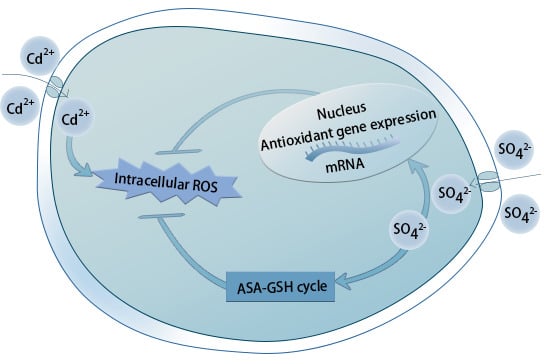
Sulfur Protects Pakchoi (Brassica chinensis L.) Seedlings against Cadmium Stress by Regulating Ascorbate-Glutathione Metabolism
Abstract
:1. Introduction
2. Results
2.1. Plant Growth Parameters
2.2. Cadmium Contents, Bioconcentration Factor (BCF) and TF in the Shoots and Roots of Pakchoi Cultivars Subjected to Different Treatments
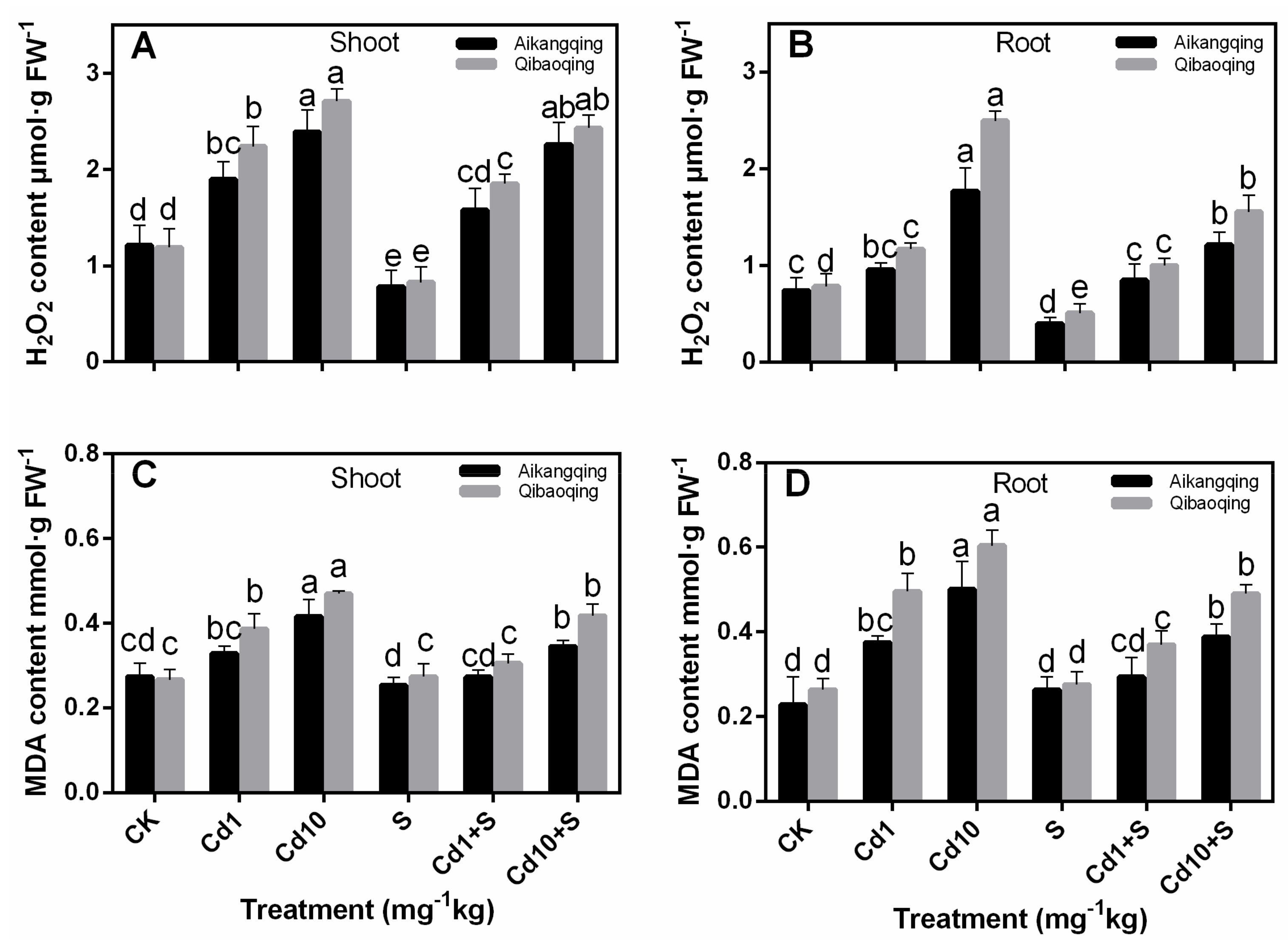
2.3. H2O2 and MDA Contents
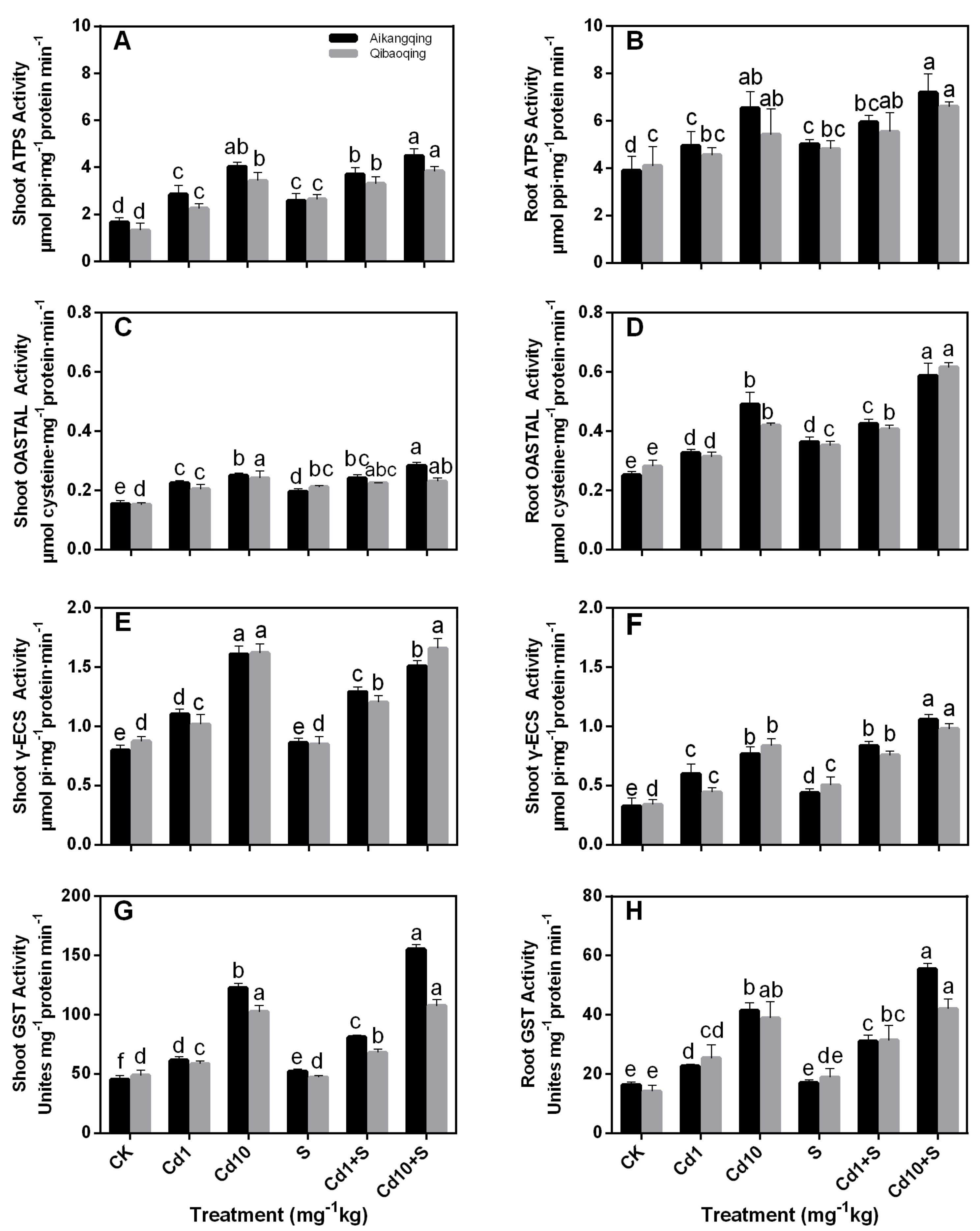
2.4. Enzymes of S Assimilation Pathway
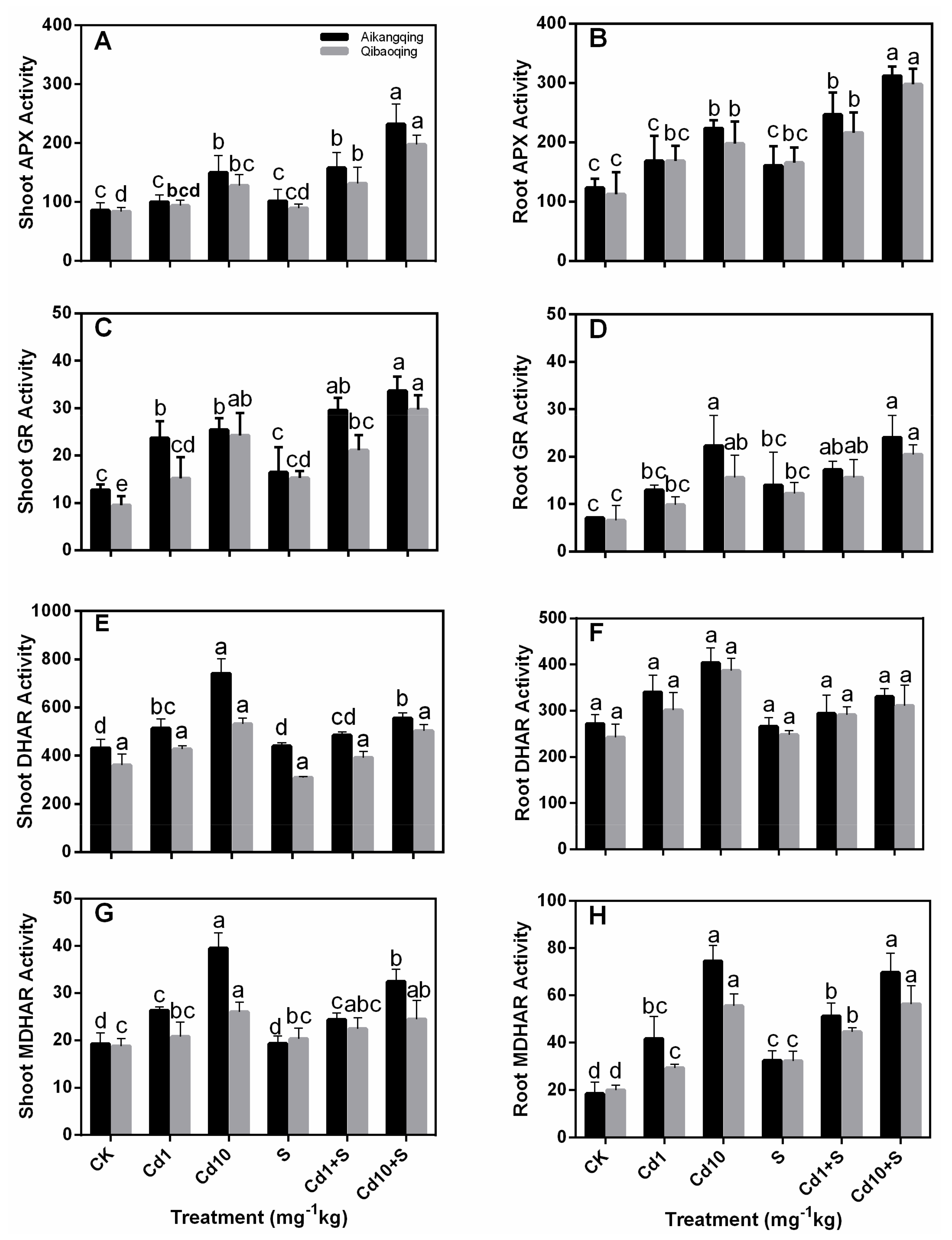
2.5. Determination of the Activity of Antioxidant Enzymes in the ASA-GSH Cycle
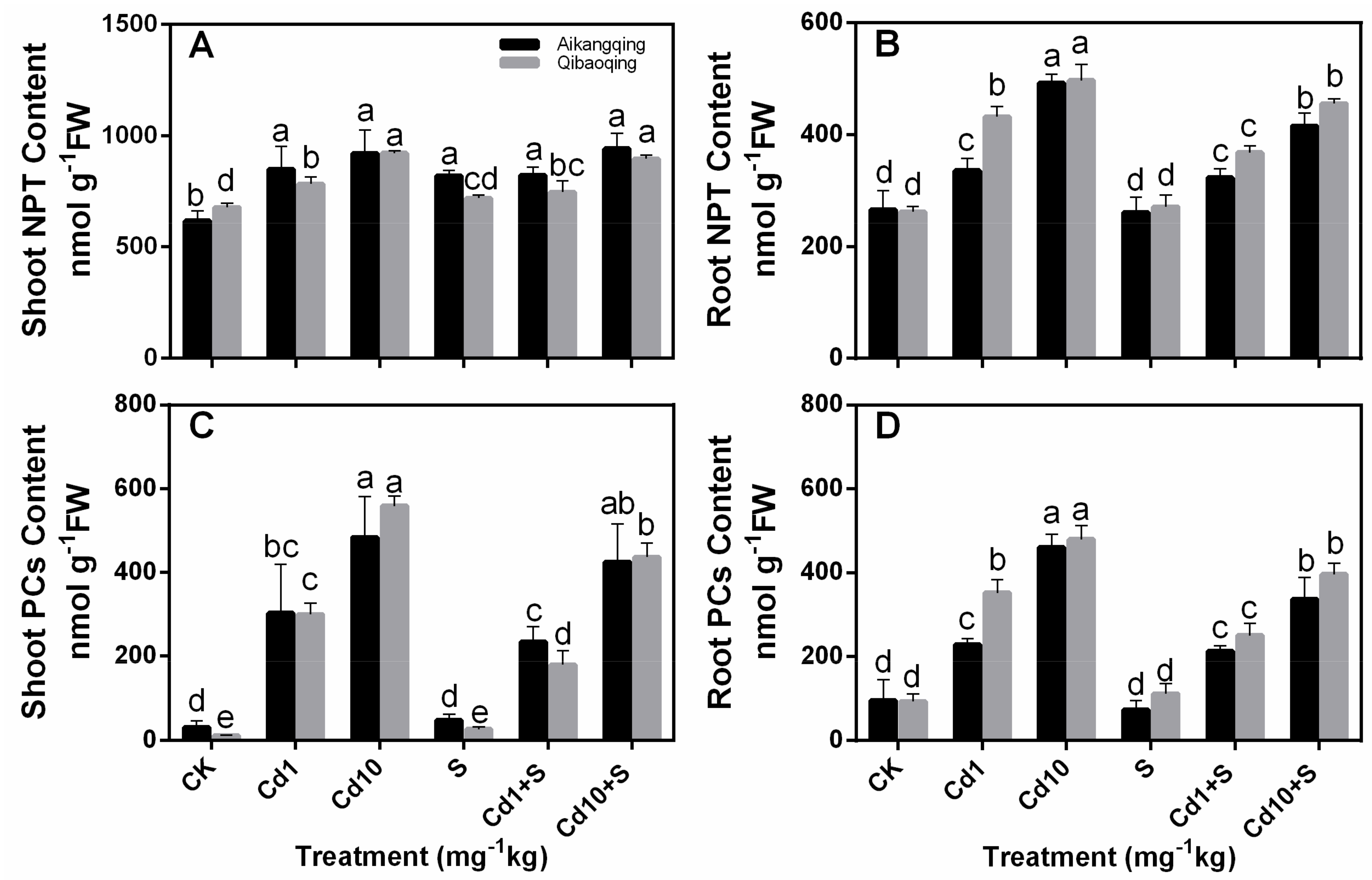
2.6. Determination of Glutathione, Ascorbate, PCs, and NPT
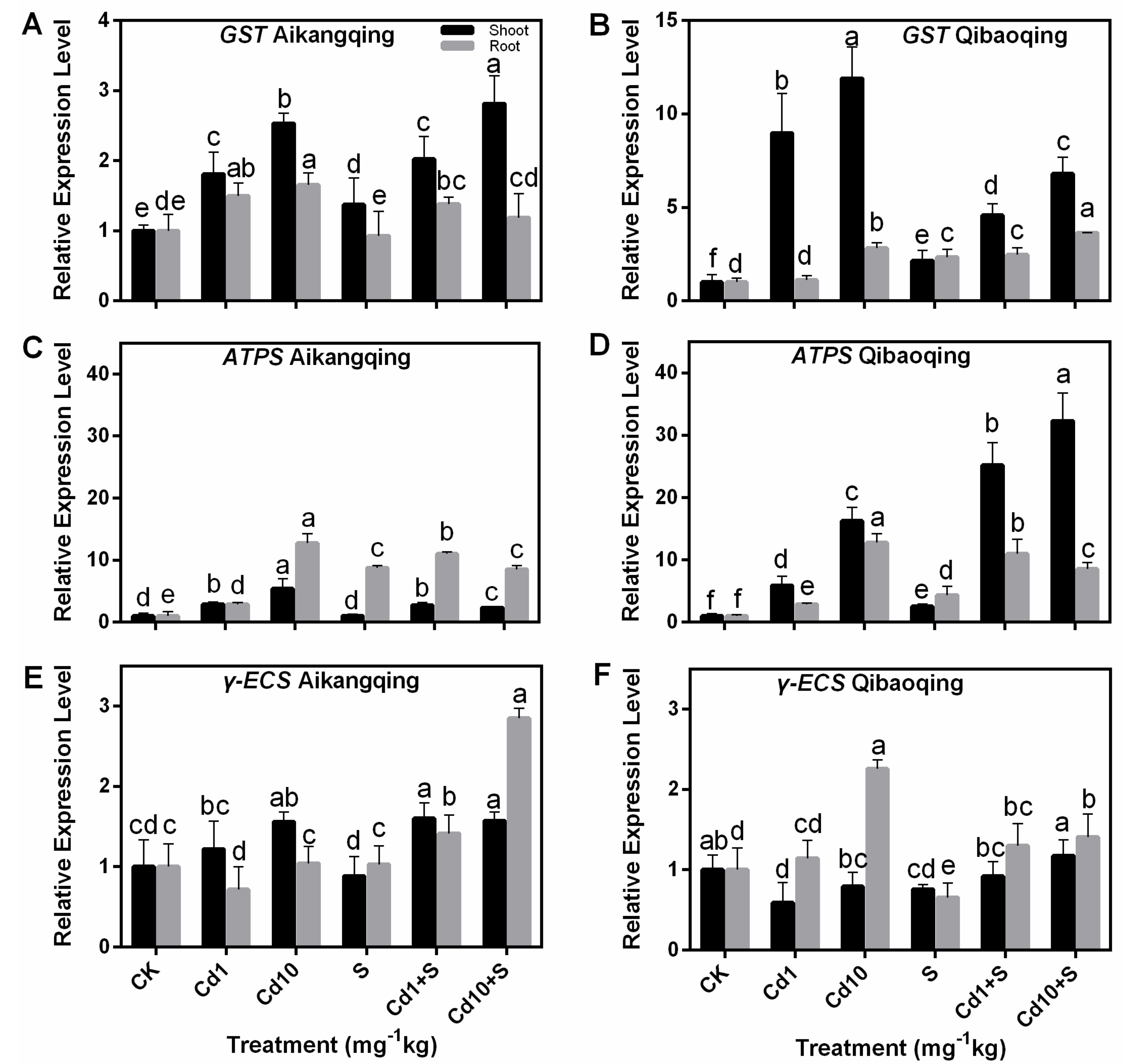
2.7. Transcript Levels of Gene-Encoding Enzymes Involved in S Assimilation Pathway in the Shoots and Roots of Pakchoi Seedlings
2.8. Transcript Levels of Gene-Encoding Enzymes Involved in ASA-GSH Cycle
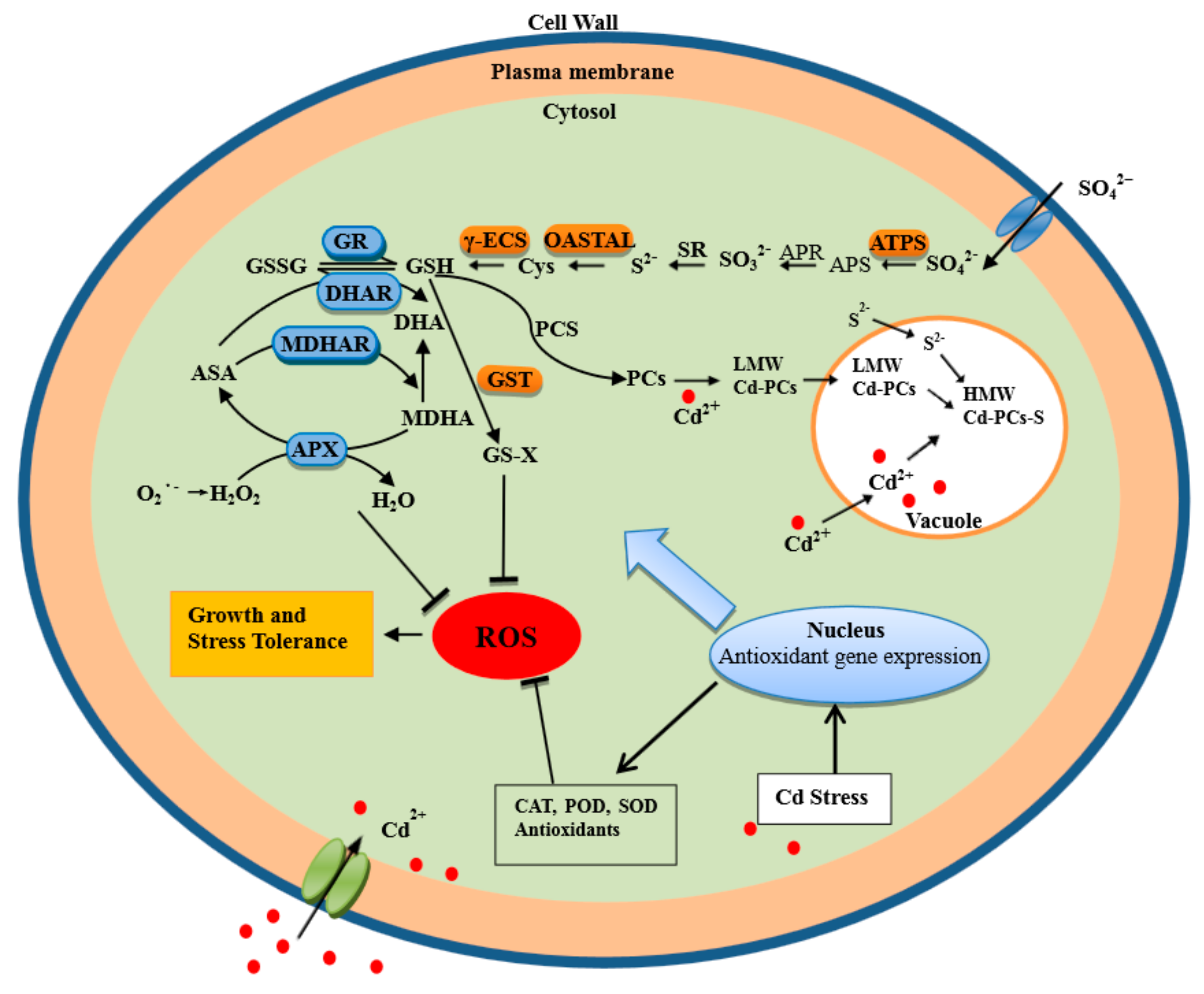
3. Discussion
4. Materials and Methods
4.1. Plant Cultivation
- (1)
- Control (CK), 0 mg·kg−1 CdCl2·2.5H2O + 0 mg·kg−1 Na2SO4;
- (2)
- Cd1, 1 mg·kg−1 CdCl2·2.5H2O + 0 mg·kg−1 Na2SO4;
- (3)
- Cd10, 10 mg·kg−1 CdCl2·2.5H2O + 0 mg·kg−1 Na2SO4;
- (4)
- S, 0 mg·kg−1 CdCl2·2.5H2O + 50 mg·kg−1 Na2SO4;
- (5)
- Cd1 + S, 1 mg·kg−1 CdCl2·2.5H2O + 50 mg·kg−1 Na2SO4;
- (6)
- Cd10 + S, 10 mg·kg−1 CdCl2·2.5H2O + 50 mg·kg−1 Na2SO4.
4.2. Measurement of Morphological Features
4.3. Cd Determination by Atomic Absorption Spectroscopy
4.4. Bioconcentration and Translocation Factors
4.5. Measurement of Malondialdehyde (MDA) and Hydrogen Peroxide (H2O2) Content
4.6. Activity of Enzymes of S Assimilation Pathway and ASA-GSH Cycle Metabolism
4.7. RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| Cd | cadmium |
| S | sulfur |
| ASA | ascorbate |
| GSH | glutathione |
| PCs | phytochelatins |
| NPT | nonprotein thiol |
| ROS | reactive oxygen species |
| APX | ascorbate peroxidase |
| GR | glutathione reductase |
| DHAR | dehydroascorbate reductase |
| MDHAR | monodehydroascorbate reductase |
| GSSG | oxidized glutathione |
| DHA | dehydroascorbate |
| GST | glutathione-S-transferases |
| ATPS | ATP sulfurylase |
| OASTAL | O-acetylserine(thiol)lyase |
| γ-ECS | γ-glutamylcysteine synthetase |
| H2O2 | hydrogen peroxide |
| MDA | malondialdehyde |
| BCF | bioconcentration factor |
| TF | translocation factor |
References
- Chen, J.; Yang, L.; Gu, J.; Bai, X.; Ren, Y.; Fan, T.; Han, Y.; Jiang, L.; Xiao, F.; Liu, Y. Man3 gene regulates cadmium tolerance through the glutathione-dependent pathway in arabidopsis thaliana. New Phytol. 2015, 205, 570–582. [Google Scholar] [CrossRef]
- Lin, Y.F.; Aarts, M.G.M. The molecular mechanism of zinc and cadmium stress response in plants. Cell. Mol. Life Sci. 2012, 69, 3187–3206. [Google Scholar] [CrossRef]
- Singh, S.; Parihar, P.; Singh, R.; Singh, V.P.; Prasad, S.M. Heavy metal tolerance in plants: Role of transcriptomics, proteomics, metabolomics, and ionomics. Front. Plant Sci. 2015, 6, 1143. [Google Scholar] [CrossRef]
- Tripathi, R.D.; Tripathi, P.; Dwivedi, S.; Dubey, S.; Chatterjee, S.; Chakrabarty, D.; Trivedi, P.K. Arsenomics: Omics of arsenic metabolism in plants. Front. Physiol. 2012, 3, 275. [Google Scholar] [CrossRef] [PubMed]
- Benavides, M.P.; Gallego, S.M.; Tomaro, M.L. Cadmium toxicity in plants. Braz. J. Plant Physiol. 2005, 17, 21–34. [Google Scholar] [CrossRef]
