Effect of Ultrasonic Extraction on Production and Structural Changes of C-Phycocyanin from Marine Spirulina maxima
Abstract
:1. Introduction
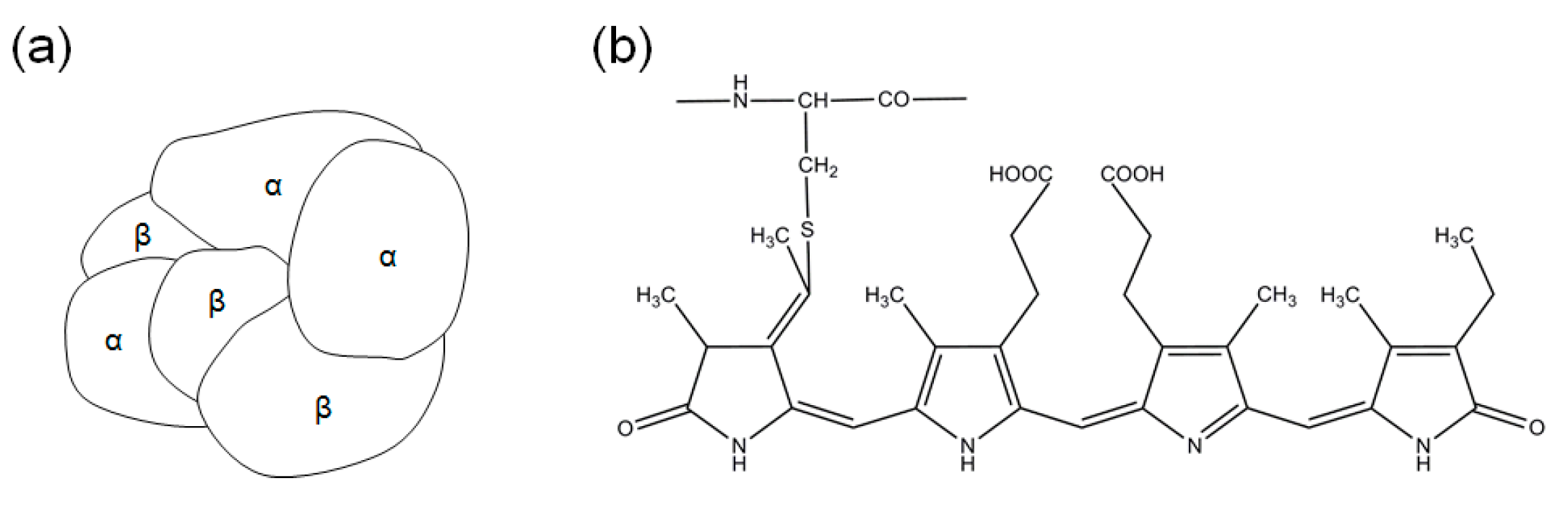
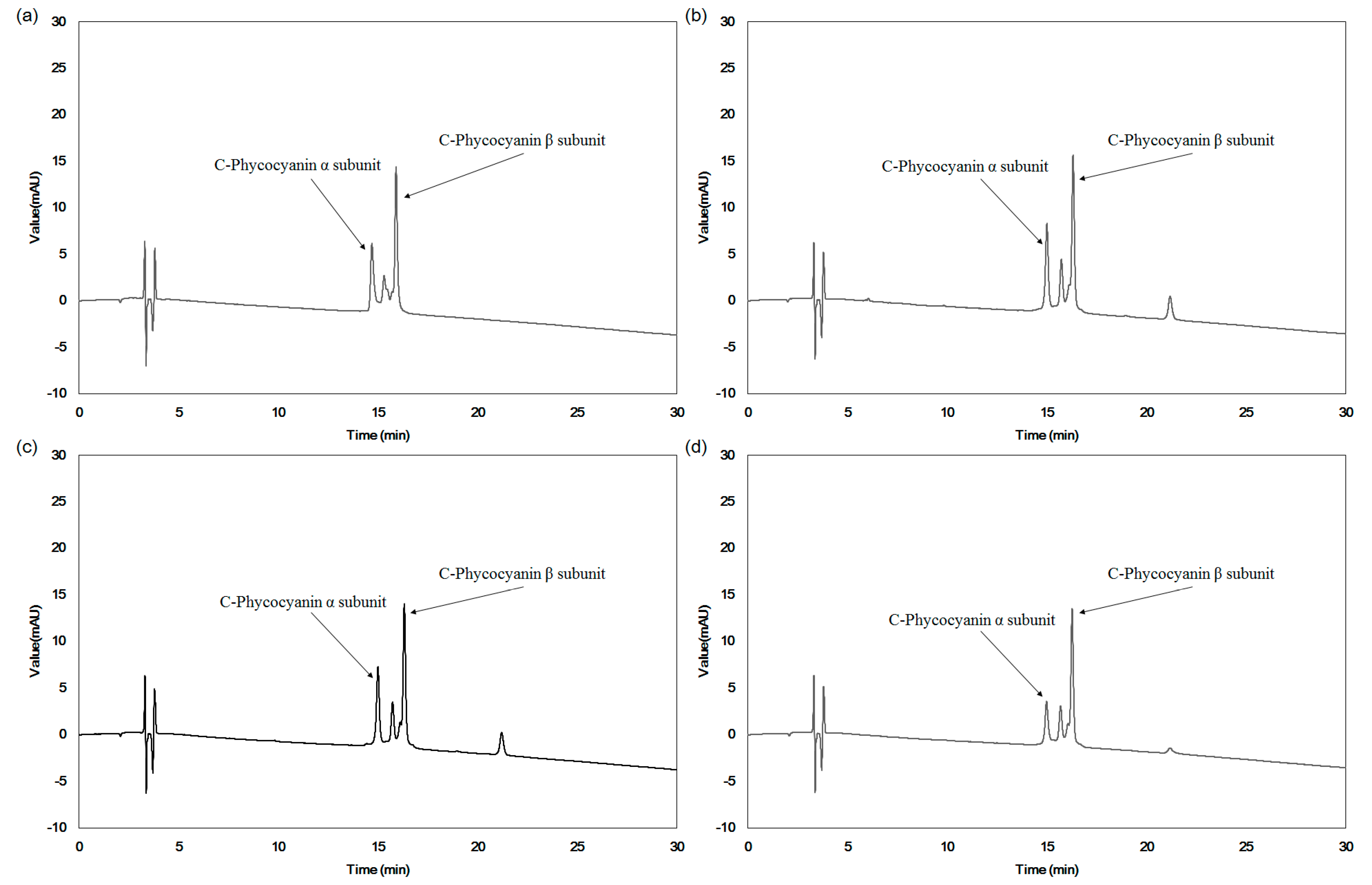
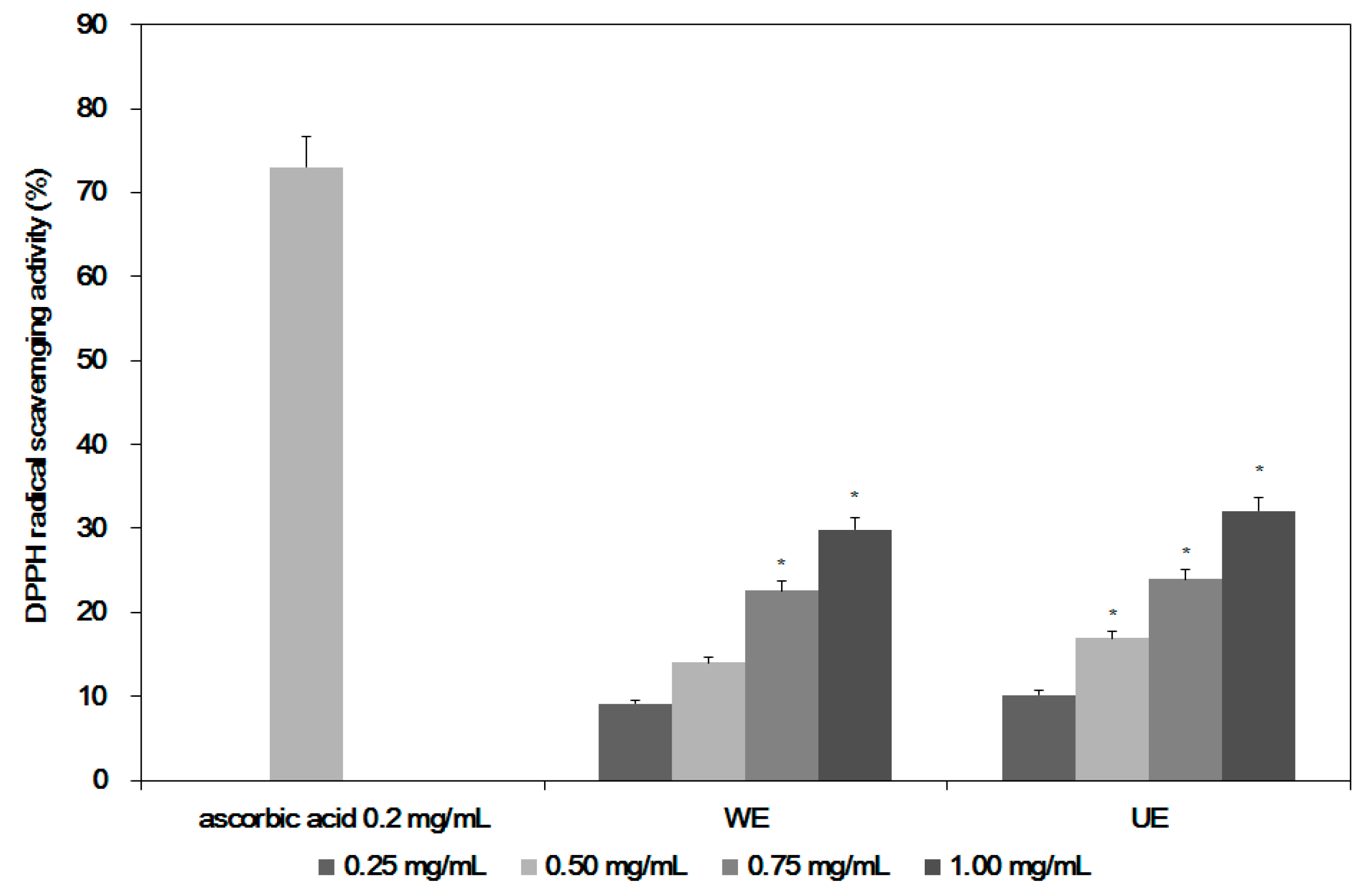
2. Results
3. Discussion
4. Materials and Methods
4.1. Materials and Preparation of the Samples
4.2. Measurement of C-Phycocyanin, Allophycocyanin, and Phycoerythrin Content in the Extracts
4.3. Structural Changes of C-PC in the Extracts from Two Different Extraction Processes
4.4. Cell Cytotoxicity of the Extracts from Two Different Extraction Processes
4.5. Measurement of Anti-Inflammatory Activities of Two Different Extracts
4.6. Determination of Antioxidant Activities of the Extracts from Two Different Processes
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Conflicts of Interest
Abbreviations
| C-PC | C-phycocyanin |
| APC | allophycocyanin |
| PE | phycoerythrin |
| UE | ultrasonic extraction |
| WE | water extraction |
References
- Kay, R.A. Microalgae as food and supplement. Crit. Rev. Food Sci. Nutr. 1991, 30, 555–783. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Kim, S.H.; Kim, J.S.; Han, J.A.; Seo, H.J.; Lim, H.J.; Choi, S.Y. Studies on Simultaneous Determination of Chlorophyll a and b, Pheophorbide a, and β-Carotene in Chlorella and Spirulina Products. J. Food Hyg. Saf. 2005, 20, 141–146. [Google Scholar]
