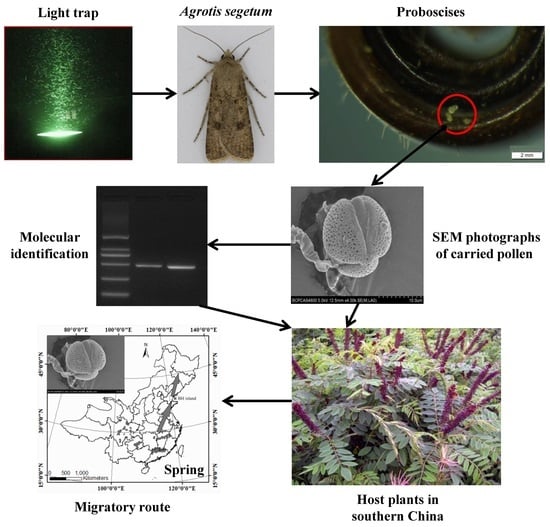
Molecular-Assisted Pollen Grain Analysis Reveals Spatiotemporal Origin of Long-Distance Migrants of a Noctuid Moth
Abstract
1. Introduction
2. Results
2.1. Plant Hosts Inferred from Pollen
2.2. Annual and Seasonal Differences in Pollen Adherence Ratio
2.3. Intra-Annual Shifts in Pollen Taxa
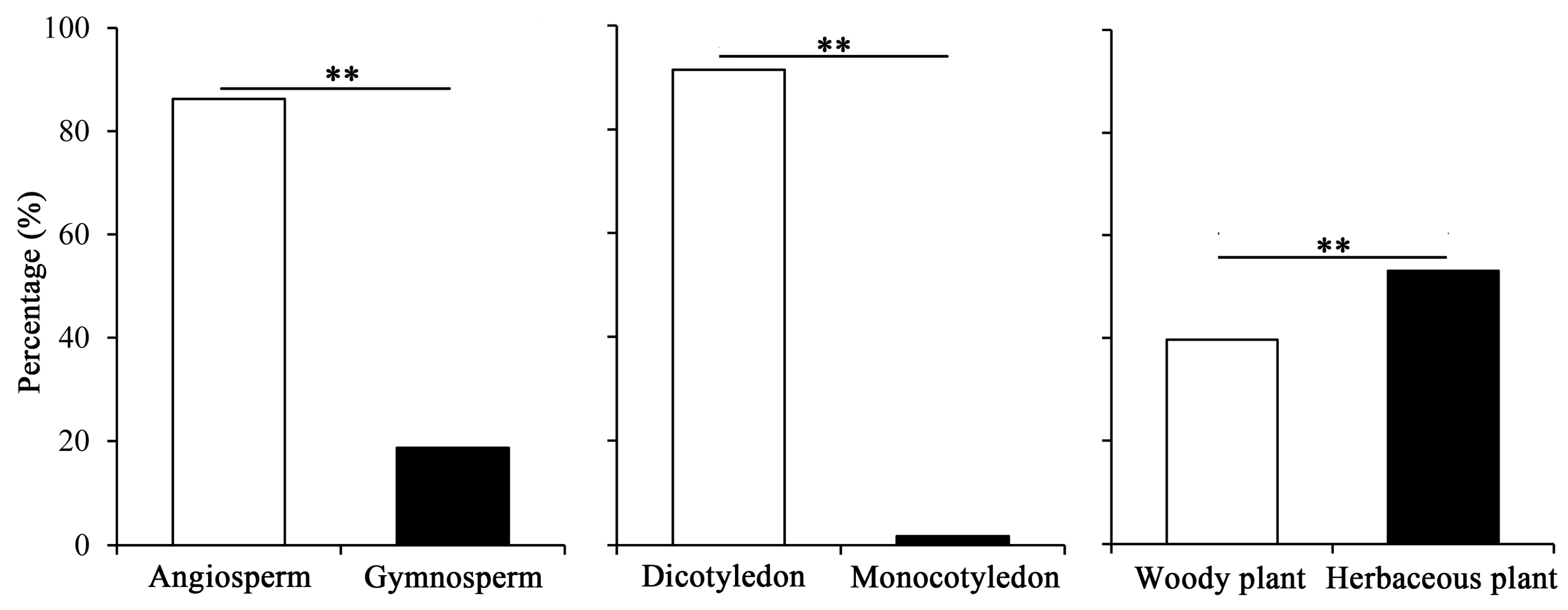
2.4. Characteristics of Pollen-Bearing Host Plants
3. Discussion
4. Materials and Methods
4.1. Moth Collection
4.2. Pollen Examination and SEM Preparation
4.3. Pollen Lysis and Single Pollen PCR
4.4. Pollen Identification and Characteristics of Pollen Source Plants
4.5. Data Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Weiner, C.N.; Werner, M.; Linsenmair, K.E.; Blüthgen, N. Land-use impacts on plant-pollinator networks: Interaction strength and specialization predict pollinator declines. Ecology 2014, 95, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Schoonhoven, L.M.; Loon, J.J.A.V.; Dicke, M. Evolution: Insects and plants forever in combat. In Insect-Plant Biology; Oxford University Press: Oxford, UK, 1998; Chapter 11; pp. 278–300. [Google Scholar]
- Jones, G.D. Pollen analyses for pollination research, unacetolyzed pollen. J. Pollinat. Ecol. 2012, 9, 96–107. [Google Scholar]
- Jones, G.D. Pollen analyses for pollination research, acetolysis. J. Pollinat. Ecol. 2014, 13, 203–217. [Google Scholar]
- Hendrix, W.H., III; Mueller, T.F.; Phillips, J.R.; Davis, O.K. Pollen as an indicator of long-distance lovement of Heliothis zea (Lepidoptera: Noctuidae). Environ. Entomol. 1987, 16, 1148–1151. [Google Scholar] [CrossRef]
- Lysenkov, S.N. Movement patterns of the main insect pollinator groups during foraging on generalized plants. Entomol. Rev. 2014, 94, 829–838. [Google Scholar] [CrossRef]
- Marcin, Z. On flower visitors and true pollinators: The case of protandrous Heracleum sphondylium L. (Apiaceae). Plant Syst. Evol. 2007, 263, 159–179. [Google Scholar]
- Lysenkov, S.N.; Galinskaya, T.V. Comparison of the pollen content on the body and in the gut of hoverflies (Diptera, Syrphidae). Entomol. Rev. 2017, 97, 10–16. [Google Scholar] [CrossRef]
- Bryant, V.M.; Pendleton, M.; Murry, R.E.; Lingren, P.D.; Raulston, J.R. Techniques for studying pollen adhering to nectar-feeding corn earworm (Lepidoptera: Noctuidae) moths using scanning electron microscopy. J. Econ. Entomol. 1991, 84, 237–240. [Google Scholar] [CrossRef]
- Mackenzie, G.; Boa, A.; Taboada, A.; Atkin, S.; Sathyapalan, T. Sporopollenin, the least known yet toughest natural biopolymer. Front. Mater. 2015, 2, 66. [Google Scholar] [CrossRef]
- Kapp, R.O. How to Know Pollen and Spores; Brown: Dubuque, IA, USA, 1969. [Google Scholar]
- Hagler, J.R.; Jackson, C.G. Methods for marking insects: Current techniques and future prospects. Annu. Rev. Entomol. 2001, 46, 511–543. [Google Scholar] [CrossRef] [PubMed]