- Shim, D.; Hwang, J.U.; Lee, J.; Lee, S.; Choi, Y.; An, G.; Martinoia, E.; Lee, Y. Orthologs of the class a4 heat shock transcription factor HsfA4a confer cadmium tolerance in wheat and rice. Plant Cell 2009, 21, 4031–4043. [Google Scholar] [CrossRef]
- Clemens, S.; Ma, J.F. Toxic heavy metal and metalloid accumulation in crop plants and foods. Annu. Rev. Plant Biol. 2016, 67, 489–512. [Google Scholar] [CrossRef] [PubMed]
- Roth, U.; von Roepenack-Lahaye, E.; Clemens, S. Proteome changes in arabidopsis thaliana roots upon exposure to CD2+. J. Exp. Bot. 2006, 57, 4003–4013. [Google Scholar] [CrossRef] [PubMed]
- Djebali, W.; Gallusci, P.; Polge, C.; Boulila, L.; Galtier, N.; Raymond, P.; Chaibi, W.; Brouquisse, R. Modifications in endopeptidase and 20 s proteasome expression and activities in cadmium treated tomato (Solanum lycopersicum L.) plants. Planta 2008, 227, 625–639. [Google Scholar] [CrossRef]
- Iqbal, N.; Khan, N.A. Variation in growth, photosynthesis functions and yield of five mustard (Brassica juncea L.) cultivars under high cadmium stress. Plant Stress 2010, 4, 87–93. [Google Scholar]
- Foyer, C.H.; Noctor, G. Redox homeostasis and antioxidant signaling: A metabolic interface between stress perception and physiological responses. Plant Cell 2005, 17, 1866–1875. [Google Scholar] [CrossRef] [PubMed]
- Anjum, N.A.; Umar, S.; Ahmad, A.; Iqbal, M.; Khan, N.A. Ontogenic variation in response of Brassica campestris L. To cadmium toxicity. J. Plant Interact. 2008, 3, 189–198. [Google Scholar] [CrossRef]
- Sandalio, L.M.; Dalurzo, H.C.; Gómez, M.; Romero-Puertas, M.C.; Río, L.A.D. Cadmium-induced changes in the growth and oxidative metabolism of pea plants. J. Exp. Bot. 2001, 52, 2115–2126. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ma, J.; He, C.; Li, X.; Zhang, W.; Xu, F.; Lin, Y.; Wang, L. Inhibition of cadmium ion uptake in rice (Oryza sativa L.) cells by a wall-bound form of silicon. New Phytol. 2013, 200, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Meyer, C.L.; Juraniec, M.; Huguet, S.; Chaves-Rodriguez, E.; Salis, P.; Isaure, M.P.; Goormaghtigh, E.; Verbruggen, N. Intraspecific variability of cadmium tolerance and accumulation, and cadmium-induced cell wall modifications in the metal hyperaccumulator Arabidopsis halleri. J. Exp. Bot. 2015, 66, 3215–3227. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.P.; Jiang, R.F.; Ueno, D.; Ma, J.F.; Schat, H.; McGrath, S.P.; Zhao, F.J. Variation in root-to-shoot translocation of cadmium and zinc among different accessions of the hyperaccumulators thlaspi caerulescens and thlaspi praecox. New Phytol. 2008, 178, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, S.; Landberg, T.; Greger, M. Cadmium uptake and interaction with phytochelatins in wheat protoplasts. Plant Physiol. Biochem. 2007, 45, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Greger, M.; Kabir, A.H.; Landberg, T.; Maity, P.J.; Lindberg, S. Silicate reduces cadmium uptake into cells of wheat. Environ. Pollut. 2015, 211, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Cobbett, C.S. Phytochelatins and their roles in heavy metal detoxification. Plant Physiol. 2000, 123, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.L. Cellular mechanisms for heavy metal detoxification and tolerance. J. Exp. Bot. 2002, 53, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bashri, G.; Prasad, S.M. Exogenous IAA differentially affects growth, oxidative stress and antioxidants system in cd stressed Trigonella foenum-graecum L. Seedlings: Toxicity alleviation by up-regulation of ascorbate-glutathione cycle. Ecotoxicol. Environ. Saf. 2016, 132, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.P.; Singh, S.; Kumar, J.; Prasad, S.M. Investigating the roles of ascorbate-glutathione cycle and thiol metabolism in arsenate tolerance in ridged luffa seedlings. Protoplasma 2015, 252, 1217–1229. [Google Scholar] [CrossRef] [PubMed]
