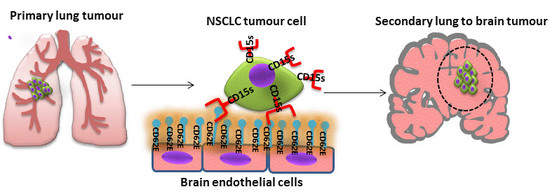
CD15s/CD62E Interaction Mediates the Adhesion of Non-Small Cell Lung Cancer Cells on Brain Endothelial Cells: Implications for Cerebral Metastasis
Abstract
:1. Introduction
1.1. The Role of CD15s in Cancer Metastasis
1.2. Role of CD62E (E-Selectin) in Cancer Metastasis
2. Results
2.1. Characterisation of CD15s in Cultured Cells
2.2. The Absence of CD62E Reduced Cancer Cell–Brain Endothelium Adhesion
2.3. Immunoblocking of CD15s Reduced Adhesion of Cancer Cell–Brain Endothelium under Static Conditions
2.4. CD15s mAb Blocking Decreases Adhesion of NSCLC Cells under Shear Stress Condition
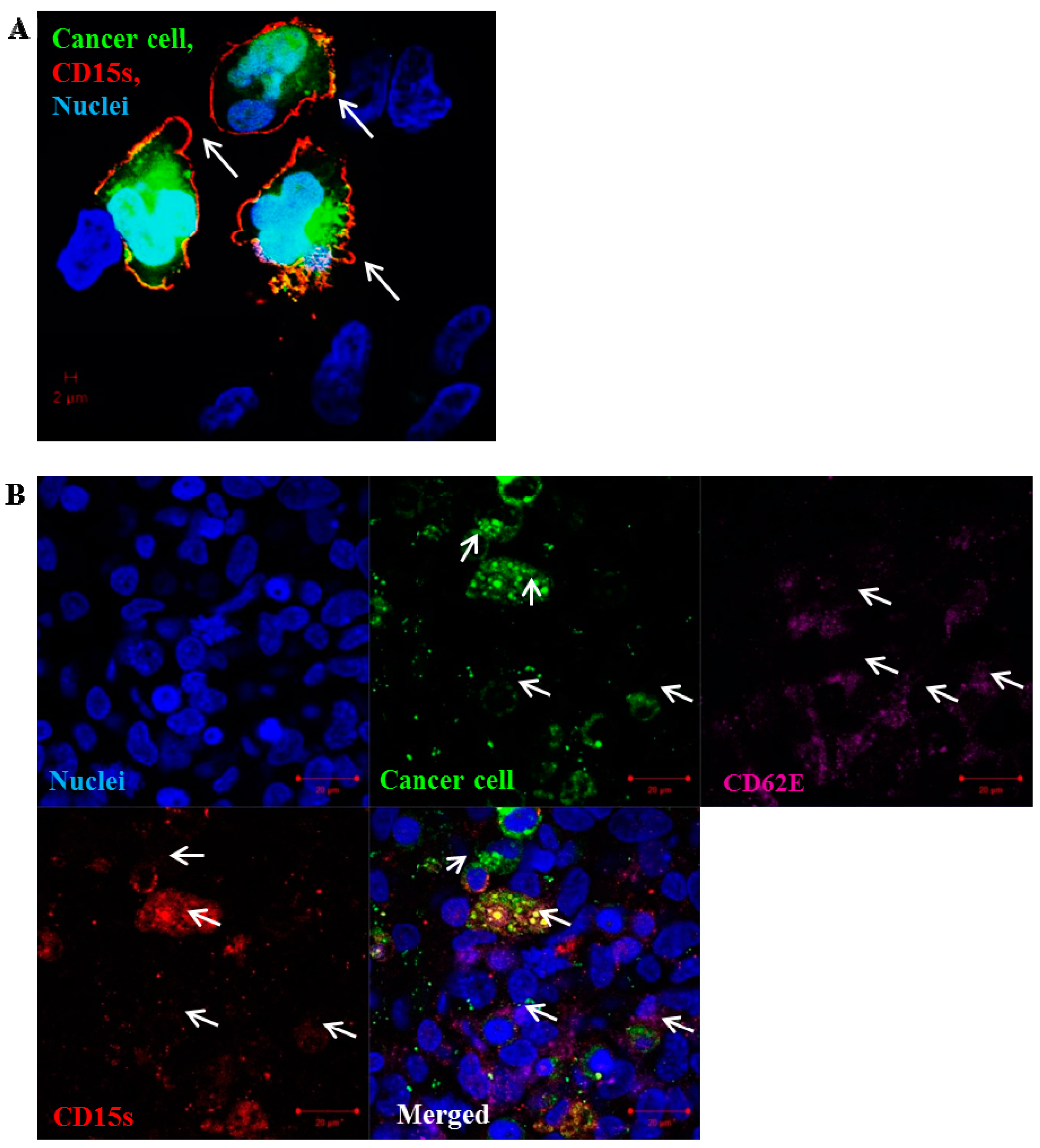
2.5. CD15s Expression Was Localised at the Peripheral Adhesion Sites of Cancer Cell–Brain Endothelium
2.6. CD15s Expression in Human Biopsy of Lung to Brain Metastasis
3. Discussion
4. Materials and methods
4.1. Ethics Statement
4.2. Cell Culture
4.3. Antibodies
4.3.1. Primary Antibodies
4.3.2. Secondary Antibodies
4.3.3. Isotype CONTROLS
4.4. Immunocytochemistry (ICC)
4.5. Flow Cytometry (FC)
4.6. Western Blotting (WB)
4.7. Adhesion Assays
4.7.1. Quantitative Adhesion Assay
4.7.2. Qualitative Adhesion Assay
4.8. Viability Assays
4.9. Cancer Cell Adhesion under Shear Stress and Live Cell Imaging Microscopy
4.10. Immunohistochemistry (IHC)
4.11. Confocal Microscopy
4.12. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Al-Mehdi, A.B.; Tozawa, K.; Fisher, A.B.; Shientag, L.; Lee, A.; Muschel, R.J. Intravascular origin of metastasis from the proliferation of endothelium-attached tumour cells: A new model for metastasis. Nat. Med. 2000, 6, 100–102. [Google Scholar] [CrossRef] [PubMed]
- Narimatsu, H.; Iwasaki, H.; Nishihara, S.; Kimura, H.; Kudo, T.; Yamauchi, Y.; Hirohashi, S. Genetic evidence for the Lewis enzyme, which synthesizes type-1 Lewis antigens in colon tissue and intracellular localization of the enzyme. Cancer Res. 1996, 56, 330–338. [Google Scholar] [PubMed]
- Martin, K.; Akinwunmi, J.; Rooprai, H.K.; Kennedy, A.J.; Linke, A.; Ognjenovic, N.; Pilkington, G.J. Non expression of CD15 by neoplastic glia: A barrier to metastasis? Anticancer Res. 1995, 15, 1159–1166. [Google Scholar] [PubMed]
- Soejima, M.; Kuda, Y. Molecular mechanisms of Lewis antigen expression. Leg. Med. 2005, 7, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Polley, M.J.; Phillips, M.L.; Wayner, E. CD62 and endothelial cell-leucocyte adhesion molecule-1 (ELAM-1) recognize the same carbohydrate ligand. Proc. Natl. Acad. Sci. USA 1991, 88, 6224–6228. [Google Scholar] [CrossRef] [PubMed]
