The Possible Importance of β3 Integrins for Leukemogenesis and Chemoresistance in Acute Myeloid Leukemia
Abstract
:1. Introduction
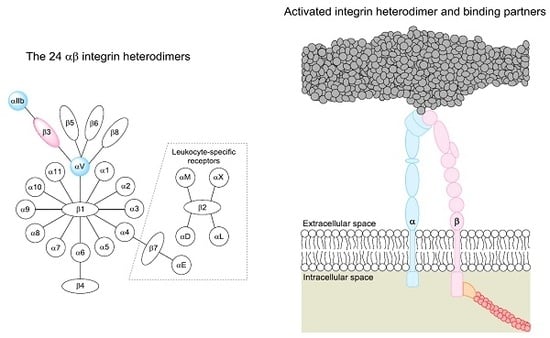
2. The Integrin Family
3. The β3 Integrin (ITGB3) Associations with Clinico-Pathological Features in AML
3.1. Regulation of ITGB3 Expression in AML Cells; the Importance of Troponin, PI3K and Monocytic Differentiation
3.2. Associations between ITGB3 Expression and Clinico-Pathological Features
4. The Promiscuity of β3 Integrin Ligand Binding
5. β3 Integrins and Spleen Tyrosine Kinase (SYK) Activation in Murine Models of AML
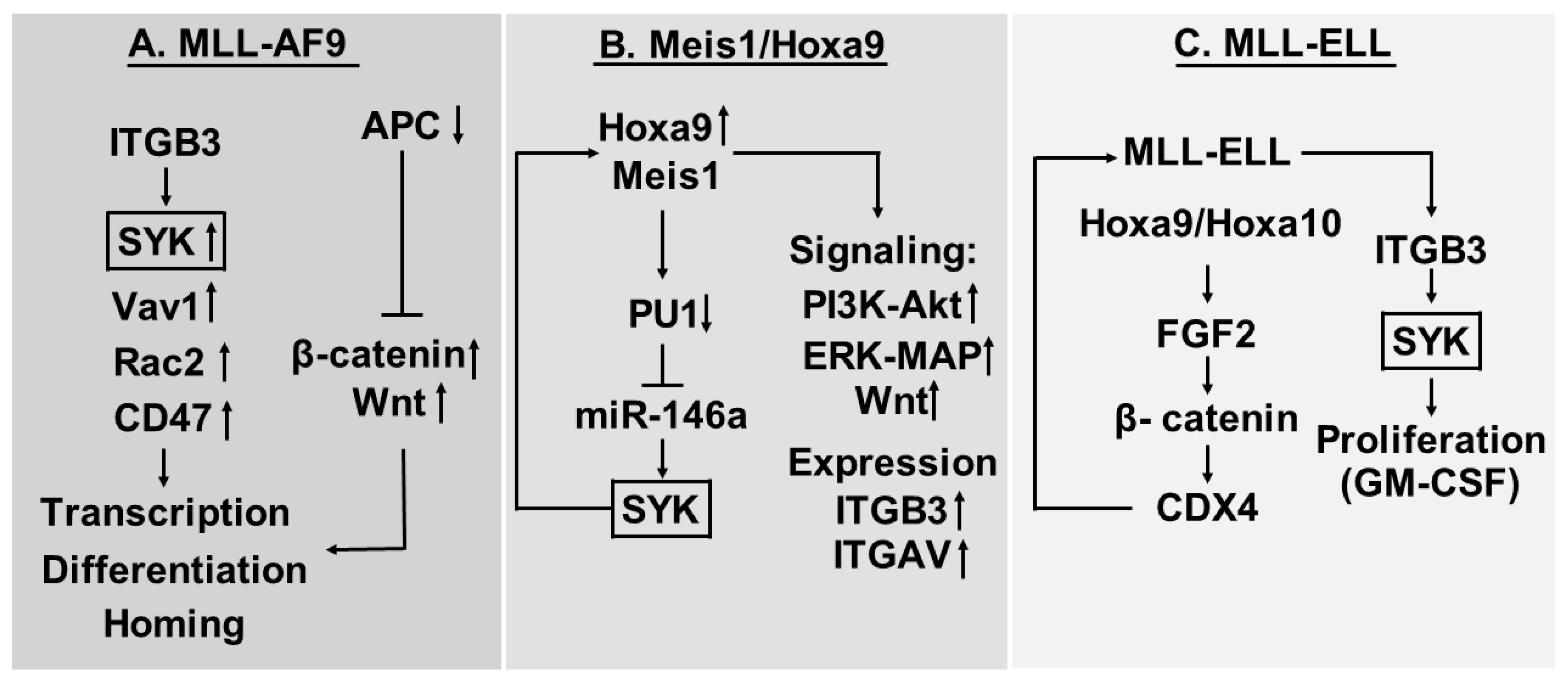
5.1. ITGB3 Shows High Expression in the MLL-AF9 Mouse Model of AML
5.2. Myeloid Ectopic (Viral) Insertion Site-1 (Meis1)/Hoxa9 Driven AML Cells Depend on Meis1-Induced Syk Expression and ITGAV/ITGB3 Induced Syk Activation
5.3. Proliferation of AML Cells Expressing the MLL-ELL Fusion Protein is Increased by Cooperation between Fibroblast Growth Factor (FGF) 2 and ITGAV/ITGB3 Integrins
6. The Role of αVβ3 Integrins Human AML
6.1. HOX Genes and β-Catenin
6.2. Modulation of Syk and Focal Adhesion Kinase (FAK) Activation by β3 Ligation
6.3. Clinical Evidence for a Role of β3 Integrins in Human AML; the Stories of SPARC and TRIM62
7. β3-Integrins, Intracellular Signaling and Transcriptional Regulation—A Summary of Our Current Evidence
- The extracellular SPARC molecule may be important for regulation of cytokine responses (e.g., FGF2 and possibly GM-CSF responses) and thereby interact with the functional effects of β3-integrin signaling.
- Several cell surface molecules seem to be important for the signaling, including CD47 and β-catenin; this last protein has a dual function and is important both for cell adhesion and transcriptional regulation. Another cell surface proteins being important for the signaling is CD47.
- Syk seems to be an important downstream mediator, possibly the most important.