- Capaldi, F.R.; Gratão, P.L.; Reis, A.R.; Lima, L.W.; Azevedo, R.A. Sulfur metabolism and stress defense responses in plants. Trop. Plant Biol. 2015, 8, 60–73. [Google Scholar] [CrossRef]
- Noctor, G.; Foyer, C.H. Ascorbate and glutathione: Keeping active oxygen under control. Plant Biol. 1998, 49, 249–279. [Google Scholar] [CrossRef] [PubMed]
- Shigeoka, S.; Ishikawa, T.; Tamoi, M.; Miyagawa, Y.; Takeda, T.; Yabuta, Y.; Yoshimura, K. Regulation and function of ascorbate peroxidase isoenzymes. J. Exp. Bot. 2002, 53, 1305–1319. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.C.; Reddy, A.R. Glutathione reductase: A putative redox regulatory system in plant cells. In Sulfur Assimilation and Abiotic Stress in Plants; Springer: Berlin/Heidelberg, Germany, 2008; pp. 111–147. [Google Scholar]
- Liu, S.; Liu, S.; Wang, M.; Wei, T.; Meng, C.; Wang, M.; Xia, G. A wheat similar to rcd-one gene enhances seedling growth and abiotic stress resistance by modulating redox homeostasis and maintaining genomic integrity. Plant Cell 2014, 26, 164–180. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Anjum, N.A.; Hasanuzzaman, M.; Gill, R.; Trivedi, D.K.; Ahmad, I.; Pereira, E.; Tuteja, N. Glutathione and glutathione reductase: A boon in disguise for plant abiotic stress defense operations. Plant Physiol. Biochem. 2013, 70, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Xiong, S.; Tu, S.; Liu, J.; Chen, C. Interactive effects of selenium and arsenic on growth, antioxidant system, arsenic and selenium species of Nicotiana tabacum L. Environ. Exp. Bot. 2015, 117, 12–19. [Google Scholar] [CrossRef]
- Nazar, R.; Iqbal, N.; Masood, A.; Khan, M.I.R.; Syeed, S.; Khan, N.A. Cadmium toxicity in plants and role of mineral nutrients in its alleviation. Am. J. Plant Sci. 2012, 3, 1476–1489. [Google Scholar] [CrossRef]
- Sarwar, N.; Saifullah; Malhi, S.S.; Zia, M.H.; Naeem, A.; Bibi, S.; Farid, G. Role of mineral nutrition in minimizing cadmium accumulation by plants. J. Sci. Food Agric. 2010, 90, 925–937. [Google Scholar] [PubMed]
- Uraguchi, S.; Watanabe, I.; Yoshitomi, A.; Kiyono, M.; Kuno, K. Characteristics of cadmium accumulation and tolerance in novel cd-accumulating crops, Avena strigosa and Crotalaria juncea. J. Exp. Bot. 2006, 57, 2955–2965. [Google Scholar] [CrossRef] [PubMed]
- Farshidi, M.; Abdolzadeh, A.; Sadeghipour, H.R. Silicon nutrition alleviates physiological disorders imposed by salinity in hydroponically grown canola (Brassica napus L.) plants. J. Acta Physiol. Plant. 2012, 34, 1779–1788. [Google Scholar] [CrossRef]
- Dixit, G.; Singh, A.P.; Kumar, A.; Mishra, S.; Dwivedi, S.; Kumar, S.; Trivedi, P.K.; Pandey, V.; Tripathi, R.D. Reduced arsenic accumulation in rice (Oryza sativa L.) shoot involves sulfur mediated improved thiol metabolism, antioxidant system and altered arsenic transporters. Plant Physiol. Biochem. 2016, 99, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Astolfi, S.; Zuchi, S.; Neumann, G.; Cesco, S.; Sanità, D.T.L.; Pinton, R. Response of barley plants to fe deficiency and cd contamination as affected by s starvation. J. Exp. Bot. 2012, 63, 1241. [Google Scholar] [CrossRef] [PubMed]