- Salmaki, Y.; Jamzad, Z.; Zarre, S.; Bräuchler, C. Pollen morphology of Stachys (Lamiaceae) in Iran and its systematic implication. Flora 2008, 203, 627–639. [Google Scholar] [CrossRef]
- Khansari, E.; Zarre, S.; Alizadeh, K.; Attara, F.; Aghabeigic, F.; Salmaki, Y. Pollen morphology of Campanula (Campanulaceae) and allied genera in Iran with special focus on its systematic implication. Flora 2012, 207, 203–211. [Google Scholar] [CrossRef]
- Wilson, E.E.; Sidhu, C.S.; Levan, K.E.; Holway, D.A. Pollen foraging behaviour of solitary Hawaiian bees revealed through molecular pollen analysis. Mol. Ecol. 2010, 19, 4823–4829. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.J.; Pei, K.Q.; Zhou, B.; Ma, K.P. A molecular approach to species identification of Chenopodiaceae pollen grains in surface soil. Am. J. Bot. 2007, 94, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, T.; Haile, J.; Möller, P.; Andreev, A.; Boessenkool, S.; Rasmussen, M.; Kienast, F.; Coissac, E.; Taberlet, P.; Brochmann, C.; et al. A comparative study of ancient sedimentary DNA, pollen and macrofossils from permafrost sediments of northern Siberia reveals long-term vegetational stability. Mol. Ecol. 2012, 21, 1989–2003. [Google Scholar] [CrossRef] [PubMed]
- Parducci, L.; Matetovici, I.; Fontana, S.L.; Bennett, K.D.; Suyama, Y.; Haile, J.; Kjær, K.H.; Larsen, N.K.; Drouzas, A.D.; Willerslev, E. Molecular- and pollen-based vegetation analysis in lake sediments from central Scandinavia. Mol. Ecol. 2013, 23, 3511–3524. [Google Scholar] [CrossRef] [PubMed]
- Galimberti, A.; Mattia, F.D.; Bruni, I.; Scaccabarozzi, D.; Sandionigi, A.; Barbuto, M.; Casiraghi, M.; Labra, M. A DNA barcoding approach to characterize pollen collected by honeybees. PLoS ONE 2014, 9, e109363. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, J.; Vere, N.D.; Griffith, A.; Ford, C.R.; Allainguillaume, J.; Hegarty, M.J.; Baillie, L.; Adams-Groom, B. Using DNA metabarcoding to identify the floral composition of honey: A new tool for investigating honey bee foraging preferences. PLoS ONE 2015, 10, e0134735. [Google Scholar] [CrossRef] [PubMed]
- Galliot, J.N.; Brunel, D.; Bérard, A.; Chauveau, A.; Blanchetête, A.; Lanore, L.; Farruggia, A. Investigating a flower-insect forager network in a mountain grassland community using pollen DNA barcoding. J. Insect Conserv. 2017, 21, 827–837. [Google Scholar] [CrossRef]
- Schoonhoven, L.M.; Jermy, T.; Loon, J.J.A.V. Herbivorous insects: Something for everyone. In Insect-Plant Biology: From Physiology to Evolution; Chapman and Hall: London, UK, 1998; Chapter 2; pp. 5–25. [Google Scholar]
- Norris, M.J. The feeding-habits of the adult Lepidoptera heteroneura. Ecol. Entomol. 2010, 85, 61–90. [Google Scholar] [CrossRef]