- However, several pathways seem to be involved, including Wnt-signaling, PI3K-Akt, and Erk-MAP. The importance of Vav1 (guanine nucleotide exchange factor) and Rac2 (a GTPase) in the MLL-AF9 model also suggest that G-protein dependent signaling may be involved.
- Several transcriptional regulators also seem to be involved, including β-catenin, Meis1, miR-146a, PU1, HoxA9, HoxA10 and CDX4.
8. The Soluble Isoform of β3 Integrins
9. The Possible Importance of Cooperation between Different Integrins
10. Summarizing and Concluding Comments
Acknowledgments
Conflict of Interests
Abbervations
| AML | Acute myelogen leukemia |
| APL | Acute promyelocytic leukemia |
| COMP | Cartilage oligomeric matrix protein |
| CREBBP | CREB binding protein |
| CTGF | Connective tissue growth factor |
| Cyr61 | Cystein-rich61 |
| Ctnnb1 | β–catenin |
| CXCR4 | CXC chemokine receptor 4 |
| Del-1 | Developmental endothelial locus-1 |
| ELL | Eleven-nineteen lysine-rich leukemia |
| FAK | Focal adhesion kinase |
| FLT3 | Fms-like receptor tyrosin kinase 3 |
| GSK3-β | Glycogen synthase kinase-3 β |
| GTPase | Guanosine triphosphatase |
| GM-CSF | Granulocyte-macrophage colony-stimulatingfactor |
| HEL | Erythroleukemia cell line |
| HoxA9 | Homebox A9 |
| Hmgb3 | High mobility group box 3 |
| ICAM-4 | Intercellular adhesion molecule-4 |
| IL2 | Interleukin-2 |
| IFN-γ | Interferon-γ |
| ITD | Internal tandem duplications |
| ITGAV | Integrin αV |
| ITGB3 | Beta3 intergrin |
| MAP/ERK | Mitogen activated protein/extracellular signal-regulated kinase |
| MEIS1 | Myeloid ectopic (viral) insertion site-1 |
| MFG-E8 | Milk fat globule-EGF-factor 8 protein |
| MLL | Mixed lineage leukemia |
| MMP-2 | Matrixmetalloprotease 2 |
| MOZ | Monocytic leukaemia zinc finger protein |
| NK | Natural killer (cell) |
| NPM1 | Nucleophosmin 1 |
| NUP98 | Nucleoporin98 |
| PDGF | Platelet derived growth factor |
| PCAM | Platelet cell adhesion molecule |
| PtdIns | Phosphatidyl inositol |
| PI3K | Phosphatidylinositol-3-kinases |
| RGD | Arg-Gly-Asp |
| Ser/Thr | Serine/Threonine |
| S β3 | Soluble β3 |
| SPARC | Secreted-Protein-Acidic-Cysteine Rich |
| STAT5 | Signal transducer and activator of transcription-5 |
| SYK | Spleen tyrosin kinase |
| TGF-β | Transforming growth factor-β |
| TNF-α | Tumor necrosis factor-α |
| TRIM62 | Tripartite motif-62 |
| TSH | Thyroid stimulating hormon |
| Tyr | Tyrosine |
| VEGF | Vascular endothelial growth factor |
| vWF | Von Willebrand Factor |
References
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 revision to the world health organization classification of myeloid neoplasms and acute leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef] [PubMed]
- Döhner, H.; Weisdorf, D.J.; Bloomfield, C.D. Acute myeloid leukemia. N. Engl. J. Med. 2015, 373, 1136–1152. [Google Scholar] [CrossRef] [PubMed]
- Döhner, H.; Estey, E.; Grimwade, D.; Amadori, S.; Appelbaum, F.R.; Buchner, T.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Larson, R.A.; et al. Diagnosis and mrnaanagement of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017, 129, 424–447. [Google Scholar] [CrossRef] [PubMed]
- Brenner, A.K.; Nepstad, I.; Bruserud, O. Mesenchymal stem cells support survival and proliferation of primary human acute myeloid leukemia cells through heterogeneous molecular mechanisms. Front. Immunol. 2017, 8, 106. [Google Scholar] [CrossRef] [PubMed]
- Reikvam, H.; Brenner, A.K.; Hagen, K.M.; Liseth, K.; Skrede, S.; Hatfield, K.J.; Bruserud, O. The cytokine-mediated crosstalk between primary human acute myeloid cells and mesenchymal stem cells alters the local cytokine network and the global gene expression profile of the mesenchymal cells. Stem Cell Res. 2015, 15, 530–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, S.; Barrett, A.J.; Dutra, A.; Pak, E.; Miner, S.; Keyvanfar, K.; Hensel, N.F.; Rezvani, K.; Muranski, P.; Liu, P.; et al. Long term maintenance of myeloid leukemic stem cells cultured with unrelated human mesenchymal stromal cells. Stem Cell Res. 2015, 14, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.A.; Shim, J.S.; Lee, G.Y.; Yim, H.W.; Kim, T.M.; Kim, M.; Leem, S.H.; Lee, J.W.; Min, C.K.; Oh, I.H. Microenvironmental remodeling as a parameter and prognostic factor of heterogeneous leukemogenesis in acute myelogenous leukemia. Cancer Res. 2015, 75, 2222–2231. [Google Scholar] [CrossRef] [PubMed]
- Hynes, R.O. Integrins: Bidirectional, allosteric signaling machines. Cell 2002, 110, 673–687. [Google Scholar] [CrossRef]
- Humphries, J.D.; Byron, A.; Humphries, M.J. Integrin ligands at a glance. J. Cell Sci. 2006, 119, 3901–3903. [Google Scholar] [CrossRef] [PubMed]
- Arnaout, M.A.; Mahalingam, B.; Xiong, J.P. Integrin structure, allostery, and bidirectional signaling. Annu. Rev. Cell Dev. Biol. 2005, 21, 381–410. [Google Scholar] [CrossRef] [PubMed]