- Saito, K. Sulfur assimilatory metabolism. The long and smelling road. Plant Physiol. 2004, 136, 2443–2450. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G.; Mhamdi, A.; Chaouch, S.; Han, Y.; Neukermans, J.; Marquez-Garcia, B.; Queval, G.; Foyer, C.H. Glutathione in plants: An integrated overview. Plant Cell Environ. 2011, 35, 454–484. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Li, H.; Ma, C.; Zhang, Y.; Polle, A.; Rennenberg, H.; Cheng, X.; Luo, Z.B. Overexpression of bacterial gamma-glutamylcysteine synthetase mediates changes in cadmium influx, allocation and detoxification in poplar. New Phytol. 2015, 205, 240–254. [Google Scholar] [CrossRef] [PubMed]
- Anjum, N.A.; Gill, R.; Kaushik, M.; Hasanuzzaman, M.; Pereira, E.; Ahmad, I.; Tuteja, N.; Gill, S.S. Atp-sulfurylase, sulfur-compounds, and plant stress tolerance. Front. Plant Sci. 2015, 6, 210. [Google Scholar] [CrossRef] [PubMed]
- Dhankher, O.P.; Li, Y.; Rosen, B.P.; Shi, J.; Salt, D.; Senecoff, J.F.; Sashti, N.A.; Meagher, R.B. Engineering tolerance and hyperaccumulation of arsenic in plants by combining arsenate reductase and γ-glutamylcysteine synthetase expression. Nat. Biotechnol. 2002, 20, 1140–1145. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Guo, J.J.; He, C.T.; Shen, C.; Huang, Y.Y.; Chen, J.X.; Guo, J.H.; Yuan, J.G.; Yang, Z.Y. Comparative transcriptome analysis between low- and high-cadmium-accumulating genotypes of pakchoi (Brassica chinensis L.) in response to cadmium stress. Environ. Sci. Technol. 2016, 50, 6485–6494. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Yang, X.; Li, Y.; Hou, X. Molecular cloning and characterisation of cytoplasmic glutamine synthetase gene bcgs1 from non-heading chinese cabbage. J. Sci. Food Agric. 2010, 90, 891–897. [Google Scholar] [PubMed]
- Yan, S.; Ling, Q.; Bao, Z.; Chen, Z.; Yan, S.; Dong, Z.; Zhang, B.; Deng, B. Cadmium accumulation in pak choi (Brassica chinensis L.) and estimated dietary intake in the suburb of hangzhou city, China. Food Addit. Contam. Part B Surveill. 2009, 2, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, T.Q.; Han, X.; Ding, Z.L.; Yang, X.E.; Jin, Y.F. Cadmium accumulation in different pakchoi cultivars and screening for pollution-safe cultivars. J. Zhejiang Univ. Sci. B 2012, 13, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Tuteja, N. Cadmium stress tolerance in crop plants: Probing the role of sulfur. Plant Signal. Behav. 2011, 6, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Astolfi, S.; Ortolani, M.R.; Catarcione, G.; Paolacci, A.R.; Cesco, S.; Pinton, R.; Ciaffi, M. Cadmium exposure affects iron acquisition in barley (Hordeum vulgare) seedlings. Physiol. Plant. 2014, 152, 646–659. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Khan, N.A.; Tuteja, N. Cadmium at high dose perturbs growth, photosynthesis and nitrogen metabolism while at low dose it up regulates sulfur assimilation and antioxidant machinery in garden cress (Lepidium sativum L.). Plant Sci. Int. J. Exp. Plant Biol. 2012, 182, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.A.; Singh, S.; Nazar, R. Activities of antioxidative enzymes, sulphur assimilation, photosynthetic activity and growth of wheat (Triticum aestivum) cultivars differing in yield potential under cadmium stress. J. Agron. Crop Sci. 2007, 193, 435–444. [Google Scholar] [CrossRef]