- Vestweber, D.; Blanks, J.E. Mechanisms that regulate the function of the selectins and their ligands. Physiol. Rev. 1999, 79, 181–213. [Google Scholar] [PubMed]
- Giavazzi, R.; Foppolo, M.; Dossi, R.; Remuzzi, A. Rolling and adhesion of human tumor cells on vascular endothelium under physiological flow conditions. J. Clin. Investig. 1993, 92, 3038–3044. [Google Scholar] [CrossRef] [PubMed]
- Kitayama, J.; Tsuno, N.; Sunami, E.; Osada, T.; Muto, T.; Nagawa, H. E-selectin can mediate the arrest type of adhesion of colon cancer cells under physiological shear flow. Eur. J. Cancer 2000, 36, 121–127. [Google Scholar] [CrossRef]
- Burdick, M.M.; McCaffery, J.M.; Kim, Y.S.; Bochner, B.S.; Konstantopoulos, K. Colon carcinoma cell glycolipids, integrins, and other glycoproteins mediate adhesion to HUVECs under flow. Am. J. Physiol. Cell Physiol. 2003, 284, C977–C987. [Google Scholar] [CrossRef] [PubMed]
- Munro, J.M. Endothelial-leukocyte adhesive interactions in inflammatory diseases. Eur. Heart J. 1993, 14, 72–77. [Google Scholar] [PubMed]
- Aruffo, A.; Glycosci, T. Drug Discovery: Saccharides in Medicinal Chemistry. Glycotechnology 1992, 4, 146. [Google Scholar] [CrossRef]
- Wang, Q.Y.; Wu, S.L.; Chen, J.H.; Liu, F.; Chen, H.L. Expressions of Lewis Antigens in Human Non-Small Cell Pulmonary Cancer and Primary Liver Cancer with Different Pathological Conditions. J. Exp. Clin. Cancer Res. 2003, 22, 431–440. [Google Scholar] [PubMed]
- Ikeda, Y.; Mori, M.; Kajiyama, K.; Haraguchi, O.; Sasaki, K. Sugimachi Immunohistochemical expression of sialyl Tn, SialylLewis a, SialylLewis a-b-, and SialylLewisx in primary tumor and metastatic lymph nodes in human gastric cancer. J. Surg. Oncol. 1996, 62, 171–176. [Google Scholar] [CrossRef]
- Nakagoe, T.; Sawai, T.; Tsuji, T.; Jibiki, M.A.; Nanashima, A.; Yamaguchi, H.; Yasutake, T.; Ayabe, H.; Arisawa, K.; Ishikawa, H. Predictive factors for preoperative serum levels of Sialy Lewis(x), Sialyl Lewis(a) and sialyl T antigens in gastric cancer patients. Anticancer Res. 2002, 22, 451–458. [Google Scholar] [PubMed]
- Jassam, S.A.; Maherally, Z.; Chairta, P.; Fillmore, H.L.; Pilkington, G.J. Expression of CD15 and CD15s is correlated with glioma cell arrest at G1-phase. Neuro-Oncology 2015, 17 (Suppl. S8), viii11. [Google Scholar]
- Zukerberg, L.R.; Collins, A.B.; Ferry, J.A.; Harris, N.L. Coexpression of CD15 and CD20 by Reed-Sternberg cells in Hodgkin’s disease. Am. J. Pathol. 1991, 139, 475–483. [Google Scholar] [PubMed]
