355–1362. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Low, B.G.; Nagel, R.J.; Kramer, K.K.; Anthony, R.V.; Zoli, A.P.; Beckers, J.F.; Roberts, R.M. Identification of the major pregnancy-specific antigens of cattle and sheep as inactive members of the aspartic proteinase family. Proc. Natl. Acad. Sci. USA 1991, 88, 10247–10251. [Google Scholar] [CrossRef] [PubMed]
- Guruprasad, K.; Blundell, T.L.; Xie, S.; Green, J.; Szafranska, B.; Nagel, R.J.; McDowell, K.; Baker, C.B.; Roberts, R.M. Comparative modelling and analysis of amino acid substitutions suggests that the family of pregnancy-associated glycoproteins includes both active and inactive aspartic proteinases. Protein Eng. 1996, 9, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Szafranska, B.; Panasiewicz, G. The placental expression of the porcine pregnancy-associated glycoprotein (pPAG) gene family examined in situ and in vitro. Anim. Reprod. Sci. 2002, 72, 95–113. [Google Scholar] [CrossRef]
- Klisch, K.; Jeanrond, E.; Pang, P.C.; Pich, A.; Schuler, G.; Dantzer, V.; Kowalewski, M.P.; Dell, A. A tetraantennary glycan with bisecting N-acetylglucosamine and the Sda antigen is the predominant N-glycan on bovine pregnancy-associated glycoproteins. Glycobiology 2008, 18, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Kiewisz, J.; de Sousa, N.M.; Beckers, J.F.; Vervaecke, H.; Panasiewicz, G.; Szafranska, B. Isolation of pregnancy-associated glycoproteins from placenta of the American bison (Bison bison) at first half of pregnancy. Gen. Comp. Endocrinol. 2008, 155, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Beriot, M.; Tchimbou, A.F.; Barbato, O.; Beckers, J.F.; de Sousa, N.M. Identification of pregnancy-associated glycoproteins and alpha-fetoprotein in fallow deer (Dama dama) placenta. Acta Vet. Scand. 2014, 56, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Lipka, A.; Panasiewicz, G.; Majewska, M.; Bieniek-Kobuszewska, M.; Saveljev, A.P.; Pankratov, A.P.; Szafranska, B. Identification of the Pregnancy-Associated Glycoprotein family (PAGs) and some aspects of placenta development in the European moose (Alces alces L.). Theriogenology 2016, 86, 2119–2135. [Google Scholar] [CrossRef] [PubMed]
- Szafranska, B.; Panasiewicz, G.; Majewska, M. Porcine pregnancy-associated glycoprotein family (pPAGs)—As in vitro-produced chorionic ligands for luteal and uterine gonadotropin receptors. Reprod. Biol. 2006, 6, 105–111. [Google Scholar] [PubMed]
- Szafranska, B.; Majewska, M.; Panasiewicz, G. N-glycodiversity of the Pregnancy-Associated Glycoprotein family (PAG) produced in vitro by trophoblast and trophectoderm explants during implantation, placentation and advanced pregnancy in the pig. Reprod. Biol. 2004, 4, 67–89. [Google Scholar] [PubMed]
- Wooding, F.B.; Roberts, R.M.; Green, J.A. Light and electron microscope immunocytochemical studies of the distribution of pregnancy associated glycoproteins (PAGs) throughout pregnancy in the cow: Possible functional implications. Placenta 2005, 26, 807–827. [Google Scholar] [CrossRef] [PubMed]
- Majewska, M.; Panasiewicz, G.; Szafranska, B.; Gizejewski, Z.; Majewski, M.; Borkowski, K. Cellular localization of the pregnancy-associated glycoprotein family (PAGs) in the synepitheliochorial placenta of the European bison. Gen. Comp. Endocrinol. 2008, 155, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Majewska, M.; Panasiewicz, G.; Majewski, M.; Szafranska, B. Localization of chorionic pregnancy-associated glycoprotein family in the pig. Reprod. Biol. 2006, 6, 205–230. [Google Scholar] [PubMed]
- Majewska, M.; Panasiewicz, G.; Szafranska, B. Pregnancy-associated glycoprotein (PAG) family localized in chorionic cells within the epitheliochorial/diffuse placenta of the alpaca (Lama pacos). Acta Histochem. 2011, 113, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Majewska, M.; Panasiewicz, G.; Szafranska, B. Expression of pregnancy-associated glycoprotein family in the epitheliochorial placenta of two Camelidae species (C. dromedarius and C. bactrianus). Acta Histochem. 2013, 115, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Wooding, F.B. The role of the binucleate cell in ruminant placental structure. J. Reprod. Fertil. Suppl. 1982, 31, 31–39. [Google Scholar] [PubMed]
- Gozdziewski, J. Polish Hunting Association, Suwałki, Poland. Unpublished work. 2018. [Google Scholar]
- Kibbe, WA. OligoCalc: An online oligonucleotide properties calculator. Nucleic Acids Res. 2007, 35, (webserver issue). Available online: www.basic.northwestern.edu/biotools/oligocalc.html (accessed on 25 May 2017). [CrossRef] [PubMed]
- Petersen, T.N.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 4.0: Discriminating signal peptides from transmembrane regions. Nat. Methods 2011, 8, 785–786. [Google Scholar] [CrossRef] [PubMed]
- Sigrist, C.J.A.; de Castro, E.; Cerutti, L.; Cuche, B.A.; Hulo, N.; Bridge, A.; Bougueleret, L.; Xenarios, I. New and continuing developments at PROSITE. Nucleic Acids Res. 2012. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Jung, E.; Brunak, S. Prediction of N-glycosylation sites in human proteins. 2004. Available online: www.cbs.dtu.dk/services/NetNGlyc/ (accessed on 31 January 2018).