- Akesson, A.; Barregard, L.; Bergdahl, I.A.; Nordberg, G.F.; Nordberg, M.; Skerfving, S. Non-renal effects and the risk assessment of environmental cadmium exposure. Environ. Health Perspect. 2014, 122, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Matraszek, R.; Hawrylak-Nowak, B.; Chwil, S.; Chwil, M. Macroelemental composition of cadmium stressed lettuce plants grown under conditions of intensive sulphur nutrition. J. Environ. Manag. 2016, 180, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Sheng, H.; Zeng, J.; Liu, Y.; Wang, X.; Wang, Y.; Kang, H.; Fan, X.; Sha, L.; Zhang, H.; Zhou, Y. Sulfur mediated alleviation of mn toxicity in polish wheat relates to regulating mn allocation and improving antioxidant system. Front. Plant Sci. 2016, 7, 1382. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Noctor, G. Ascorbate and glutathione: The heart of the redox hub. Plant Physiol. 2011, 155, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Krantev, A.; Yordanova, R.; Janda, T.; Szalai, G.; Popova, L. Treatment with salicylic acid decreases the effect of cadmium on photosynthesis in maize plants. J. Plant Physiol. 2008, 165, 920–931. [Google Scholar] [CrossRef] [PubMed]
- Dixit, G.; Singh, A.P.; Kumar, A.; Singh, P.K.; Kumar, S.; Dwivedi, S.; Trivedi, P.K.; Pandey, V.; Norton, G.J.; Dhankher, O.P.; et al. Sulfur mediated reduction of arsenic toxicity involves efficient thiol metabolism and the antioxidant defense system in rice. J. Hazard. Mater. 2015, 298, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Anjum, N.A.; Umar, S.; Ahmad, A.; Iqbal, M.; Khan, N.A. Sulphur protects mustard (Brassica campestris L.) from cadmium toxicity by improving leaf ascorbate and glutathione. Plant Growth Regul. 2008, 54, 271–279. [Google Scholar] [CrossRef]
- Shi, G.; Cai, Q. Cadmium tolerance and accumulation in eight potential energy crops. Biotechnol. Adv. 2009, 27, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Wang, G.; Lou, L.; Lv, J. Physiological responses and tolerance of kenaf (Hibiscus cannabinus L.) exposed to chromium. Ecotoxicol. Environ. Saf. 2016, 133, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Odjegba, V.; Fasidi, I. Accumulation of trace elements by pistia stratiotes: Implications for phytoremediation. Ecotoxicology 2004, 13, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Zayed, A.; Gowthaman, S.; Terry, N. Phytoaccumulation of trace elements by wetland plants: I. Duckweed. J. Environ. Qual. 1998, 27, 715–721. [Google Scholar] [CrossRef]
- Fotopoulos, V.; Ziogas, V.; Tanou, G.; Molassiotis, A. Involvement of asa/dha and gsh/gssg ratios in gene and protein expression and in the activation of defence mechanisms under abiotic stress conditions. In Ascorbate-Glutathione Pathway and Stress Tolerance in Plants; Springer: Dordrecht, The Netherlands, 2010; pp. 265–302. [Google Scholar]
- Dietz, K.J.; Mittler, R.; Noctor, G. Recent progress in understanding the role of reactive oxygen species in plant cell signaling. Plant Physiol. 2016, 171, 1535–1539. [Google Scholar] [CrossRef] [PubMed]
- Baxter, A.; Mittler, R.; Suzuki, N. Ros as key players in plant stress signalling. J. Exp. Bot. 2014, 65, 1229–1240. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zhang, J.; Chen, X.; Gao, Z.; Xuan, W.; Xu, S.; Ding, X.; Shen, W. Carbon monoxide alleviates cadmium-induced oxidative damage by modulating glutathione metabolism in the roots of Medicago sativa. New Phytol. 2007, 177, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.K.; Palma, J.M.; Corpas, F.J. Reactive Oxygen Species and Oxidative Damage in Plants Under Stress; Springer International Publishing: Berlin/Heidelberg, Germany, 2015; p. 621. [Google Scholar]