- Read, T.; Fogarty, M.P.; Markant, S.L.; McLendon, R.E.; Wei, Z.W.; Febbo, P.G.; Wechsler-Reya, R. Identification of CD15 as a Marker for Tumour-Propagating Cells in a Mouse Model of Medulloblastoma. Cancer Cell 2009, 15, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Graves, B.J.; Crowther, R.L.; Chandran, C.; Rumberger, J.M.; Li, S.; Huang, K.S.; Presky, D.H.; Familletti, P.C.; Wolitzky, A.; Burns, D.K. Insight in to E-selectin/ligand interaction from the crystal structure and mutagenesis of the lec/EGF domains. Nature 1994, 367, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Somers, W.S.; Tang, J.; Shaw, G.D.; Camphausen, R.T. Insights into the molecular basis of leukocyte tethering and rolling revealed by structures of P- and E-selectin bound to SLex and PSGL-1. Cell 2000, 103, 467–479. [Google Scholar] [CrossRef]
- Tomlinson, J.; Wang, J.L.; Barsky, S.H. Human colon cancer cells express multiple glycoprotein ligands for E-selectin. Int. J. Oncol. 2000, 16, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Gout, S.; Morin, C.; Houle, F.; Huot, J. Death receptor-3, a new E-Selectin counter-receptor that confers migration and survival advantages to colon carcinoma cells by triggering p38 and ERK MAPK activation. Cancer Res. 2006, 66, 9117–9124. [Google Scholar] [CrossRef] [PubMed]
- Napier, S.L.; Healy, Z.R.; Schnaar, R.L.; Konstantopoulous, K. Selectin ligand expression regulates the initial vascular interactions of colon carcinoma cells: The roles of CD44V and alternative sialofucosylated selectin ligands. J. Biol. Chem. 2007, 282, 3433–3441. [Google Scholar] [CrossRef] [PubMed]
- Zarbock, A.; Ley, K.; McEver, R.; Hidalgo, A. Leukocyte ligands for endothelial selectins: Specialized glycoconjugates that mediate rolling and signalling under flow. Blood 2011, 118, 6743–6751. [Google Scholar] [CrossRef] [PubMed]
- Laferriere, J.; Houle, F.; Taher, M.M.; Valerie, K.; Huot, J. Transendothelial migration of colon carcinoma cells requires expression of Eselectin by endothelial cells and activation of stress-activated protein kinase-2 (SAPK2/p38) in the tumour cells. J. Biol. Chem. 2001, 276, 33762–33772. [Google Scholar] [CrossRef] [PubMed]
- Hiratsukaa, S.; Goela, S.; Kamouna, W.S.; Marub, Y.; Fukumuraa, D.; Dudaa, D.; Jain, R. Endothelial focal adhesion kinase mediates cancer cell homing to discrete regions of the lungs via E-selectin up-regulation. Proc. Natl. Acad. Sci. USA 2011, 108, 3725–3730. [Google Scholar] [CrossRef] [PubMed]
- Tabatabai, G.; Herrmann, C.; von Kurthy, G.; Mittelbronn, M.; Grau, S.; Frank, B.; Mohle, R.; Weller, M.; Wicky, W. VEGF-dependent induction of CD62E on endothelial cells mediates glioma tropism of adult haematopoietic progenitor cells. Brain 2008, 131, 2579–2595. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Goffin, J.R.; Arnold, A.; Ellis, P.M. Survival of patients with non-small- cell lung cancer after a diagnosis of brain metastases. Curr. Oncol. 2013, 20, e300–e306. [Google Scholar] [CrossRef] [PubMed]
