The Combination of Arginine Deprivation and 5-Fluorouracil Improves Therapeutic Efficacy in Argininosuccinate Synthetase Negative Hepatocellular Carcinoma
Abstract
:1. Introduction
2. Results
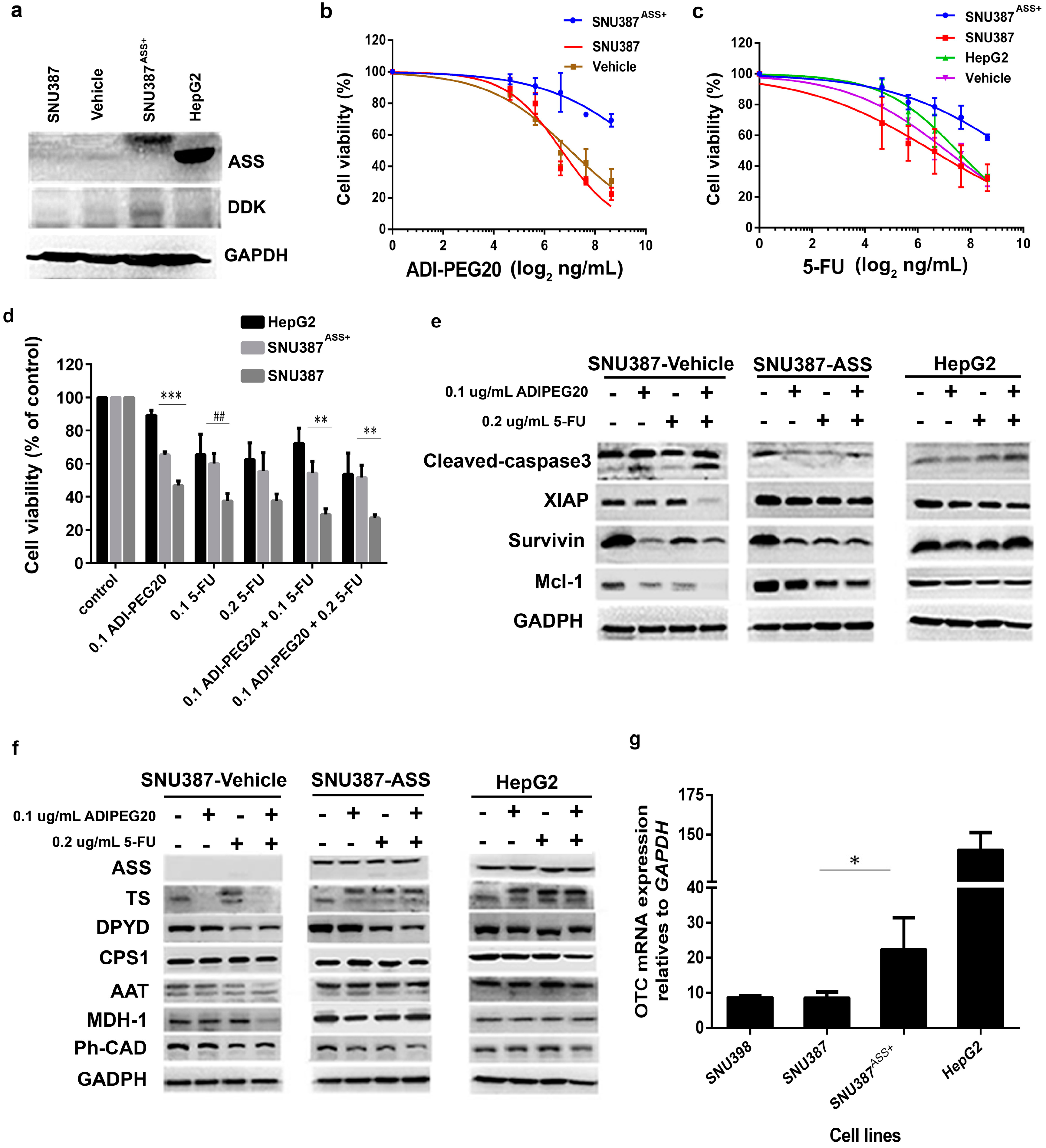