- Khan, Z.; Bhadouria, P.; Bisen, P.S. Nutritional and therapeutic potential of Spirulina. Curr. Pharm. Biotechnol. 2005, 6, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.M.; Shahbazizadeh, S.; Khosravi-Darani, K.; Mozafari, M.R. Spirulina paltensis: Food and Function. Curr. Nutr. Food Sci. 2013, 9, 189–193. [Google Scholar] [CrossRef]
- Romay, C.; Gonzalez, R.; Ledon, N.; Remirez, D.; Rimbau, V. C-Phycocyanin: A biliprotein with antioxidant, anti-Inflammatory and neuroprotective effects. Curr. Protein Pept. Sci. 2003, 4, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Hou, M.F.; Huang, H.W.; Chang, F.R.; Yeh, C.C.; Tang, J.Y.; Chang, H.W. Marine algal natural products with anti-oxidative, anti-inflammatory, and anti-cancer properties. Cancer Cell. Int. 2013, 13, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Bennett, A.; Bogorad, L. Complementary chromatic adaptation in a filamentous blue-green alga. J. Cell Biol. 1973, 58, 419–435. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Gao, M.H.; Zhang, X.C.; Chu, X.M. Molecular immune mechanism of C-phycocyanin from Spirulina platensis induces apoptosis in HeLa cells in vitro. Biotechnol. Appl. Biochem. 2006, 43, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Sarada, R.; Pillai, M.G.; Ravishankar, G.A. Phycocyanin from Spirulina sp: Influence of processing of biomass on phycocyanin yield, analysis of efficacy of extraction methods and stability studies on phycocyanin. Process Biochem. 1999, 34, 795–801. [Google Scholar] [CrossRef]