- Haselhorst, T.; Weimar, T.; Peters, T. Molecular recognition of Sialyl Lewis(x) and related saccharides by two lectins. J. Am. Shem. Soc. 2001, 123, 10705–10714. [Google Scholar] [CrossRef]
- Turunen, J.P.; Paavonen, T.; Majuri, M.L.; Tiisala, S.; Mattila, P.; Mennander, A.; Gahmberg, C.G.; Hayry, P.; Tamatani, T.; Miyasaka, M.; et al. Sialyl Lewis(x)-and l-selectin-dependent site-specific lymphocyte extravasation into renal transplants during acute rejection. Eur. J. Immunol. 1994, 24, 1130–1136. [Google Scholar] [CrossRef] [PubMed]
- Fukuoka, K.; Narita, N.; Saijo, N. Increase expression of Sialyl Lewis(x) antigen in associated with distant metastasis in lung cancer patients: Immunohistochemical study on bronchofiberscopic biopsy specimens. Lung Cancer 1998, 20, 109–116. [Google Scholar] [CrossRef]
- Nakamori, S.; Kameyama, M.; Imaoka, S.; Firukawa, H.; Ishikawa, O.; Sasaki, Y.; Izumi, Y.; Irimura, T. Involvement of carbohydrate antigen Sialyl Lewis(x) in colorectal cancer metastasis. Dis. Colon Rectum 1997, 40, 420–431. [Google Scholar] [CrossRef] [PubMed]
- Izumi, Y.; Taniuchi, Y.; Tsuji, T.; Smith, C.W.; Nakamori, S.; Fifler, I.J.; Irimura, T. Characterization of human colon carcinoma variant cells selected for sialyl Lex carbohydrate antigen: Liver colonization and adhesion to vascular endothelial cells. Exp. Cell Res. 1995, 216, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Renkonen, J.; Makitie, A.; Paavonen, T.; Renkonen, R. Sialyl-Lewis (x/a)-decorated selectin ligands in head and neck tumours. J. Cancer Res. Clin. Oncol. 1999, 125, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Kadota, A.; Masutani, M.; Takei, M.; Horie, T. Evaluation of expression of CD15 and CD15s in non-samll cell lung cancer. Int. J. Oncol. 1999, 15, 1081–1089. [Google Scholar] [PubMed]
- Komatsu, H.; Mizuguchi, S.; Izumi, N.; Chung, K.; Hnada, S.; Inoue, H.; Suehiro, S.; Nishiyama, N. Sialyl Lewis X as a predictor of skip N2 metastasis in clinical stage IA non-small cell lung cancer. World J. Surg. Oncol. 2013, 11, 309. [Google Scholar] [CrossRef] [PubMed]
- Jassam, S.A.; Maherally, Z.; Smith, J.R.; Ashkan, K.; Roncaroli, F.; Fillmore, H.L.; Pilkington, G.J. TNF-α enhancement of CD26E mediates adhesion of non-small cell lung cancer cells to brain endothelium via CD15 in lung-brain metastasis. Neuro-Oncology 2015, 18, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Soto, M.S.; Serres, S.; Anthony, D.C.; Sibson, N.R. Functional role of endothelial adhesion molecules in the early stages of brain metastasis. Neuro-Oncology 2014, 16, 540–551. [Google Scholar] [CrossRef] [PubMed]