2.1. Determination of ASS Expression Level among Cancer Cell Types
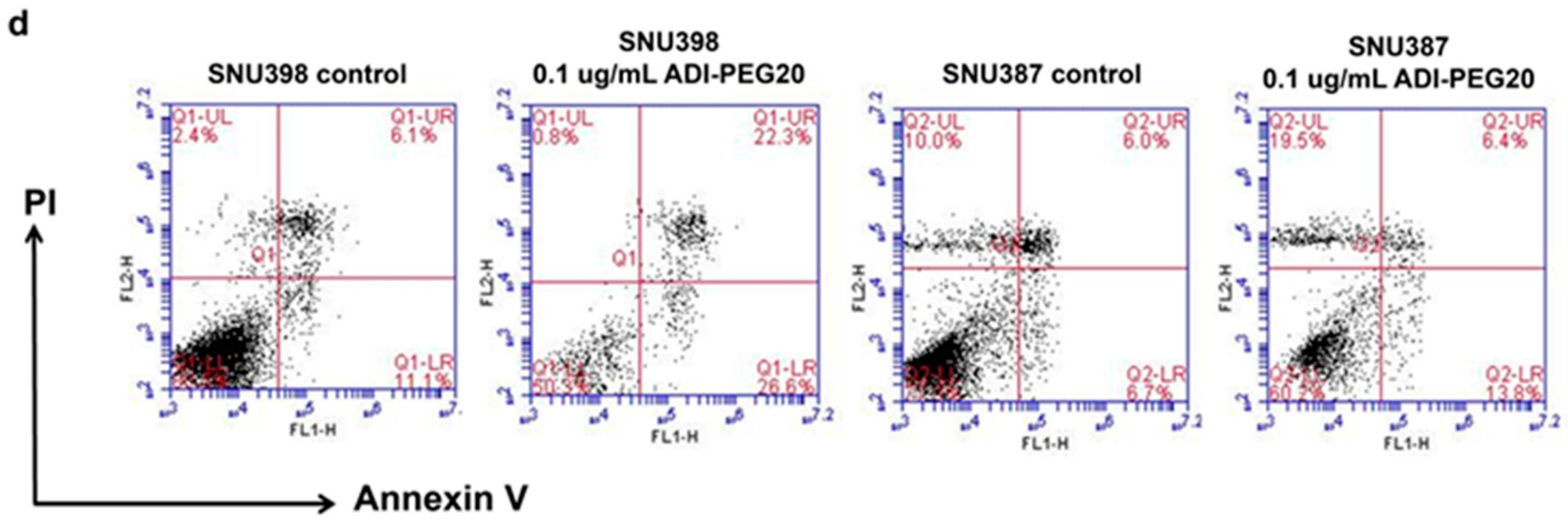
2.2. The Levels of ASS Expression Dictate the Sensitivity to Arginine Deprivation