- Springer, T.A.; Wang, J.H. The three-dimensional structure of integrins and their ligands, and conformational regulation of cell adhesion. Adv. Protein Chem. 2004, 68, 29–63. [Google Scholar] [PubMed]
- Barczyk, M.; Carracedo, S.; Gullberg, D. Integrins. Cell Tissue Res. 2010, 339, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Tohyama, Y.; Tohyama, K.; Tsubokawa, M.; Asahi, M.; Yoshida, Y.; Yamamura, H. Outside-in signaling of soluble and solid-phase fibrinogen through integrin alphaiibbeta3 is different and cooperative with each other in a megakaryoblastic leukemia cell line, cmk. Blood 1998, 92, 1277–1286. [Google Scholar] [PubMed]
- Pigneux, A.; Bene, M.C.; Guardiola, P.; Recher, C.; Hamel, J.F.; Sauvezie, M.; Harousseau, J.L.; Tournilhac, O.; Witz, F.; Berthou, C.; et al. Addition of androgens improves survival in elderly patients with acute myeloid leukemia: A goelams study. J. Clin. Oncol. 2017, 35, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Favreau, A.J.; Vary, C.P.; Brooks, P.C.; Sathyanarayana, P. Cryptic collagen IV promotes cell migration and adhesion in myeloid leukemia. Cancer Med. 2014, 3, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.J.; Prazak, L.; Fajardo, M.; Yu, S.; Tyagi, N.; Di Cesare, P.E. Leukemia/lymphoma-related factor, a POZ domain-containing transcriptional repressor, interacts with histone deacetylase-1 and inhibits cartilage oligomeric matrix protein gene expression and chondrogenesis. J. Biol. Chem. 2004, 279, 47081–47091. [Google Scholar] [CrossRef] [PubMed]
- Battula, V.L.; Chen, Y.; Cabreira Mda, G.; Ruvolo, V.; Wang, Z.; Ma, W.; Konoplev, S.; Shpall, E.; Lyons, K.; Strunk, D.; et al. Connective tissue growth factor regulates adipocyte differentiation of mesenchymal stromal cells and facilitates leukemia bone marrow engraftment. Blood 2013, 122, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Cheung, L.C.; Strickland, D.H.; Howlett, M.; Ford, J.; Charles, A.K.; Lyons, K.M.; Brigstock, D.R.; Goldschmeding, R.; Cole, C.H.; Alexander, W.S.; et al. Connective tissue growth factor is expressed in bone marrow stromal cells and promotes interleukin-7-dependent B lymphopoiesis. Haematologica 2014, 99, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Long, X.; Yu, Y.; Perlaky, L.; Man, T.K.; Redell, M.S. Stromal CYR61 confers resistance to mitoxantrone via spleen tyrosine kinase activation in human acute myeloid leukaemia. Br. J. Haematol. 2015, 170, 704–718. [Google Scholar] [CrossRef] [PubMed]
- Mitroulis, I.; Chen, L.S.; Singh, R.P.; Kourtzelis, I.; Economopoulou, M.; Kajikawa, T.; Troullinaki, M.; Ziogas, A.; Ruppova, K.; Hosur, K.; et al. Secreted protein Del-1 regulates myelopoiesis in the hematopoietic stem cell niche. J. Clin. Investig. 2017, 127, 3624–3639. [Google Scholar] [CrossRef] [PubMed]
- Smaldone, S.; Bigarella, C.L.; Del Solar, M.; Ghaffari, S.; Ramirez, F. Fibrillin-1 microfibrils influence adult bone marrow hematopoiesis. Matrix Biol. 2016, 52–54, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.D.; Heini, A.D.; Seipel, K.; Mueller, B.; Angelillo-Scherrer, A.; Pabst, T. Increased fibrinogen levels at diagnosis are associated with adverse outcome in patients with acute myeloid leukemia. Hematol. Oncol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Vidal, A.; Ysebaert, L.; Didier, C.; Betous, R.; De Toni, F.; Prade-Houdellier, N.; Demur, C.; Contour-Galcera, M.O.; Prevost, G.P.; Ducommun, B.; et al. Cell adhesion regulates CDC25A expression and proliferation in acute myeloid leukemia. Cancer Res. 2006, 66, 7128–7135. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Lo, A.; Short, S.A.; Spring, F.; Parsons, S.F.; Yazdanbakhsh, K.; Mohandas, N.; Anstee, D.J.; Chasis, J.A. Targetes gene deletion demonstrates that the cell adhesion molecule ICAM-4 is critical for erythroblastic island foramtion. Blood 2006, 15, 2064–2071. [Google Scholar] [CrossRef] [PubMed]
- Ebeling, O.; Duczmal, A.; Aigner, S.; Geiger, C.; Schollhammer, S.; Kemshead, J.T.; Moller, P.; Schwartz-Albiez, R.; Altevogt, P. L1 adhesion molecule on human lymphocytes and monocytes: Expression and involvement in binding to alpha v beta 3 integrin. Eur. J. Immunol. 1996, 26, 2508–2516. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Choi, B.I.; Park, S.Y.; An, S.Y.; Han, J.; Kim, J.H. Fetal hematopoietic stem cells express MFG-E8 during mouse embryogenesis. Exp. Mol. Med. 2015, 47, e174. [Google Scholar] [CrossRef] [PubMed]