- Weksler, B.B.; Subileau, E.A.; Perriere, N.; Charneau, P.; Holloway, K.; Leveque, M.; Tricoire-Leignel, H.; Nicotra, A.; Bourdoulous, S.; Turowski, P.; et al. Blood-brain barrier-specific properties of a human adult brain endothelial cell line. FASEB J. 2005, 19, 1872–1874. [Google Scholar] [CrossRef] [PubMed]
- Maherally, Z.; Fillmore, H.L.; Tan, S.L.; Tan, S.F.; Jassam, S.A.; Quack, F.I.; Hatherell, K.E.; Pilkington, G.J. Real-time acquisition of trans-endothelial electrical resistance in an all human in vitro 3D blood brain model exemplifies tight junction integrity. FASEB J. 2017, in press. [Google Scholar]
- Hatherell, K.; Couraud, P.; Romero, I.A.; Weksler, B.; Pilkington, G.J. Development of a three-dimensional, all-human in vitro model of the blood-brain barrier using mono-, co-, and tri-cultivation Transwell models. J. Neurosci. Methods 2011, 199, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Tateishi, K.; Ando, W.; Higuchi, C.; Hart, D.A.; Hashimoto, J.; Nakata, K.; Yoshikawa, H.; Nakamura, N. Comparison of human serum with fetal bovine serum for expression and differention of human synovial MSC: Potential feasibility for clinical applications. Cell Transplant. 2008, 17, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Murray, S.A. Significance of Serum Supplementation Type to Gene and Protein Expression in Monolayer and Three-Dimensional In Vitro Culture Systems of Human Glioma. Ph.D. Thesis, University of Portsmouth, Portsmouth, UK, 2010. [Google Scholar]
- Martin-Satue, M.; Marrugat, R.; Cancelas, J.A.; Blanco, J. Enhanced expression of a (1,3)-fucosyltransferase genes correlates with E-selectin-mediated adhesion and metastatic potential of human lung adenocarcinoma cells. Cancer Res. 1998, 58, 1544–1550. [Google Scholar] [PubMed]
- Man, S.; Ubogu, E.E.; Williams, K.A.; Tucky, B.; Callahan, M.K.; Ransohoff, R.M. Human Brain Microvascular Endothelial Cells and Umbilical Vein Endothelial Cells Differentially Facilitate Leukocyte Recruitment and Utilize Chemokines for T Cell Migration. Clin. Dev. Immunol. 2008, 2008, 384982. [Google Scholar] [CrossRef] [PubMed]
- Munro, J.M.; Lo, S.K.; Corless, C.; Robertson, M.J.; Lee, N.C.; Barnhill, R.L.; Weinberg, D.S.; Bevilacqua, M.P. Expression of Sialyl-Lewis X, an E-selectin ligand, in inflammation, immune processes, and lymphoid tissues. Am. J. Pathol. 1992, 141, 1397–1408. [Google Scholar] [PubMed]
- Matsumura, R.; Hirakawa, J.; Sato, K.; Ikeda, T.; Nagai, M.; Fududa, M.; Imai, Y.; Kawashima, H. Novel Antibodies Reactive with Sialyl Lewis X in Both Humans and Mice Define Its Critical Role in Leukocyte Trafficking and Contact Hypersensitivity Responses. J. Biol. Chem. 2015, 290, 15313–15326. [Google Scholar] [CrossRef] [PubMed]