- Gilbert, L.E. Pollen feeding and reproductive biology of Heliconius butterflies. Proc. Natl. Acad. Sci. USA 1972, 69, 1403–1407. [Google Scholar] [CrossRef] [PubMed]
- Baker, H.G.; Baker, I. Studies of Nectar-Constitution and Pollinator-Plant Coevolution; University of Texas Press: Austin, TX, USA, 1975; pp. 131–171. [Google Scholar]
- Erhardt, A.; Baker, I. Pollen amino acids-an additional diet for a nectar feeding butterfly? Plant Syst. Evol. 1990, 169, 111–121. [Google Scholar] [CrossRef]
- Hendrix, W.H., III; Showers, W.B. Tracing black cutworm and armyworm (Lepidoptera: Noctuidae) northward migration using Pithecellobium and Calliandra pollen. Environ. Entomol. 1992, 21, 1092–1096. [Google Scholar] [CrossRef]
- Lingren, P.D.; Bryant, V.M.; Raulston, J.R.; Pendleton, M.; Westbrook, J.; Jones, G.D. Adult feeding host range and migratory activities of com earworm, cabbage looper, and celery looper (Lepidoptera: Noctuidae) moths as evidenced by attached pollen. J. Econ. Entomol. 1993, 86, 1429–1439. [Google Scholar] [CrossRef]
- Lü, Z.Z.; Wang, P.; Zhang, Q.; Gong, Z.; Ding, H. Relationships between overwintering Agrotis segetum population and snow. Chin. J. Ecol. 2006, 25, 1532–1534. [Google Scholar]
- Lemic, D.; Drmić, Z.; Bažok, R. Population dynamics of noctuid moths and damage forecasting in sugar beet. Agric. For. Entomol. 2016, 18, 128–136. [Google Scholar] [CrossRef]
- Esbjerg, P.; Sigsgaard, L. Phenology and pest status of Agrotis segetum in a changing climate. Crop Prot. 2014, 62, 64–71. [Google Scholar] [CrossRef]
- Guo, J.L.; Fu, X.W.; Wu, X.; Zhao, X.Y.; Wu, K.M. Annual migration of Agrotis segetum (Lepidoptera: Noctuidae): Observed on a small isolated island in northern China. PLoS ONE 2015, 10, e0131639. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Q.; Fu, X.W.; Mao, L.M.; Xing, Z.L.; Wu, K.M. Host plants identification for adult Agrotis ipsilon, a long-distance migratory insect. Int. J. Mol. Sci. 2016, 17, 851. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Q.; Fu, X.W.; Mao, L.M.; Xing, Z.L.; Wu, K.M. Identification of host plant use of adults of a long-distance migratory insect, Mythimna separata. PLoS ONE 2017, 12, e0184116. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, D. The role of nourishment in oogenesis. Annu. Rev. Entomol. 1996, 41, 407–431. [Google Scholar] [CrossRef] [PubMed]
- Scheirs, J.; Bruyn, L.D. Integrating optimal foraging and optimal oviposition theory in plant-insect research. Oikos 2002, 96, 187–191. [Google Scholar] [CrossRef]
- Ghaffar, A.; Attique, M.R.; Naveed, M.R.; Jan, M.T. Effect of different hosts on the development and survival of Spodoptera exigua (Hubner) (Noctuidae: Lepidoptera). Pak. J. Zool. 2002, 34, 229–231. [Google Scholar]
- Rijn, P.C.J.V.; Bruin, J.; Wäckers, F.L. Nectar- and Pollen- Feeding by Adult Herbivorous Insects; Cambridge University Press: Cambridge, UK, 2005; pp. 178–219. [Google Scholar]