- Moraes, C.C.; Sala, L.; Cerveira, G.P.; Kalil, S.J. C-phycocyanin extraction from Spirulina platensis wet biomass. Braz. J. Chem. Eng. 2011, 28, 45–49. [Google Scholar] [CrossRef]
- Chung, K.W.; Kim, W.I.; Hong, I.K.; Park, K.A. Ultrasonic energy effects on squalene extraction from amaranth seed. Appl. Chem. 2000, 4, 149–152. [Google Scholar]
- Oh, S.H.; Ahn, J.H.; Kang, D.H.; Lee, H.Y. The effect of ultrasonificated extracts of Spirulina maxima on the Anticancer activity. Mar. Biotechnol. 2011, 13, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Boussiba, S.; Richmond, A.E. Isolation and characterization of phycocyanins from the blue-Green alga Spirulina platensis. Arch. Microbiol. 1979, 120, 155–159. [Google Scholar] [CrossRef]
- Kumar, D.; Dhar, D.W.; Pabbi, S.; Kumar, N.; Walia, S. Extraction and purification of C-phycocyanin from Spirulina platensis (CCC540). Indian J. Plant Physiol. 2014, 19, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Romay, C.; Armesto, J.; Remirez, D.; González, R.; Ledon, N.; García, I. Antioxidant and anti-inflammatory properties of C-phycocyanin from blue-green algae. Inflamm. Res. 1998, 47, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Hirata, T.; Tanaka, M.; Ooike, M.; Tsunomura, T.; Sakaguchi, M. Antioxidant activities of phycocyanobilin prepared from Spirulina platensis. J. Appl. Phycol. 