- Wei, L.; Wang, L.; Yang, Y.; Wang, P.; Guo, T.; Kang, G. Abscisic acid enhances tolerance of wheat seedlings to drought and regulates transcript levels of genes encoding ascorbate-glutathione biosynthesis. Front. Plant Sci. 2015, 6, 458. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Wang, J.; Shafi, M.; Liu, Y.; Wang, J.; Wu, J.; Wu, A. Hypobaric treatment effects on chilling injury, mitochondrial dysfunction, and the ascorbate-glutathione (ASA-GSH) cycle in postharvest peach fruit. J. Agric. Food Chem. 2016, 64, 4665–4674. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Khan, N.A.; Masood, A.; Per, T.S.; Asgher, M. Hydrogen peroxide alleviates nickel-inhibited photosynthetic responses through increase in use-efficiency of nitrogen and sulfur, and glutathione production in mustard. Front. Plant Sci. 2016, 7, 44. [Google Scholar] [CrossRef] [PubMed]
- Shu, S.; Gao, P.; Li, L.; Yuan, Y.; Sun, J.; Guo, S. Abscisic acid-induced H2O2 accumulation enhances antioxidant capacity in pumpkin-grafted cucumber leaves under Ca(NO3)2 stress. Front. Plant Sci. 2016, 7, 1489. [Google Scholar] [CrossRef] [PubMed]
- Bashir, H.; Ibrahim, M.M.; Bagheri, R.; Ahmad, J.; Arif, I.A.; Baig, M.A.; Qureshi, M.I. Influence of sulfur and cadmium on antioxidants, phytochelatins and growth in indian mustard. AoB Plants 2015. [Google Scholar] [CrossRef] [PubMed]
- Astolfi, S.; Zuchi, S.; Passera, C. Role of sulphur availability on cadmium-induced changes of nitrogen and sulphur metabolism in maize (Zea mays L.) leaves. J. Plant Physiol. 2004, 161, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhang, C.; Dai, C.; Ge, Y. Sufficient sulfur supply promotes seedling growth, alleviates oxidation stress, and regulates iron uptake and translocation in rice. Biol. Plant. 2015, 59, 788–792. [Google Scholar] [CrossRef]
- Matraszek, R.; Hawrylak-Nowak, B.; Chwil, S.; Chwil, M. Interaction between cadmium stress and sulphur nutrition level on macronutrient status of Sinapis alba L. Water Air Soil Pollut. 2016, 227, 355. [Google Scholar] [CrossRef] [PubMed]
- Anjum, N.A.; Umar, S.; Iqbal, M.; Khan, N.A. Cadmium causes oxidative stress in mung bean by affecting the antioxidant enzyme system and ascorbate-glutathione cycle metabolism. Russ. J. Plant Physiol. 2011, 58, 92–99. [Google Scholar] [CrossRef]
- Nocito, F.F.; Lancilli, C.; Crema, B.; Fourcroy, P.; Davidian, J.C.; Sacchi, G.A. Heavy metal stress and sulfate uptake in maize roots. Plant Physiol. 2006, 141, 1138–1148. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, P.; Zanella, L.; De Paolis, A.; Di Litta, D.; Cecchetti, V.; Falasca, G.; Barbieri, M.; Altamura, M.M.; Costantino, P.; Cardarelli, M. Cadmium-inducible expression of the ABC-type transporter AtABCC3 increases phytochelatin-mediated cadmium tolerance in Arabidopsis. J. Exp. Bot. 2015, 66, 3815–3829. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yang, L.; Yan, X.; Liu, Y.; Wang, R.; Fan, T.; Ren, Y.; Tang, X.; Xiao, F.; Liu, Y.; et al. Zinc-finger transcription factor zat6 positively regulates cadmium tolerance through the glutathione-dependent pathway in arabidopsis. Plant Physiol. 2016, 171, 707–719. [Google Scholar] [CrossRef] [PubMed]