- Wäckers, F.L.; Romeis, J.; Rijn, P.V. Nectar and pollen feeding by insect herbivores and implications for multitrophic interactions. Annu. Rev. Entomol. 2007, 52, 301–323. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.D.; Jones, S.D. The uses of pollen and its implication for entomology. Neotrop. Entomol. 2001, 30, 341–350. [Google Scholar] [CrossRef]
- Hartstack, A.W.; Lopez, J.D.; Muller, R.A.; Sterling, W.L.; King, E.G.; Witz, J.A.; Eversull, A.C. Evidence of long range migration of Heliothis zea (Boddie) into Texas and Arkansas. Cancer Res. 1982, 59, 6132–6613. [Google Scholar]
- Mikkola, K. Pollen analysis as a means of studying the migrations of Lepidoptera. Ann. Entomol. Fenn. 1971, 37, 136–139. [Google Scholar]
- Fang, J.Y.; Wang, Z.H.; Tang, Z.Y. Atlas of Woody Plant in China: Distribution and Climate; Springer: Berlin, Germany, 2009. [Google Scholar]
- Jones, G.D.; Coppedge, J.R. Foraging resources of boll weevils (Coleoptera: Curculionidae). J. Econ. Entomol. 1999, 92, 860–869. [Google Scholar] [CrossRef]
- Dong, J.T. The biology study of Agrotis segetum. Entomol. Knowl. 1983, 1, 17–20. [Google Scholar]
- Xia, Z.X.; Ding, F.L. The occurrence characteristics and comprehensive control measures of Agrotis segetum. China Cotton 1989, 2, 45–46. [Google Scholar]
- Heath, R.R.; Landolt, P.J.; Dueben, B.; Lenczewski, B. Identification of Floral Compounds of Night-Blooming Jessamine Attractive to Cabbage Looper Moths. Environ. Entomol. 1992, 21, 854–859. [Google Scholar] [CrossRef]
- Zhu, Y.C.; Keaster, A.J.; Gerhardt, K.O. Field observations on attractiveness of selected blooming plants to noctuid noths and electroantennogram responses of black cutworm (Lepidoptera: Noctuidae) moths to flower volatiles. Environ. Entomol. 1993, 22, 162–166. [Google Scholar] [CrossRef]
- Cunningham, J.P.; Moore, C.J.; Zalucki, M.P.; West, S.A. Learning, odour preference and flower foraging in moths. J. Exp. Biol. 2004, 207, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Tingle, F.C.; Mitchell, E.R. Attraction of Heliothis virescens (F.) (Lepidoptera: Noctuidae) to volatiles from extracts of cotton flowers. J. Chem. Ecol. 1992, 18, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Udayagiri, S.; Mason, C.E. Host plant constituents as oviposition stimulants for a generalist herbivore: European corn borer. Entomol. Exp. Appl. 1995, 76, 59–65. [Google Scholar] [CrossRef]
- Williams, N.M.; Kremen, C. Resource distributions among habitats determine solitary bee offspring production in a mosaic landscape. Ecol. Appl. Publ. Ecol. Soc. Am. 2007, 17, 910–921. [Google Scholar] [CrossRef]
- CBOL Plant Working Group. A DNA barcode for land plants. Proc. Natl. Acad. Sci. USA 2009, 106, 12794–12797. [Google Scholar]