2000, 12, 435–439. [Google Scholar] [CrossRef]
- Shalaby, E.A.; Shanab, S.M.M. Comparison of DPPH and ABTS assays for determining antioxidant potential of water and methanol extracts of Spirulina platensis. Indian J. Geo-Mar. Sci. 2013, 42, 556–564. [Google Scholar]
- Kang, S.M.; Kim, K.N.; Lee, S.H.; Ahn, G.; Cha, S.H.; Kim, A.D.; Yang, X.D.; Kang, M.C.; Jeon, Y.J. Anti-inflammatory activity of polysaccharide purified from AMG-assistant extract of Ecklonia cava in LPS-stimulated RAW 264.7 macrophages. Carbohydr. Polym. 2011, 85, 80–85. [Google Scholar] [CrossRef]
- Patel, A.; Mishra, S.; Pawar, R.; Ghosh, P.K. Purification and characterization of C-Phycocyanin from cyanobacterial species of marine and freshwater habitat. Protein Expr. Purif. 2005, 40, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Muthulakshmi, M.; Saranya, A.; Selvakumar, G. Extraction, parital purification, and antibacterial activity of phycocyanin from Spirulina isolated from fresh water body agisnt various human pathogens. J. Algal Biomass Util. 2012, 3, 7–11. [Google Scholar]