- Reikvam, H.; Hatfield, K.J.; Oyan, A.M.; Kalland, K.H.; Kittang, A.O.; Bruserud, O. Primary human acute myelogenous leukemia cells release matrix metalloproteases and their inhibitors: Release profile and pharmacological modulation. Eur. J. Haematol. 2010, 84, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Yu, G.; Meng, W.; Wang, Z.; Meng, F.; Ma, W. Overexpression of amyloid precursor protein in acute myeloid leukemia enhances extramedullary infiltration by MMP-2. Tumour Biol. 2013, 34, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Song, J.H.; Kim, S.H.; Cho, D.; Lee, I.K.; Kim, H.J.; Kim, T.S. Enhanced invasiveness of drug-resistant acute myeloid leukemia cells through increased expression of matrix metalloproteinase-2. Int. J. Cancer 2009, 125, 1074–1081. [Google Scholar] [CrossRef] [PubMed]
- Liersch, R.; Gerss, J.; Schliemann, C.; Bayer, M.; Schwoppe, C.; Biermann, C.; Appelmann, I.; Kessler, T.; Lowenberg, B.; Buchner, T.; et al. Osteopontin is a prognostic factor for survival of acute myeloid leukemia patients. Blood 2012, 119, 5215–5220. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.B.; Ren, S.M.; Li, S.D.; Du, Z. Prognostic significance of osteopontin in acute myeloid leukemia: A meta-analysis. Mol. Clin. Oncol. 2017, 7, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Somerman, M.J.; Berry, J.E.; Khalkhali-Ellis, Z.; Osdoby, P.; Simpson, R.U. Enhanced expression of alpha v integrin subunit and osteopontin during differentiation of hl-60 cells along the monocytic pathway. Exp. Cell Res. 1995, 216, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Ayatollahi, M.; Geramizadeh, B.; Zakerinia, M.; Ramzi, M.; Yaghobi, R.; Hadadi, P.; Rezvani, A.R.; Aghdai, M.; Azarpira, N.; Karimi, H. Human bone marrow-derived mesenchymal stem cell: A source for cell-based therapy. Int. J. Organ Transplant. Med. 2012, 3, 32–41. [Google Scholar] [PubMed]
- Alachkar, H.; Santhanam, R.; Maharry, K.; Metzeler, K.H.; Huang, X.; Kohlschmidt, J.; Mendler, J.H.; Benito, J.M.; Hickey, C.; Neviani, P.; et al. Sparc promotes leukemic cell growth and predicts acute myeloid leukemia outcome. J. Clin. Investig. 2014, 124, 1512–1524. [Google Scholar] [CrossRef] [PubMed]
- Lei, U.; Wohlfahrt, J.; Hjalgrim, H.; Hjalgrim, L.L.; Simonsen, H.; Melbye, M. Neonatal level of thyroid-stimulating hormone and acute childhood leukemia. Int. J. Cancer 2000, 88, 486–488. [Google Scholar] [CrossRef]
- Li, K.; Yang, M.; Yuen, P.M.; Chik, K.W.; Li, C.K.; Shing, M.M.; Lam, H.K.; Fok, T.F. Thrombospondin-1 induces apoptosis in primary leukemia and cell lines mediated by CD36 and caspase-3. Int. J. Mol. Med. 2003, 12, 995–1001. [Google Scholar] [CrossRef] [PubMed]
- Foss, B.; Nesthus, I.; Bergheim, J.; Bruserud, O. Serum levels of thrombopoietin and stem cell factor in acute leukemia patients with chemotherapy-induced cytopenia and complicating infections. Platelets 1999, 10, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Shah, C.A.; Bei, L.; Wang, H.; Altman, J.K.; Platanias, L.C.; Eklund, E.A. Cooperation between alphavbeta3 integrin and the fibroblast growth factor receptor enhances proliferation of hox-overexpressing acute myeloid leukemia cells. Oncotarget 2016, 7, 54782–54794. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.J.; Han, Y.; Ma, Z.N.; Wang, Q.; Tang, Y.Q.; Wang, J.; Su, J.; Sun, A.N.; Wang, Z.Y.; Ruan, C.G.; et al. Changes of adamts13 activity and VWF antigen level in patients with acute myelogenous leukemia and their significance. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2014, 22, 1503–1507. (in Chinese). [Google Scholar] [PubMed]
- Liu, C.; Zhao, L.; Zhao, J.; Xu, Q.; Song, Y.; Wang, H. Reduced adamts-13 level negatively correlates with inflammation factors in plasma of acute myeloid leukemia patients. Leuk. Res. 2017, 53, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Winograd-Katz, S.E.; Fassler, R.; Geiger, B.; Legate, K.R. The integrin adhesome: From genes and proteins to human disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 273–288. [Google Scholar] [CrossRef] [PubMed]
- Benedetto, S.; Pulito, R.; Crich, S.G.; Tarone, G.; Aime, S.; Silengo, L.; Hamm, J. Quantification of the expression level of integrin receptor alpha(v)beta3 in cell lines and mr imaging with antibody-coated iron oxide particles. Magn. Reson. Med. 2006, 56, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Zhai, J.; Wang, Y.; Xu, C.; Zheng, L.; Wang, M.; Feng, W.; Gao, L.; Zhao, L.; Liu, R.; Gao, F.; et al. Facile approach to observe and quantify the alpha(IIb)beta3 integrin on a single-cell. Anal. Chem. 2015, 87, 2546–2549. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.; Zeng, D.; Shen, Z.; Liao, J.; Wang, X.; Liu, Y.; Zhang, X.; Kong, P. Integrin alphavbeta3 enhances beta-catenin signaling in acute myeloid leukemia harboring FMS-like tyrosine kinase-3 internal tandem duplication mutations: Implications for microenvironment influence on sorafenib sensitivity. Oncotarget 2016, 7, 40387–40397. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.C.; Chou, Y.L.; Ch’ang, L.Y. Down-regulation of human NDR gene in megakaryocytic differentiation of erythroleukemia K562 cells. J. Biomed. Sci. 2004, 11, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Helleberg, C.; Knudsen, H.; Hansen, P.B.; Nikolajsen, K.; Kjaersgaard, E.; Ralfkiaer, E.; Johnsen, H.E. CD34+ megakaryoblastic leukaemic cells are CD38-, but CD61+ and glycophorin A+: Improved criteria for diagnosis of AML-M7? Leukemia 1997, 11, 830–834. [Google Scholar] [CrossRef] [PubMed]