- Chen, S.L.; Yao, H.; Han, J.P.; Liu, C.; Song, J.Y.; Shi, L.C.; Zhu, Y.J.; Ma, X.Y.; Gao, T.; Pang, X.H.; et al. Validation of the ITS2 region as a novel DNA barcode for identifying medicinal plant species. PLoS ONE 2010, 5, e8613. [Google Scholar] [CrossRef] [PubMed]
- Li, X.W.; Yang, Y.; Henry, R.J.; Rossetto, M.; Wang, Y.T.; Chen, S.L. Plant DNA barcoding: From gene to genome. Biol. Rev. 2015, 90, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Sickel, W.; Ankenbrand, M.J.; Grimmer, G.; Holzschuh, A.; Härtel, S.; Lanzen, J.; Steffan-Dewenter, I.; Keller, A. Increased efficiency in identifying mixed pollen samples by meta-barcoding with a dual-indexing approach. BMC Ecol. 2015, 15, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Macgregor, C.J.; Pocock, M.J.O.; Fox, R.; Evans, D.M. Pollination by nocturnal Lepidoptera, and the effects of light pollution: A review. Ecol. Entomol. 2015, 40, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.H.; Pan, Y.B.; Chen, R.K. High-throughput procedure for single pollen grain collection and polymerase chain reaction in plants. J. Integr. Plant Biol. 2008, 50, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Fay, M.F.; Swensen, S.M.; Chase, M.W. Taxonomic affinities of Medusagyne oppositifolia (Medusagynaceae). Kew Bull. 1997, 52, 111–120. [Google Scholar] [CrossRef]
- Fazekas, A.J.; Burgess, K.S.; Kesanakurti, P.R.; Graham, S.W.; Newmaster, S.G.; Husband, B.C.; Percy, D.M.; Hajibabaei, M.; Barrett, S.C.H. Multiple multilocus DNA barcodes from the plastid genome discriminate plant species equally well. PLoS ONE 2008, 3, e2802. [Google Scholar] [CrossRef] [PubMed]
- Baamrane, M.A.A.; Shehzad, W.; Ouhammou, A.; Abbad, A.; Naimi, M.; Coissac, E.; Taberlet, P.; Znari, M. Assessment of the food habits of the Moroccan dorcas gazelle in M’Sabih Talaa, West Central Morocco, using the trnL approach. PLoS ONE 2012, 7, e35643. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Bruns, T.D.; Lee, S.B.; Taylor, J.W. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Wang, F.X.; Qian, N.F.; Zhang, Y.L.; Yang, H.Q. Pollen Flora of China; Science Press: Beijing, China, 1995; 461p. [Google Scholar]
- Li, T.Q.; Cao, H.J.; Kang, M.S.; Zhang, Z.X.; Zhao, N.; Zhang, H. Pollen Flora of China, Woody Plants by SEM; Science Press: Beijing, China, 2010; 1233p. [Google Scholar]
- Ma, D.W.; Zhang, C.H.; Gao, S.Z.; Ma, N.; Liu, H.H.; Zhang, Y.P.; Sun, L. Pollen Flora of China Vegetables by SEM; China Agriculture Press: Beijing, China, 1999; 166p. [Google Scholar]
- Martin, P.S.; Drew, C.M. Scanning electron photomicrographs of southwestern pollen grains. J. Ariz. Acad. Sci. 1969, 5, 147–176. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Ross, H.A.; Murugan, S.; Li, W.L. Testing the reliability of genetic methods of species identification via simulation. Syst. Biol. 2008, 57, 216–230. [Google Scholar] [CrossRef] [PubMed]