- Silveira, S.T.; Burkert, J.F.M.; Costa, J.A.V.; Burkert, C.A.V.; Kalil, S.J. Optimization of phycocyanin extraction from Spirulina platensis using factorial design. Bioresour. Technol. 2007, 98, 1629–1634. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite and [15N] nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Dietz, B.M.; Kang, Y.H.; Liu, G.; Eggler, A.L.; Yao, P.; Chadwick, L.R.; Pauli, G.F.; Farnsworth, N.R.; Mesecar, A.D.; Breemen, R.B.; et al. Xanthohumol isolated from humulus lupulus inhibits menadione-induced DNA damage through induction of quinone reductase. Chem. Res. Toxicol. 2005, 18, 1296–1305. [Google Scholar] [CrossRef] [PubMed]
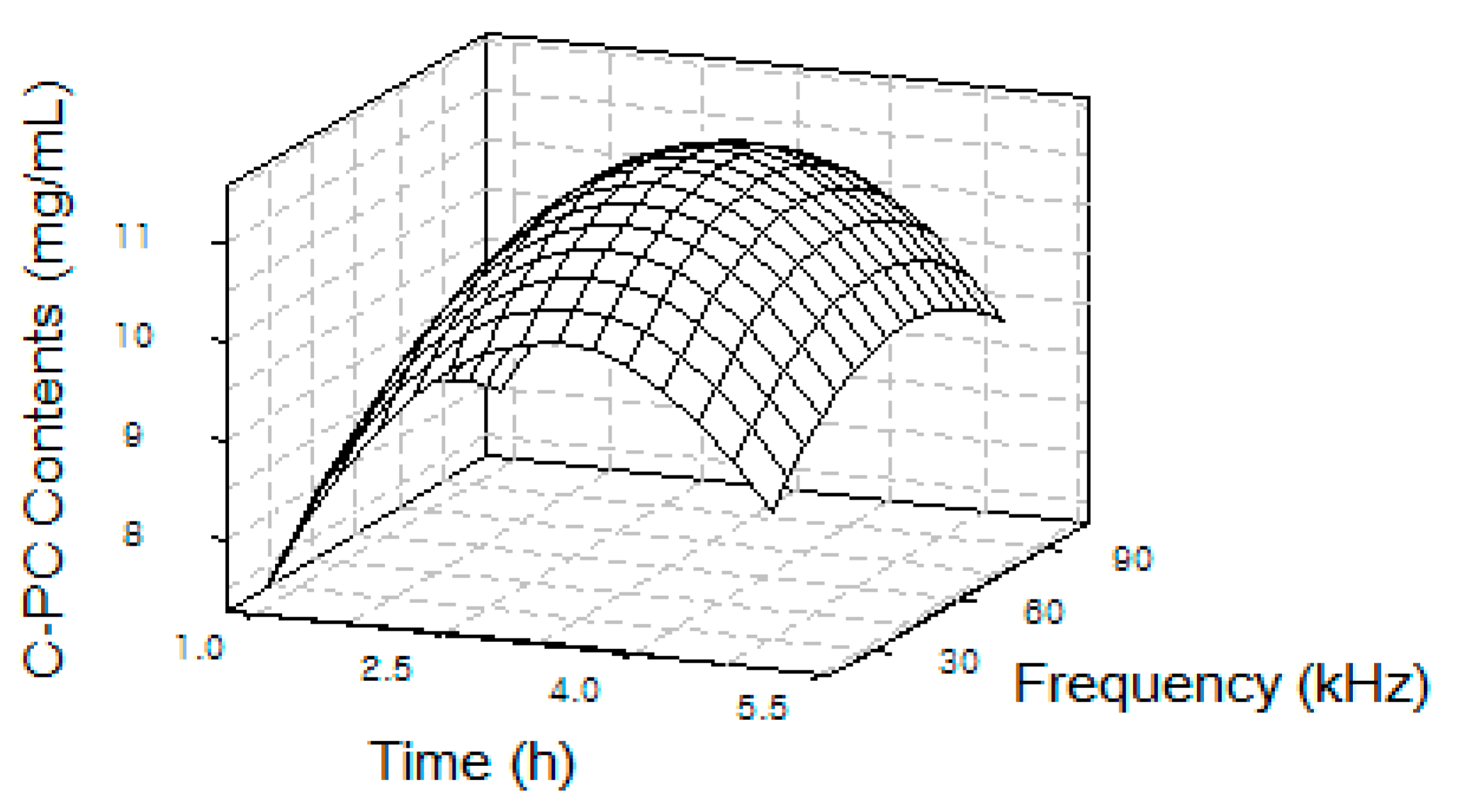


| Sample | Temperature (°C) | Frequency (kHz) | Extraction Time (h) | C-Phycocyanin (mg/mL) | Allophycocyanin (mg/mL) | Phycoerythrin (mg/mL) |
|---|---|---|---|---|---|---|
| Control 1 | 4 | - | 24 | 9.8 ± 0.02 | 2.3 ± 0.02 | 0.7 ± 0.01 |
| WE 2 | 25 | - | 24 | 5.7 ± 0.01 | 1.2 ± 0.05 | 0.3 ± 0.01 |
| UE 3 | 25 | 60 | 1 | 8.6 ± 0.04 | 2.3 ± 0.03 | 0.5 ± 0.01 |
| 2 | 9.2 ± 0.13 | 2.5 ± 0.06 | 0.7 ± 0.01 | |||
| 3 | 11.3 ± 0.06 * | 3.1 ± 0.02 * | 0.8 ± 0.01 * | |||
| 4 | 10.9 ± 0.34 * | 3.0 ± 0.02 * | 0.5 ± 0.01 | |||
| 5 | 9.9 ± 0.07 | 2.7 ± 0.01 | 0.6 ± 0.01 | |||
| 20 | 3 | 10.2 ± 0.04 | 2.9 ± 0.03 | 0.4 ± 0.01 | ||
| 40 | 11.3 ± 0.13 * | 3.1 ± 0.05 * | 0.8 ± 0.01 * | |||
| 60 | 10.9 ± 0.08 | 3.0 ± 0.07 * | 0.6 ± 0.01 | |||
| 80 | 11.2 ± 0.16 * | 2.7 ± 0.03 | 0.4 ± 0.01 | |||