- Ylanne, J.; Cheresh, D.A.; Virtanen, I. Localization of beta 1, beta 3, alpha 5, alpha v, and alpha IIb subunits of the integrin family in spreading human erythroleukemia cells. Blood 1990, 76, 570–577. [Google Scholar] [PubMed]
- Plow, E.F.; Haas, T.A.; Zhang, L.; Loftus, J.; Smith, J.W. Ligand binding to integrins. J. Biol. Chem. 2000, 275, 21785–21788. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Krivtsov, A.V.; Sinha, A.U.; North, T.E.; Goessling, W.; Feng, Z.; Zon, L.I.; Armstrong, S.A. The wnt/beta-catenin pathway is required for the development of leukemia stem cells in aml. Science 2010, 327, 1650–1653. [Google Scholar] [CrossRef] [PubMed]
- Miller, P.G.; Al-Shahrour, F.; Hartwell, K.A.; Chu, L.P.; Jaras, M.; Puram, R.V.; Puissant, A.; Callahan, K.P.; Ashton, J.; McConkey, M.E.; et al. In vivo RNAi screening identifies a leukemia-specific dependence on integrin beta 3 signaling. Cancer Cell 2013, 24, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Sheng, Y.; Li, W.; Hu, C.; Mittal, N.; Tohyama, K.; Seba, A.; Zhao, Y.Y.; Ozer, H.; Zhu, T.; et al. Beta-catenin is a candidate therapeutic target for myeloid neoplasms with del(5q). Cancer Res. 2017, 77, 4116–4126. [Google Scholar] [CrossRef] [PubMed]
- Ugarte, G.D.; Vargas, M.F.; Medina, M.A.; Leon, P.; Necunir, D.; Elorza, A.A.; Gutierrez, S.E.; Moon, R.T.; Loyola, A.; De Ferrari, G.V. Wnt signaling induces transcription, spatial proximity, and translocation of fusion gene partners in human hematopoietic cells. Blood 2015, 126, 1785–1789. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, E.A.; Golding, M.C.; Srivastava, P.; Povinelli, B.J.; James, S.R.; Ford, L.A.; Wetzler, M.; Wang, E.S.; Nemeth, M.J. Pharmacological targeting of beta-catenin in normal karyotype acute myeloid leukemia blasts. Haematologica 2015, 100, e49–e52. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.; Shanmukhaiah, C.; Marathe, S.; Mishra, P.; Babu Rao, V.; Ghosh, K.; Madkaikar, M. Differential antigen expression and aberrant signaling via PI3/AKT, MAP/ERK, JAK/STAT, and WNT/beta catenin pathways in Lin-/CD38-/CD34+ cells in acute myeloid leukemia. Eur. J. Haematol. 2016, 96, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Hu, C.; Mei, C.; Ren, Z.; Vera, J.C.; Zhuang, Z.; Jin, J.; Tong, H. Sequential combination of decitabine and idarubicin synergistically enhances anti-leukemia effect followed by demethylating wnt pathway inhibitor promoters and downregulating wnt pathway nuclear target. J. Transl. Med. 2014, 12, 167. [Google Scholar] [CrossRef] [PubMed]
- Mohr, S.; Doebele, C.; Comoglio, F.; Berg, T.; Beck, J.; Bohnenberger, H.; Alexe, G.; Corso, J.; Strobel, P.; Wachter, A.; et al. Hoxa9 and Meis1 cooperatively induce addiction to syk signaling by suppressing miR-146a in acute myeloid leukemia. Cancer Cell 2017, 31, 549–562 e511. [Google Scholar] [CrossRef] [PubMed]
- Collins, C.T.; Hess, J.L. Deregulation of the hoxa9/Meis1 axis in acute leukemia. Curr. Opin. Hematol. 2016, 23, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Eklund, E. The role of hox proteins in leukemogenesis: Insights into key regulatory events in hematopoiesis. Crit. Rev. Oncog. 2011, 16, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, H.J.; Heyn, H.; Vizoso, M.; Moutinho, C.; Vidal, E.; Gomez, A.; Martinez-Cardus, A.; Simo-Riudalbas, L.; Moran, S.; Jost, E.; et al. DNMT3A mutations mediate the epigenetic reactivation of the leukemogenic factor MEIS1 in acute myeloid leukemia. Oncogene 2016, 35, 3079–3082. [Google Scholar] [CrossRef] [PubMed]
- Ogawara, Y.; Katsumoto, T.; Aikawa, Y.; Shima, Y.; Kagiyama, Y.; Soga, T.; Matsunaga, H.; Seki, T.; Araki, K.; Kitabayashi, I. IDH2 and NPM1 mutations cooperate to activate Hoxa9/Meis1 and hypoxia pathways in acute myeloid leukemia. Cancer Res. 2015, 75, 2005–2016. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Qin, Y.Z.; Yang, S.; Wang, Y.; Chang, Y.J.; Zhao, T.; Jiang, Q.; Huang, X.J. Meis1 is critical to the maintenance of human acute myeloid leukemia cells independent of MLL rearrangements. Ann. Hematol. 2017, 96, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Shah, C.A.; Bei, L.; Wang, H.; Platanias, L.C.; Eklund, E.A. The leukemia-associated MLL-ell oncoprotein induces fibroblast growth factor 2 (FGF2)-dependent cytokine hypersensitivity in myeloid progenitor cells. J. Biol. Chem. 2013, 288, 32490–32505. [Google Scholar] [CrossRef] [PubMed]