- Astolfi, S.; Zuchi, S.; Passera, C. Effect of cadmium on H⁺ atpase activity of plasma membrane vesicles isolated from roots of different s-supplied maize (Zea mays L.) plants. Plant Sci. 2005, 169, 361–368. [Google Scholar] [CrossRef]
- Ahmad, P.; Prasad, M. Environmental Adaptations and Stress Tolerance of Plants in the Era of Climate Change; Springer: New York, NY, USA, 2012; pp. 29–56. [Google Scholar]
- Pilon-Smits, E.A.; Hwang, S.; Lytle, C.M.; Zhu, Y.; Tai, J.C.; Bravo, R.C.; Chen, Y.; Leustek, T.; Terry, N. Over expression of atp sulfurylase in indian mustard leads to increased selenate uptake, reduction, and tolerance. Plant Physiol. 1999, 119, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Eltayeb, A.E.; Kawano, N.; Badawi, G.H.; Kaminaka, H.; Sanekata, T.; Shibahara, T.; Inanaga, S.; Tanaka, K. Overexpression of monodehydroascorbate reductase in transgenic tobacco confers enhanced tolerance to ozone, salt and polyethylene glycol stresses. Planta 2007, 225, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Wang, S.; Eltayeb, A.E.; Uddin, M.I.; Yamamoto, Y.; Tsuji, W.; Takeuchi, Y.; Tanaka, K. Overexpression of dehydroascorbate reductase, but not monodehydroascorbate reductase, confers tolerance to aluminum stress in transgenic tobacco. Planta 2010, 231, 609–621. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.Y.; Kim, M.H.; Kim, Y.H.; Park, H.M.; Yoon, H.S. Co-expression of monodehydroascorbate reductase and dehydroascorbate reductase from Brassica rapa effectively confers tolerance to freezing-induced oxidative stress. Mol. Cells 2013, 36, 304–315. [Google Scholar] [CrossRef] [PubMed]
- Johnston, E.J.; Rylott, E.L.; Beynon, E.; Lorenz, A.; Chechik, V.; Bruce, N.C. Monodehydroascorbate reductase mediates tnt toxicity in plants. Science 2015, 349, 1072–1075. [Google Scholar] [CrossRef] [PubMed]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Alexieva, V.; Sergiev, I.; Mapelli, S.; Karanov, E. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ. 2001, 24, 1337–1344. [Google Scholar] [CrossRef]
- Masato, O. An improved method for determination of l-ascorbic acid and l-dehydroascorbic acid in blood plasma. Clin. Chim. Acta 1980, 103, 259–268. [Google Scholar] [CrossRef]
- Anderson, M.E. Determination of glutathione and glutathione disulfide in biological samples. Methods Enzymol. 1985, 113, 548–555. [Google Scholar] [PubMed]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Duan, G.-L.; Hu, Y.; Liu, W.-J.; Kneer, R.; Zhao, F.-J.; Zhu, Y.-G. Evidence for a role of phytochelatins in regulating arsenic accumulation in rice grain. Environ. Exp. Bot. 2011, 71, 416–421. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Smith, I.K.; Vierheller, T.L.; Thorne, C.A. Assay of glutathione reductase in crude tissue homogenates using 5, 5’-dithiobis (2-nitrobenzoic acid). Anal. Biochem. 1988, 175, 408–413. [Google Scholar] [CrossRef]
- Li, Z.-S.; Zhen, R.-G.; Rea, P.A. 1-Chloro-2,4-dinitrobenzene-elicited increase in vacuolar glutathione-S-conjugate transport activity. Plant Physiol. 1995, 109, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Lappartient, A.G.; Touraine, B. Demand-driven control of root atp sulfurylase activity and so42-uptake in intact canola (the role of phloem-translocated glutathione). Plant Physiol. 1996, 111, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Riemenschneider, A.; Riedel, K.; Hoefgen, R.; Papenbrock, J.; Hesse, H. Impact of reduced O-acetylserine (thiol) lyase isoform contents on potato plant metabolism. Plant Physiol. 2005, 137, 892–900. [Google Scholar] [CrossRef] [PubMed]