| Pollen Grain Type | Identified Plants | Molecular Identification | Morphology-Based Identification | Geographic Distribution in China |
|---|---|---|---|---|
| 1 | Citrus sinensis | Sister to Citrus limon/Citrus maxima/Citrus sinensis | Citrus sinensis | Zhejiang, Taiwan, Fujian, Jiangxi, Hubei, Hunan, Guangdong, Giangxi, Yunnan, Guihzhou, Sichuan |
| 2 | Heliotropium L. | Sister to Heliotropium stenophyllum/Heliotropium huascoense | Heliotropium L. | From south to southeast of China |
| 3 | Liliaceae | Unidentifiable | Liliaceae | The nationwide distribution |
| 4 | Cupressaceae | Unidentifiable | Cupressaceae | The nationwide distribution |
| 5 | Robinia pseudoacacia | Robinia pseudoacacia | Robinia L. | Gansu, Qinghai, Neimenggu, Xinjiang, Shanxi, Shaanxi, Hebei, Henan, Shandong et al. |
| 6 | Leguminosae | Unidentifiable | Leguminosae | The nationwide distribution |
| 7 | Amorpha fruticosa | Sister to Amorpha nana/Amorpha fruticosa | Amorpha fruticosa | The nationwide distribution |
| 8 | Cercidium L. | Sister to Cercidium andicola/Parkinsonia africana | Cercidium L. | The nationwide distribution |
| 9 | Pterocarya rhoifolia | Pterocarya rhoifolia | Pterocarya Kunth. | Shandong |
| 10 | Elaeagnus umbellata | Elaeagnus umbellata | Elaeagnus L. | North China, east China, southwest of China and Shaanxi, Gansu, Qinghai, Ningxia, Liaoning, Hubei |
| 11 | Fendlera Engelm. & Gray | Sister to Fendlera rupicola/Hydrangea quercifolia | Fendlera Engelm. & Gray | The nationwide distribution |
| 12 | Corylus L. | Sister to Corylus avellana/Ostryopsis nobilis | Corylus L. | From southwest to northeast of China |
| 13 | Betula L. | Sister to Betula pendula/Betula alba | Betula L. | The nationwide distribution |
| 14 | Melia azedarach | Melia azedarach | Melia L. | South of the Yellow River |
| 15 | Olea europaea | Olea europaea | Olea L. | Jiangsu, Anhui, Hubei, Hunan, Guizhou, Sichuan, Yunnan, Guangxi, Guangdong et al. |
| 16 | Ligustrum lucidum | Sister to Ligustrum lucidum/Forsythia suspensa | Ligustrum L. | Jiangsu, Zhejiang, Jiangxi, Anhui, Shandong, Hubei, Hunan, Guizhou, Sichuan, Fujian, Guangxi, Guangdong |
| 17 | Brassicaceae | Unidentifiable | Brassicaceae | The nationwide distribution |
| 18 | Brassica L. | Sister to Brassica rapa/Brassica napus/Brassica oleracea/Brassica juncea | Brassica L. | The nationwide distribution |
| 19 | Alliaceae | Unidentifiable | Alliaceae | The nationwide distribution |
| 20 | Pinus L. | Sister to Pinus tabuliformis/Pinus thunbergii/Pinus densata/Pinus hwangshanensis/Pinus kesiya/Pinus yunnanensisu | Pinus L. | The nationwide distribution |
| 21 | Pinaceae | Unidentifiable | Pinaceae | The nationwide distribution |