- Roche, J.; Zeng, C.; Baron, A.; Gadgil, S.; Gemmill, R.M.; Tigaud, I.; Thomas, X.; Drabkin, H.A. Hox expression in aml identifies a distinct subset of patients with intermediate cytogenetics. Leukemia 2004, 18, 1059–1063. [Google Scholar] [CrossRef] [PubMed]
- Argiropoulos, B.; Humphries, R.K. Hox genes in hematopoiesis and leukemogenesis. Oncogene 2007, 26, 6766–6776. [Google Scholar] [CrossRef] [PubMed]
- Tohyama, Y.; Yanagi, S.; Sada, K.; Yamamura, H. Translocation of p72syk to the cytoskeleton in thrombin-stimulated platelets. J. Biol. Chem. 1994, 269, 32796–32799. [Google Scholar] [PubMed]
- Despeaux, M.; Chicanne, G.; Rouer, E.; De Toni-Costes, F.; Bertrand, J.; Mansat-De Mas, V.; Vergnolle, N.; Eaves, C.; Payrastre, B.; Girault, J.A.; et al. Focal adhesion kinase splice variants maintain primitive acute myeloid leukemia cells through altered WNT signaling. Stem Cells 2012, 30, 1597–1610. [Google Scholar] [CrossRef] [PubMed]
- Recher, C.; Ysebaert, L.; Beyne-Rauzy, O.; Mansat-De Mas, V.; Ruidavets, J.B.; Cariven, P.; Demur, C.; Payrastre, B.; Laurent, G.; Racaud-Sultan, C. Expression of focal adhesion kinase in acute myeloid leukemia is associated with enhanced blast migration, increased cellularity, and poor prognosis. Cancer Res. 2004, 64, 3191–3197. [Google Scholar] [CrossRef] [PubMed]
- Carter, B.Z.; Mak, P.Y.; Wang, X.; Yang, H.; Garcia-Manero, G.; Mak, D.H.; Mu, H.; Ruvolo, V.R.; Qiu, Y.; Coombes, K.; et al. Focal adhesion kinase as a potential target in AML and MDS. Mol. Cancer Ther. 2017, 16, 1133–1144. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.; Ghosh, J.; Ramdas, B.; Mali, R.S.; Martin, H.; Kobayashi, M.; Vemula, S.; Canela, V.H.; Waskow, E.R.; Visconte, V.; et al. Regulation of STAT5 by FAK and PAK1 in oncogenic FLT3- and KIT-driven leukemogenesis. Cell Rep. 2014, 9, 1333–1348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tavernier-Tardy, E.; Cornillon, J.; Campos, L.; Flandrin, P.; Duval, A.; Nadal, N.; Guyotat, D. Prognostic value of CXCR4 and fak expression in acute myelogenous leukemia. Leuk. Res. 2009, 33, 764–768. [Google Scholar] [CrossRef] [PubMed]
- Quintas-Cardama, A.; Zhang, N.; Qiu, Y.H.; Post, S.; Creighton, C.J.; Cortes, J.; Coombes, K.R.; Kornblau, S.M. Loss of TRIM62 expression is an independent adverse prognostic factor in acute myeloid leukemia. Clin. Lymphoma Myeloma Leuk. 2015, 15, 115–127 e115. [Google Scholar] [CrossRef] [PubMed]
- Skaik, Y.; Vahlsing, S.; Goudeva, L.; Eiz-Vesper, B.; Battermann, A.; Blasczyk, R.; Figueiredo, C. Secreted beta3-integrin enhances natural killer cell activity against acute myeloid leukemia cells. PLoS ONE 2014, 9, e98936. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, L.; Mancusi, A.; Burchielli, E.; Aversa, F.; Martelli, M.F.; Velardi, A. Natural killer cell alloreactivity in allogeneic hematopoietic transplantation. Curr. Opin. Oncol. 2007, 19, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Blystone, S.D.; Lindberg, F.P.; LaFlamme, S.E.; Brown, E.J. Integrin beta 3 cytoplasmic tail is necessary and sufficient for regulation of alpha 5 beta 1 phagocytosis by alpha v beta 3 and integrin-associated protein. J. Cell Biol. 1995, 130, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Pacifici, R.; Roman, J.; Kimble, R.; Civitelli, R.; Brownfield, C.M.; Bizzarri, C. Ligand binding to monocyte alpha 5 beta 1 integrin activates the alpha 2 beta 1 receptor via the alpha 5 subunit cytoplasmic domain and protein kinase c. J. Immunol. 1994, 153, 2222–2233. [Google Scholar] [PubMed]
- Jiang, B.H.; Liu, L.Z. Pi3k/pten signaling in angiogenesis and tumorigenesis. Adv. Cancer Res. 2009, 102, 19–65. [Google Scholar] [CrossRef] [PubMed]
- Becker, P.S.; Kopecky, K.J.; Wilks, A.N.; Chien, S.; Harlan, J.M.; Willman, C.L.; Petersdorf, S.H.; Stirewalt, D.L.; Papayannopoulou, T.; Appelbaum, F.R. Very late antigen-4 function of myeloblasts correlates with improved overall survival for patients with acute myeloid leukemia. Blood 2009, 113, 866–874. [Google Scholar] [CrossRef] [PubMed]
- Bae, M.H.; Oh, S.H.; Park, C.J.; Lee, B.R.; Kim, Y.J.; Cho, Y.U.; Jang, S.; Lee, J.H.; Kim, N.; Park, S.H.; et al. VLA-4 and CXCR4 expression levels show contrasting prognostic impact (favorable and unfavorable, respectively) in acute myeloid leukemia. Ann. Hematol. 2015, 94, 1631–1638. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, T.; Takemoto, N.; Sato, T.; Takimoto, R.; Tanaka, I.; Fujimi, A.; Akiyama, T.; Kuroda, H.; Kawano, Y.; Kobune, M.; et al. Interaction between leukemic-cell VLA-4 and stromal fibronectin is a decisive factor for minimal residual disease of acute myelogenous leukemia. Nat. Med. 2003, 9, 1158–1165. [Google Scholar] [CrossRef] [PubMed]