| 100 | 10.8 ± 0.14 * | 3.0 ± 0.08 * | 0.7 ± 0.01 |
| Sample | Treatment Concentration (mg of S. maxima Extracts Powder/mL) | |||
|---|---|---|---|---|
| 0.25 | 0.50 | 0.75 | 1.00 | |
| WE 1 | 1.58 ± 0.009 | 6.21 ± 0.101 | 11.89 ± 1.930 | 14.06 ± 1.027 * |
| UE 2 | 2.01 ± 0.057 | 7.74 ± 0.160 | 12.52 ± 1.021 * | 15.48 ± 0.994 * |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, W.Y.; Lee, H.Y. Effect of Ultrasonic Extraction on Production and Structural Changes of C-Phycocyanin from Marine Spirulina maxima. Int. J. Mol. Sci. 2018, 19, 220. https://doi.org/10.3390/ijms19010220
Choi WY, Lee HY. Effect of Ultrasonic Extraction on Production and Structural Changes of C-Phycocyanin from Marine Spirulina maxima. International Journal of Molecular Sciences. 2018; 19(1):220. https://doi.org/10.3390/ijms19010220
Chicago/Turabian StyleChoi, Woon Yong, and Hyeon Yong Lee. 2018. "Effect of Ultrasonic Extraction on Production and Structural Changes of C-Phycocyanin from Marine Spirulina maxima" International Journal of Molecular Sciences 19, no. 1: 220. https://doi.org/10.3390/ijms19010220
APA StyleChoi, W. Y., & Lee, H. Y. (2018). Effect of Ultrasonic Extraction on Production and Structural Changes of C-Phycocyanin from Marine Spirulina maxima. International Journal of Molecular Sciences, 19(1), 220. https://doi.org/10.3390/ijms19010220