- Szafranska, B.; Ziecik, A.; Okrasa, S. Primary antisera against selected steroids or proteins and secondary antisera against gamma-globulins—An available tool for studies of reproductive processes. Reprod. Biol. 2002, 2, 187–204. [Google Scholar] [PubMed]
- Szafranska, B.; Panasiewicz, G.; Majewska, M.; Beckers, J.F. Chorionic expression of heterogeneous products of the PAG (Pregnancy-Associated Glycoprotein) gene family secreted in vitro throughout embryonic and foetal development in the pig. Reprod. Nutr. Dev. 2003, 43, 497–519. [Google Scholar] [CrossRef] [PubMed]





| Protein Name a | N-Domain b | C-Domain b |
|---|---|---|
| pep/PAG-L | VLFDTGSSNLWV | GIVDTGTSLLTV |
| hPepsinogen C | ............ | A........... |
| mPepsinogen C | ............ | ..........VM |
| hPepsinogen A | .V.......... | A..........G |
| mCathepsin D | .V.......... | A.........VG |
| mNapsin A | .V.......... | A.L......I.G |
| mPepsinogen F | .VL.....V... | ..M........G |
| fPAG | .I......D... | A.I.......IG |
| TrNothepsin | .V......D... | A........IAG |
| pPAG2, 4, 6, 10 | .V......D... | A.......M.HG |
| oPAG2 | .V......D... | AL.......IHG |
| bPAG2 | .V.....A.... | ALL.....MIYG |
| mRenin | .M.....A.... | VV....S.FISA |
| mPepsinogen A | .VL.....V... | ..M........G |
| ePAG | .I.....AD... | A.........LG |
| zPAG | .I.....AD... | A.........LG |
| Pf plasmepsin I | FI.....A.... | A...S...SI.A |
| Pr plasmepsin I | FI.....A.... | VV..S...SI.A |
| pPAG1, 3, 5 | .I...A..D... | A.L.S.SAF.LG |
| Pr plasmepsin II | FIL....A.... | C...S...AI.. |
| Pf plasmepsin II | FIL....A.... | C...S...AI.. |
| Pf histo-AP | FIL....A.... | C...S...AI.. |
| Pr histo-AP | F..H.A...V.. | V.L.SA..AI.. |
| Animal Code | Age a [years] | Mass [kg] | Gestation | Fetal Sex | Analyses |
|---|---|---|---|---|---|
| #25 | 1.5–2 | 14.92 | Single | female | RNA-seq; capillary sequencing; IHC; Western |
| #29 | 2.5–3 | 20.22 | Twin | 29/1 male | IHC |
| 29/2 female | IHC; Western | ||||
| #26 | >3 | 22.09 | Triple | 26/1 female | IHC; Western |
| 26/2 male | RNA-seq; capillary sequencing; IHC; Western | ||||
| 26/3 female | IHC; Western |
| Ordinal No. of Primer Pairs | Primers Name | Sequence (5′–3′) | Position a | Amplicon Length (bp) a |
|---|---|---|---|---|
| 1 | ATG_F | ATGAAGTGGATAGTGGTGGCC | 1–21 | ~1110 |
| 9_R | GAAGACATCWCCMAGGATCCAA | 1089–1110 | ||
| 2 | 2_F | TTCCTGAAGAMSCACAA | 124–140 | ~436 |
| 5_R | TAGGCCAGSCCCAKGATGCCATC | 538–560 | ||
| 3 | 3_F | GCTCCTCCAACCTGTGGGT | 287–305 | ~568 |
| 7_R | CAGAGAGGTGCCKGTGTCMACAA | 833–855 | ||
| 4 | 5_F | GATGGCATCMTGGGSCTGGCCTA | 538–560 | ~572 |
| 9_R | GAAGACATCWCCMAGGATCCAA | 1089–1110 | ||
| 5 | 7_F | TTGTKGACACMGGCACCTCTCTG | 833–855 | ~424 |
| 3′utr_R | CCAGAGAAGAGGCACAGATAGA | 1236–1257 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lipka, A.; Panasiewicz, G.; Majewska, M.; Paukszto, L.; Bieniek-Kobuszewska, M.; Szafranska, B. Identification of Placental Aspartic Proteinase in the Eurasian Beaver (Castor fiber L.). Int. J. Mol. Sci. 2018, 19, 1229. https://doi.org/10.3390/ijms19041229
Lipka A, Panasiewicz G, Majewska M, Paukszto L, Bieniek-Kobuszewska M, Szafranska B. Identification of Placental Aspartic Proteinase in the Eurasian Beaver (Castor fiber L.). International Journal of Molecular Sciences. 2018; 19(4):1229. https://doi.org/10.3390/ijms19041229
Chicago/Turabian StyleLipka, Aleksandra, Grzegorz Panasiewicz, Marta Majewska, Lukasz Paukszto, Martyna Bieniek-Kobuszewska, and Bozena Szafranska. 2018. "Identification of Placental Aspartic Proteinase in the Eurasian Beaver (Castor fiber L.)" International Journal of Molecular Sciences 19, no. 4: 1229. https://doi.org/10.3390/ijms19041229
APA StyleLipka, A., Panasiewicz, G., Majewska, M., Paukszto, L., Bieniek-Kobuszewska, M., & Szafranska, B. (2018). Identification of Placental Aspartic Proteinase in the Eurasian Beaver (Castor fiber L.). International Journal of Molecular Sciences, 19(4), 1229. https://doi.org/10.3390/ijms19041229