| 22 | Helianthus L. | Sister to Helianthus annuus/Helianthus argophyllus/Helianthus debilis/Helianthus tuberosus/Helianthus pauciflorus/Helianthus mollis/Helianthus petiolaris/Helianthus maximiliani | Helianthus L. | The nationwide distribution |
| 23 | Artemisia L. | Sister to Artemisia gmelinii/Artemisia vulqaris | Artemisia L. | The nationwide distribution |
| 24 | Asteraceae | Unidentifiable | Asteraceae | The nationwide distribution |
| 25 | Gnaphalium L. | Sister to Gnaphalium uliqinosum/Gnaphalium affine | Gnaphalium L. | The nationwide distribution |
| 26 | Chrysanthemum L. | Sister to Chrysanthemum mutellinum/Chrysanthemum indicum/Chrysanthemum x morifolium/Chrysanthemum lavandulifolium/Chrysanthemum maximum | Chrysanthemum L. | The nationwide distribution |
| 27 | Dendromecon Benth. | Unidentifiable | Dendromecon Benth. | The nationwide distribution |
| 28 | Eschscholtzia Cham. | Unidentifiable | Eschscholtzia Cham. | The nationwide distribution |
| 29 | Polygala L. | Sister to Polygala setacea/Polygala alba/Polygala qalapageia/Polygala sancti-qeorqii | Polygala L. | The nationwide distribution |
| 30 | Castanea henryi | Sister to Castanea sativa/Castanea mollissima/Castanea henryi | Castanea henryi | Jiangsu, Zhejiang, Jiangxi, Anhui, Shandong, Hubei, Hunan, Guizhou, Sichuan, Fujian, Guangxi, Guangdong |
| 31 | Castanopsis echinocarpa | Sister to Castanopsis echinocarpa/Castanopsis carlesii | Castanopsis echinocarpa | Southern of Yunnan province, southeast of the Tibet autonomous region |
| 32 | Ailanthus Desf. | Sister to Ailanthus fordii/ Ailanthus altissima/Ailanthus excelsa | Ailanthus Desf. | The nationwide distribution |
| 33 | Rosaceae | Unidentifiable | Rosaceae | The nationwide distribution |
| 34 | Rosaceae | Unidentifiable | Rosaceae | The nationwide distribution |
| 35 | Adenophora trachelioides | Sister to Adenophora erecta/Adenophora remotiflora/ | Adenophora trachelioides | Liaoning, Hebei, Shandong, Jiangsu, Anhui, Zhejiang |
| Adenophora trachelioides | ||||
| 36 | Chenopodium album | Sister to Chenopodium serotinum/Chenopodium album/Chenopodium acuminatum/Chenopodium quinoa | Chenopodium album | The nationwide distribution |
| 37 | Gaura L. | Sister to Gaura coccinea/Oenothera suffrutescens | Gaura L. | North of China |
| 38 | Galium L. | Sister to Galium boreale/Galium sp. | Galium L. | The nationwide distribution |
| 39 | Pilea Lindl., nom. conserv. | Sister to Pilea depressa/Pilea plataniflora/Pilea microphylla | Pilea Lindl., nom. conserv. | The nationwide distribution |
| 40 | Lauraceae | Unidentifiable | Lauraceae | The nationwide distribution |
| No./% | 2014 | 2015 | 2016 | 2017 | Total |
|---|---|---|---|---|---|
| No. adults examined | 773 | 492 | 628 | 673 | 2566 |
| No. with pollen | 72 | 132 | 158 | 75 | 437 |
| % with pollen | 9.31 | 26.83 | 25.16 | 11.14 | 17.03 |
| No. taxa | 15 | 27 | 24 | 18 | 40 |
| No. families | 14 | 17 | 19 | 13 | 26 |