- Walter, R.B.; Alonzo, T.A.; Gerbing, R.B.; Ho, P.A.; Smith, F.O.; Raimondi, S.C.; Hirsch, B.A.; Gamis, A.S.; Franklin, J.L.; Hurwitz, C.A.; et al. High expression of the very late antigen-4 integrin independently predicts reduced risk of relapse and improved outcome in pediatric acute myeloid leukemia: A report from the children's oncology group. J. Clin. Oncol. 2010, 28, 2831–2838. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.F.; Li, K.S.; Shen, Y.H.; Gao, P.T.; Dong, Z.R.; Cai, J.B.; Zhang, C.; Huang, X.Y.; Tian, M.X.; Hu, Z.Q.; et al. Galectin-1 induces hepatocellular carcinoma EMT and sorafenib resistance by activating FAK/PI3K/AKT signaling. Cell Death Dis. 2016, 7, e2201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ge, X.H.; Guo, X.J.; Guan, S.B.; Li, X.M.; Gu, W.; Xu, W.G. Bone marrow mesenchymal stem cells inhibit the function of dendritic cells by secreting galectin-1. Biomed. Res. Int. 2017, 2017, 3248605. [Google Scholar] [CrossRef] [PubMed]
- El Leithy, A.A.; Helwa, R.; Assem, M.M.; Hassan, N.H. Expression profiling of cancer-related galectins in acute myeloid leukemia. Tumour Biol. 2015, 36, 7929–7939. [Google Scholar] [CrossRef] [PubMed]
- Azzariti, A.; Mancarella, S.; Porcelli, L.; Quatrale, A.E.; Caligiuri, A.; Lupo, L.; Dituri, F.; Giannelli, G. Hepatic stellate cells induce hepatocellular carcinoma cell resistance to sorafenib through the laminin-332/alpha3 integrin axis recovery of focal adhesion kinase ubiquitination. Hepatology 2016, 64, 2103–2117. [Google Scholar] [CrossRef] [PubMed]
- Xargay-Torrent, S.; Lopez-Guerra, M.; Montraveta, A.; Saborit-Villarroya, I.; Rosich, L.; Navarro, A.; Perez-Galan, P.; Roue, G.; Campo, E.; Colomer, D. Sorafenib inhibits cell migration and stroma-mediated bortezomib resistance by interfering B-cell receptor signaling and protein translation in mantle cell lymphoma. Clin. Cancer Res. 2013, 19, 586–597. [Google Scholar] [CrossRef] [PubMed]
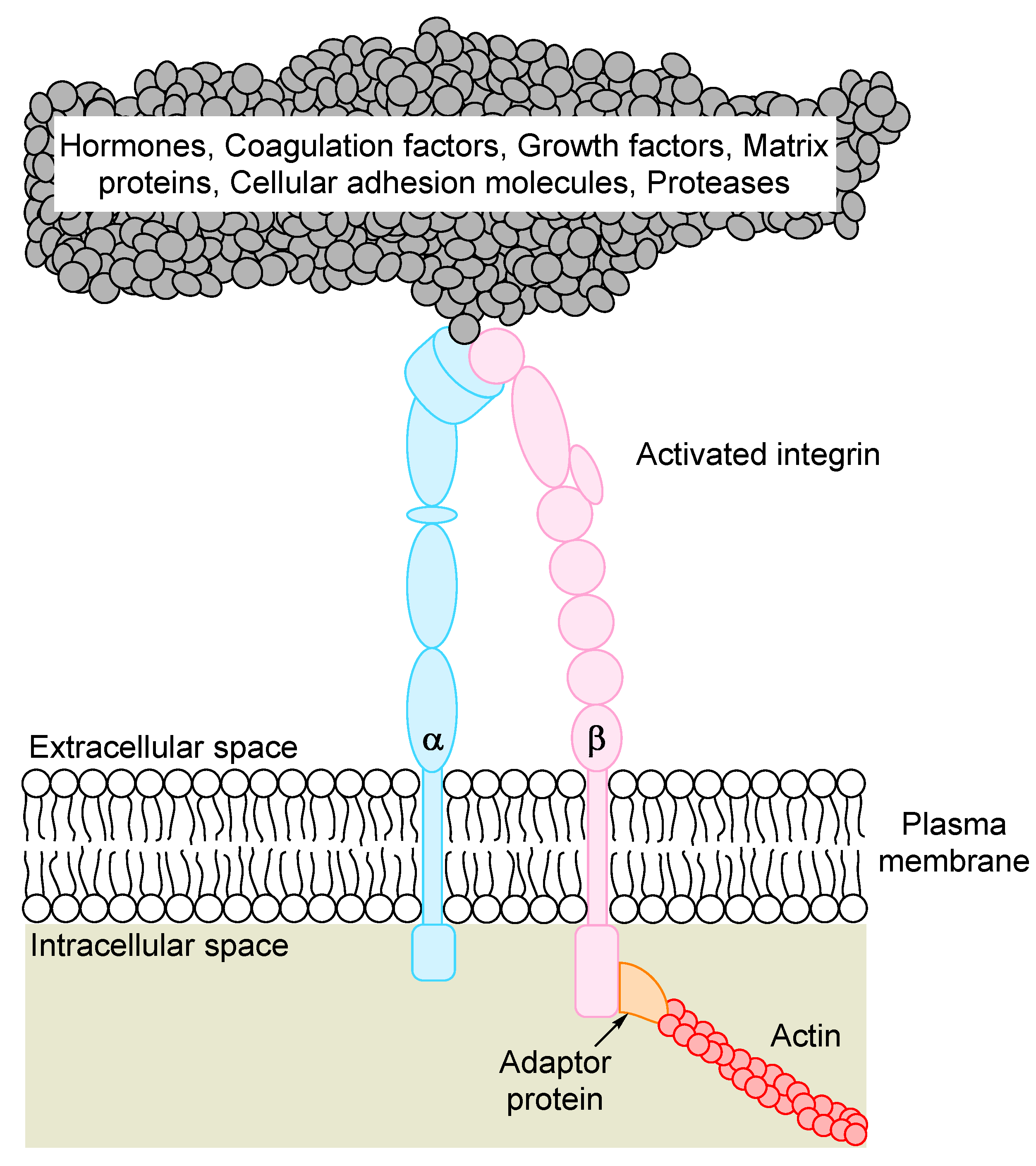
| Ligand | Integri Binding | Function of the Ligand in Human Acute Myeloid Leukemia (AML) | Key References |
|---|---|---|---|
| ADAM family members | αVβ3 | ADAMTS-13, see von Willebrand factor (vWF) below. | |
| Androgens | αVβ3 | A recent study described improved survival of elderly patients when androgens maintenance treatment was combined with intensive chemotherapy. | [14] |
| BSP | αVβ3 | Bone sialo protein (BSP). No known effect in AML. | |
| Collagen | α10β3 | Collagen IV promotes the migration and adhesion of primary human AML cells, MMP-9 is also increased. Collagen and collagen IV is present in human bone marrow. It is not known whether binding to integrins contributes to these effects or whether other receptors are responsible (e.g., the diskoid domain receptor 1). | [15] |
| COMP | αVβ3 | Cartilage oligomeric matrix protein(COMP) This mediator is synthesized by osteoblasts and may thus be expressed in the bone marrow niches. | [16] |
| Connective tissue growth factor | αVβ3, αIIbβ3 | Connective tissue growth factor (CTGF) is expressed in bone marrow stromal cells; it is regarded as a regulator of adipocyte differentiation and may influence leukemogenesis both through direct effects on the AML cells and through indirect effects on AML-supporting stromal cells. AML cells induce its expression in bone marrow mesenchymal cells. | [17,18] |
| Cyr61 | αIIbβ3, αVβ3 | Cystein-rich 61(Cyr61) is released by stromal cells, it is released as a matricellular protein and it increases the proapoptotic effects of mitoxantrone in AML-stromal cell cocultures. | [19] |
| Del-1 | αVβ3 | The secreted glycoprotein Developmental endothelial locus-1 (Del-1) is expressed endothelial cell, becomes associated with extracellular matrix or cell surfaces and regulates hematopoiesis in the bone marrow stem cell niche. | [20] |
| Fibrillin | αVβ3 | Murine studies have demonstrated that the extracellular matrix protein, fibrillin, is expressed in the bone marrow and functions as a regulator of normal hematopoiesis. | [21] |
| Fibrinogen | αIIbβ3, αVβ3 | The plasma fibrinogen levels at the time of diagnosis seem to have a prognostic impact and are associated with an adverse outcome in AML patients. This impact is not caused by increased early mortality, but it is not known whether this long-term effect is caused by a direct effect of fibrinogen on the AML cells. Both soluble and solid-phase fibrinogen induces Syk signaling in human megakaryoblastic cell lines. | [13,22] |
| Fibronectin | αIIbβ3, αVβ3 | Experimental studies suggest that AML cell adhesion to fibronectin increase leukemia cell proliferation, accelerate S-phase entry and cause accumulation of the cell cycle inhibitor CDC25A. This CDC25A accumulation was caused by decreased degradation. Activation of PI3K-Akt-mTOR seemed to be important for this adhesion-dependent growth enhancement. Fibronectin adhesion inhibited the proliferation of normal CD34+ bone hematopoietic cells. | [23] |