- Pinto, M.C.D.; Tommasi, F.; Gara, L.D. Enzymes of the ascorbate biosynthesis and ascorbate-glutathione cycle in cultured cells of tobacco bright yellow 2. Plant Physiol. Biochem. 2000, 38, 541–550. [Google Scholar] [CrossRef]



| Growth Parameters | CK | Cd1 | Cd10 | S | Cd1 + S | Cd10 + S |
|---|---|---|---|---|---|---|
| Aikangqing | ||||||
| Shoot fresh weight (g·plant−1) | 39.59 ± 4.43 b | 35.25 ± 3.61 bc | 25.66 ± 2.84 c | 52.66 ± 12.21 a | 49.91 ± 7.89 a | 38.42 ± 8.24 bc |
| Shoot height (cm) | 23.1 ± 1.18 ab | 20.67 ± 3.46 cd | 18.70 ± 2.09 d | 24.07 ± 0.81 a | 21.50 ± 0.44 bc | 20.53 ± 1.08 cd |
| Root fresh weight (g·plant−1) | 3.18 ± 0.59 b | 2.01 ± 0.38 cd | 1.52 ± 0.37 d | 4.18 ± 0.2 a | 2.35 ± 0.29 c | 1.62 ± 0.47 d |
| Root long (cm) | 33.25 ± 0.49 a | 29.68 ± 3.06 ab | 24.12 ± 4.42 c | 30.94 ± 4.95 ab | 27.43 ± 2.51 ab | 24.62 ± 4.23 bc |
| Qibaoqing | ||||||
| Shoot fresh weight(g·plant−1) | 46.05 ± 4.63 ab | 34.78 ± 5.30 cd | 26.83 ± 3.89 d | 47.70 ± 5.35 a | 37.43 ± 4.51 bc | 33.41 ± 4.37 cd |
| Shoot height (cm) | 19.73 ± 0.51 ab | 17.30 ± 1.44 bc | 15.63 ± 1.24 c | 20.97 ± 2.52 a | 17.00 ± 0.75 bc | 16.48 ± 1.03 c |
| Root fresh weight (g·plant−1) | 2.40 ± 0.46 b | 1.98 ± 0.43 bc | 1.77 ± 0.27 c | 3.00 ± 0.43 a | 2.23 ± 0.38 b | 2.01 ± 0.31 bc |
| Root long (cm) | 28.58 ± 3.57 ab | 22.46 ± 1.95 cd | 21.08 ± 2.89 d | 29.68 ± 2.66 a | 27.46 ± 1.19 ab | 25.78 ± 2.84 bc |
| Cultivars | Treatments | Cd Content (mg·kg−1 DW) | BCF (%) | TF (%) | ||
|---|---|---|---|---|---|---|
| Shoots | Roots | Shoots | Roots | |||
| Aikangqing | Cd1 | 11.83 ± 2.09 b | 16.14 ± 2.52 c | 1214 | 1656 | 73 |
| Cd10 | 45.03 ± 4.23 a | 69.92 ± 1.67 a | 521 | 756 | 69 | |
| Cd1 + S | 7.68 ± 0.39 b | 11.68 ± 0.23 d | 772 | 1175 | 66 | |
| Cd10 + S | 32.97 ± 2.71 a | 50.86 ± 3.95 b | 352 | 543 | 65 | |
| Qibaoqing | Cd1 | 9.53 ± 1.72 c | 15.16 ± 0.22 c | 971 | 1544 | 63 |
| Cd10 | 34.56 ± 2.59 a | 58.19 ± 2.65 a | 422 | 711 | 59 | |
| Cd1 + S | 6.24 ± 0.84 c | 10.76 ± 0.78 d | 647 | 1116 | 58 | |
| Cd10 + S | 24.91 ± 3.28 b | 46.31 ± 1.99 b | 268 | 499 | 54 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lou, L.; Kang, J.; Pang, H.; Li, Q.; Du, X.; Wu, W.; Chen, J.; Lv, J. Sulfur Protects Pakchoi (Brassica chinensis L.) Seedlings against Cadmium Stress by Regulating Ascorbate-Glutathione Metabolism. Int. J. Mol. Sci. 2017, 18, 1628. https://doi.org/10.3390/ijms18081628
Lou L, Kang J, Pang H, Li Q, Du X, Wu W, Chen J, Lv J. Sulfur Protects Pakchoi (Brassica chinensis L.) Seedlings against Cadmium Stress by Regulating Ascorbate-Glutathione Metabolism. International Journal of Molecular Sciences. 2017; 18(8):1628. https://doi.org/10.3390/ijms18081628
Chicago/Turabian StyleLou, Lili, Jingquan Kang, Hongxi Pang, Qiuyu Li, Xiaoping Du, Wei Wu, Junxiu Chen, and Jinyin Lv. 2017. "Sulfur Protects Pakchoi (Brassica chinensis L.) Seedlings against Cadmium Stress by Regulating Ascorbate-Glutathione Metabolism" International Journal of Molecular Sciences 18, no. 8: 1628. https://doi.org/10.3390/ijms18081628
APA StyleLou, L., Kang, J., Pang, H., Li, Q., Du, X., Wu, W., Chen, J., & Lv, J. (2017). Sulfur Protects Pakchoi (Brassica chinensis L.) Seedlings against Cadmium Stress by Regulating Ascorbate-Glutathione Metabolism. International Journal of Molecular Sciences, 18(8), 1628. https://doi.org/10.3390/ijms18081628