| No. genera | 5 | 13 | 9 | 6 | 18 |
| No. species | 7 | 8 | 8 | 7 | 12 |
| Year | Female | Male | The Value of Test | |
|---|---|---|---|---|
| No. (%) of Moths Contaminated | ||||
| 2014 | 50 (9.52) | 22 (8.87) | χ2 | 0.085 |
| df | 1 | |||
| p | 0.771 | |||
| 2015 | 68 (31.19) | 64 (23.36) | χ2 | 3.796 |
| df | 1 | |||
| p | 0.051 | |||
| 2016 | 68 (23.69) | 90 (26.39) | χ2 | 0.603 |
| df | 1 | |||
| p | 0.437 | |||
| 2017 | 38 (10.86) | 37 (11.46) | χ2 | 0.061 |
| df | 1 | |||
| p | 0.805 | |||
| 2014–2017 | 350 (25.36) | 323 (27.23) | t | 0.165 |
| df | 6 | |||
| p | 0.875 | |||
| Family | Early-Season (May–June) | Mid-Season (July–August) | Late-Season (September–October) | Overall Total |
|---|---|---|---|---|
| Pinaceae | 31.9 | 7.23 | 4.1 | 19.45 |
| Leguminosae | 10.34 | 1.2 | 5.72 | |
| Oleaceae | 9.48 | 5.03 | ||
| Brassicaceae | 8.62 | 31.33 | 10.53 | |
| Meliaceae | 8.19 | 4.35 | ||
| Rosaceae | 7.76 | 4.82 | 5.03 | |
| Elaeagnaceae | 4.74 | 2.52 | ||
| Fagaceae | 4.74 | 7.23 | 3.89 | |
| Boraginaceae | 3.02 | 1.6 | ||
| Simaroubaceae | 2.59 | 1.37 | ||
| Alliaceae | 2.16 | 1.14 | ||
| Cupressaceae | 1.72 | 0.92 | ||
| Rutaceae | 1.29 | 0.69 | ||
| Asteraceae | 0.86 | 7.22 | 68.85 | 21.28 |
| Papaveraceae | 0.86 | 0.46 | ||
| Polygalaceae | 0.43 | 0.23 | ||
| Juglandaceae | 0.43 | 0.23 | ||
| Betulaceae | 0.43 | 1.2 | 0.46 | |
| Saxifragaceae | 0.43 | 0.23 | ||
| Onagraceae | 16.87 | 3.2 | ||
| Chenopodiaceae | 16.87 | 9.02 | 5.72 | |
| Campanulaceae | 3.61 | 0.69 | ||
| Rubiaceae | 2.41 | 0.46 | ||
| Urticaceae | 14.75 | 4.12 | ||
| Lauraceae | 3.28 | 0.92 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, H.; Guo, J.; Fu, X.; Liu, Y.; Wyckhuys, K.A.G.; Hou, Y.; Wu, K. Molecular-Assisted Pollen Grain Analysis Reveals Spatiotemporal Origin of Long-Distance Migrants of a Noctuid Moth. Int. J. Mol. Sci. 2018, 19, 567. https://doi.org/10.3390/ijms19020567
Chang H, Guo J, Fu X, Liu Y, Wyckhuys KAG, Hou Y, Wu K. Molecular-Assisted Pollen Grain Analysis Reveals Spatiotemporal Origin of Long-Distance Migrants of a Noctuid Moth. International Journal of Molecular Sciences. 2018; 19(2):567. https://doi.org/10.3390/ijms19020567
Chicago/Turabian StyleChang, Hong, Jianglong Guo, Xiaowei Fu, Yongqiang Liu, Kris A. G. Wyckhuys, Youming Hou, and Kongming Wu. 2018. "Molecular-Assisted Pollen Grain Analysis Reveals Spatiotemporal Origin of Long-Distance Migrants of a Noctuid Moth" International Journal of Molecular Sciences 19, no. 2: 567. https://doi.org/10.3390/ijms19020567
APA StyleChang, H., Guo, J., Fu, X., Liu, Y., Wyckhuys, K. A. G., Hou, Y., & Wu, K. (2018). Molecular-Assisted Pollen Grain Analysis Reveals Spatiotemporal Origin of Long-Distance Migrants of a Noctuid Moth. International Journal of Molecular Sciences, 19(2), 567. https://doi.org/10.3390/ijms19020567