- Zeisig, R.; Stahn, R.; Wenzel, K.; Behrens, D.; Fichtner, I. Effect of Sialyl Lewis X-glycoliposomes on the inhibition of E-selectin-mediated tumour cell adhesion in vitro. Biochim. Biophys. Acta 2004, 1660, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Mintun, M.A.; Lundstrom, B.N.; Snyder, A.Z.; Vlassenko, A.G.; Shulman, G.L.; Raichle, M.E. Blood flow and oxygen delivery to human brain during functional activity: Theoretical modelling and experimental data. Proc. Natl. Acad. Sci. USA 2001, 98, 6859–6864. [Google Scholar] [CrossRef] [PubMed]
- Satoh, J.; Kim, S.U. Differential expression of Lewis(x) and Sialyl-Lewis(x) antigens in fetal human neural cells in culture. J. Neurosci. Res. 1994, 37, 466–474. [Google Scholar] [CrossRef] [PubMed]
- An, Q.; Fillmore, H.L.; Vouri, M.; Pilkington, G.J. Brain tumor cell line authentication, an efficient alternative to capillary electrophoresis by using a microfluidics-based system. Neuro-Oncology 2014, 16, 265–273. [Google Scholar] [CrossRef] [PubMed]





© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jassam, S.A.; Maherally, Z.; Smith, J.R.; Ashkan, K.; Roncaroli, F.; Fillmore, H.L.; Pilkington, G.J. CD15s/CD62E Interaction Mediates the Adhesion of Non-Small Cell Lung Cancer Cells on Brain Endothelial Cells: Implications for Cerebral Metastasis. Int. J. Mol. Sci. 2017, 18, 1474. https://doi.org/10.3390/ijms18071474
Jassam SA, Maherally Z, Smith JR, Ashkan K, Roncaroli F, Fillmore HL, Pilkington GJ. CD15s/CD62E Interaction Mediates the Adhesion of Non-Small Cell Lung Cancer Cells on Brain Endothelial Cells: Implications for Cerebral Metastasis. International Journal of Molecular Sciences. 2017; 18(7):1474. https://doi.org/10.3390/ijms18071474
Chicago/Turabian StyleJassam, Samah A., Zaynah Maherally, James R. Smith, Keyoumars Ashkan, Federico Roncaroli, Helen L. Fillmore, and Geoffrey J. Pilkington. 2017. "CD15s/CD62E Interaction Mediates the Adhesion of Non-Small Cell Lung Cancer Cells on Brain Endothelial Cells: Implications for Cerebral Metastasis" International Journal of Molecular Sciences 18, no. 7: 1474. https://doi.org/10.3390/ijms18071474
APA StyleJassam, S. A., Maherally, Z., Smith, J. R., Ashkan, K., Roncaroli, F., Fillmore, H. L., & Pilkington, G. J. (2017). CD15s/CD62E Interaction Mediates the Adhesion of Non-Small Cell Lung Cancer Cells on Brain Endothelial Cells: Implications for Cerebral Metastasis. International Journal of Molecular Sciences, 18(7), 1474. https://doi.org/10.3390/ijms18071474