| ICAM-4 | αVβ3, αIIbβ3 | Intercellular adhesion molecule-4 (ICAM-4) is expressed by erythroid cells and seems important in erythropoiesis, but it is not known whether it is important in AML. | [24] |
| L1 | αVβ3, αIIbβ3 | L1 is expressed by human monocytes and may thus be expressed in the bone marrow stem cell niches. | [25] |
| MFG-E8 | αVβ3, αVβ5 | The Milk fat globule-EGF-factor 8 protein (MFG-E8) is expressed and released by bone marrow macrophages and is thus present in the AML cell microenvironment. | [26] |
| MMP-2 | αVβ3 | Matrix metalloprotease 2 (MMP-2) is constitutively released by primary human AML cells for most patients and is involved in AML cell migration; it may even be important for the extracellular migration of leukemic cells. An adverse prognostic impact of constitutive MMP-2 release has been suggested. | [27,28,29] |
| Osteopontin | αVβ3 | Monocytic differentiation in human AML cells seems to be associated with increased expression of both ITGαV and osteopontin. High osteopontin serum levels seem to be associated with an adverse prognosis in human AML, but this impact differs among patients and is most clearly seen for patients with intermediate risk factors. | [30,31,32] |
| PCAM | αVβ3 | Mesenchymal stem cells express Platelet cell adhesion molecule (PCAM); this ligand is thus expressed in the bone marrow stem cell niches where leukemic stem cells locate. | [33] |
| SPARC | αVβ3? | Secreted-Protein-Acidic-Cysteine Rich (SPARC) Seems to induce β3-catenin signaling at least in subsets of human AML. | [34] |
| Thyroid hormones | αVβ3 | A matched case-control study with 28 children/patients with AML showed that extreme Thyroid stimulating hormone (TSH) levels, both high and low at neonatal screening, were associated with decreased risk of AML | [35] |
| Trombospondin | αVβ3, αIIbβ3 | Thrombospondin induces apoptosis in AML cell lines and also in primary human AML cells, but this effect may be due to ligation of CD36. The effect is antagonized by thrombopoietin, a mediator that is often increased in AML patients receiving intensive chemotherapy. | [36,37] |
| Vitronectin | αIIbβ3, αVβ3 | Adhesion of Mixed lineage leukemia-Eleven-nineteen lysine-rich leukemia (MLL-ELL) murine myeloid progenitor cells to vitronectin activates/phosphorylates β3 integrins and Syk kinase. | [38] |
| vWf | αVβ3, αIIbβ3 | ADAMTS-13 is essential for maintaining the keeping normal sized of the vWF; it cleaves the multimer into smaller forms. Low plasma levels of ADAMTS-13 seems to be associated with an adverse outcome in human AML, but it is not known whether this is due to an effect of ADAMTS-13/vWF directly on the AML cells or whether it represents a secondary reactive mechanism. | [39,40] |
| Actin and Actin Regulators (18 Members) |
|---|
| Closely related to the cytoskeleton |
| Adaptor proteins contain a variety of protein-binding modules that link protein-binding partners |
| together and facilitate formation of larger complexes. |
| The integrins are the largest subset of proteins in this group |
| Channel proteins (5 members) |
| Chaperones (3 members) |
| E3 ligases |
| GTPase activating proteins (14 members) |
| Guanine nucleotide exchange factor (16 members) |
| GTPases (6 members) |
| Proteases (4 members) |
| Phosphatidyl inositol (PtdIns) kinases (2 members) |
| PtdIns phosphatases (3 members) |
| RNA or DNA regulation (4 members) |
| Serine/Threonine (Ser/Thr) kinases (10 members) |
| Ser/Thr phosphatases (5 members) |
| Tyrosine (Tyr) kinases (10 members) |
| Tyr phosphatases |
|
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johansen, S.; Brenner, A.K.; Bartaula-Brevik, S.; Reikvam, H.; Bruserud, Ø. The Possible Importance of β3 Integrins for Leukemogenesis and Chemoresistance in Acute Myeloid Leukemia. Int. J. Mol. Sci. 2018, 19, 251. https://doi.org/10.3390/ijms19010251
Johansen S, Brenner AK, Bartaula-Brevik S, Reikvam H, Bruserud Ø. The Possible Importance of β3 Integrins for Leukemogenesis and Chemoresistance in Acute Myeloid Leukemia. International Journal of Molecular Sciences. 2018; 19(1):251. https://doi.org/10.3390/ijms19010251
Chicago/Turabian StyleJohansen, Silje, Annette K. Brenner, Sushma Bartaula-Brevik, Håkon Reikvam, and Øystein Bruserud. 2018. "The Possible Importance of β3 Integrins for Leukemogenesis and Chemoresistance in Acute Myeloid Leukemia" International Journal of Molecular Sciences 19, no. 1: 251. https://doi.org/10.3390/ijms19010251
APA StyleJohansen, S., Brenner, A. K., Bartaula-Brevik, S., Reikvam, H., & Bruserud, Ø. (2018). The Possible Importance of β3 Integrins for Leukemogenesis and Chemoresistance in Acute Myeloid Leukemia. International Journal of Molecular Sciences, 19(1), 251. https://doi.org/10.3390/ijms19010251