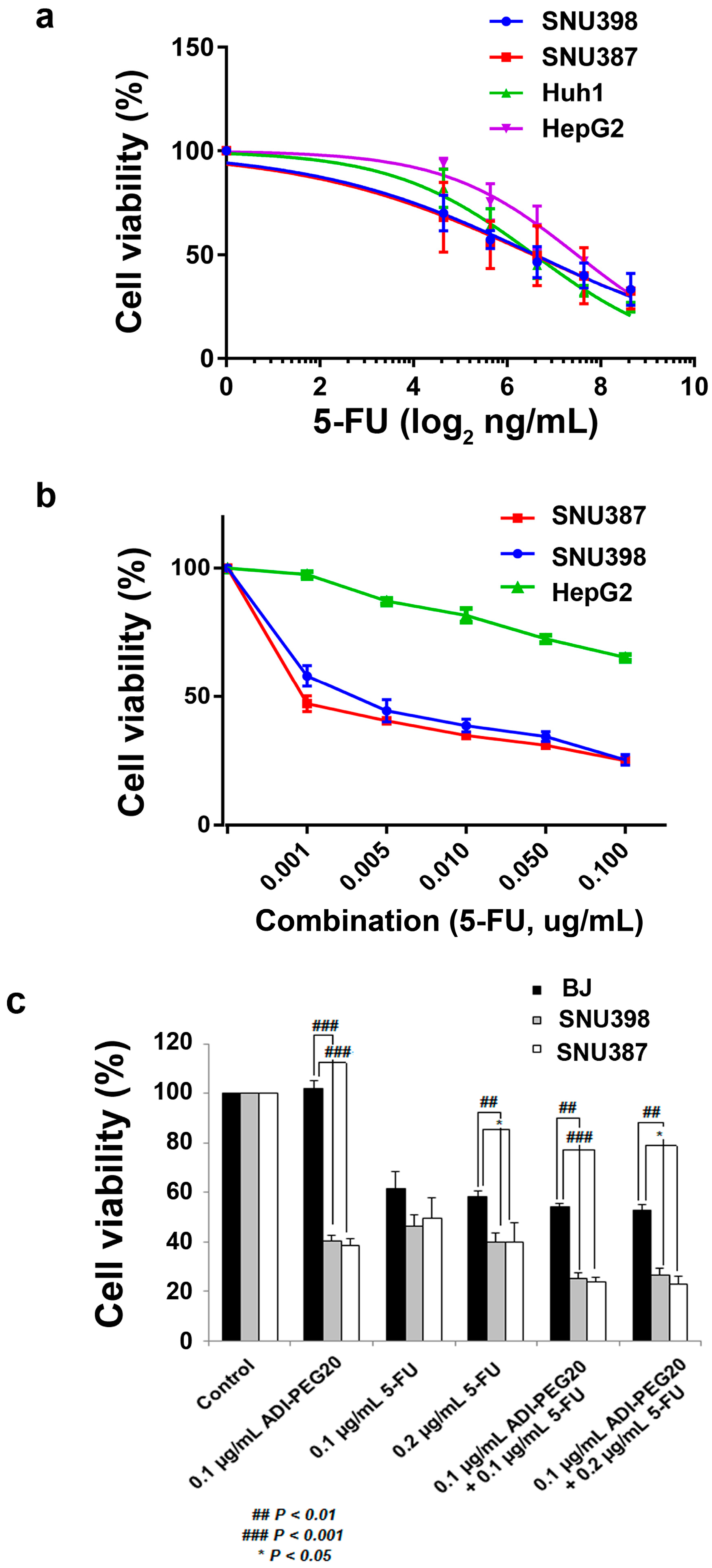
2.3. The Anti-Tumor Effect of 5-FU in Combination with ADI-PEG20
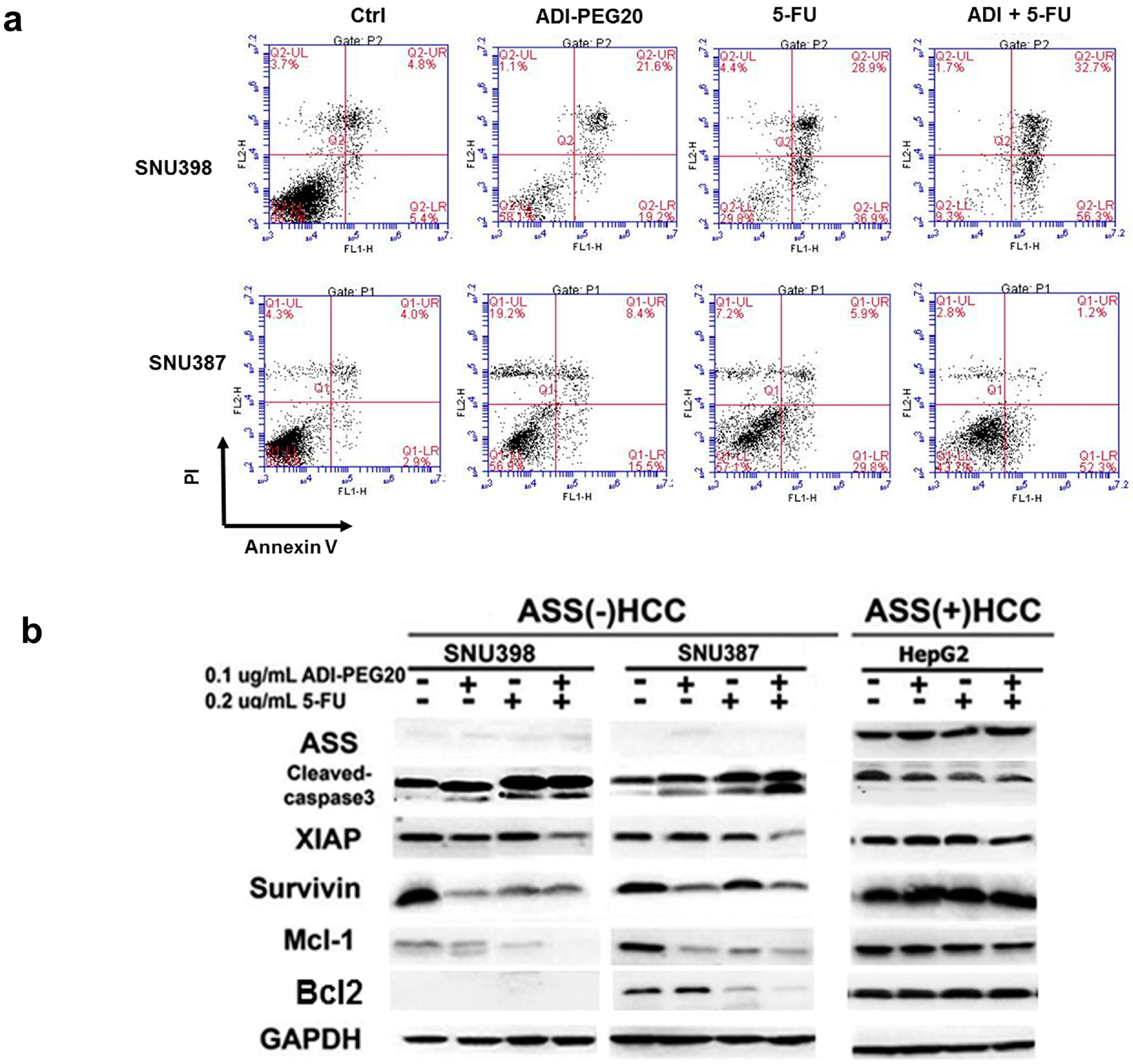
2.4. ADI-PEG20 and 5-FU Combination Effectively Increase Cell Death
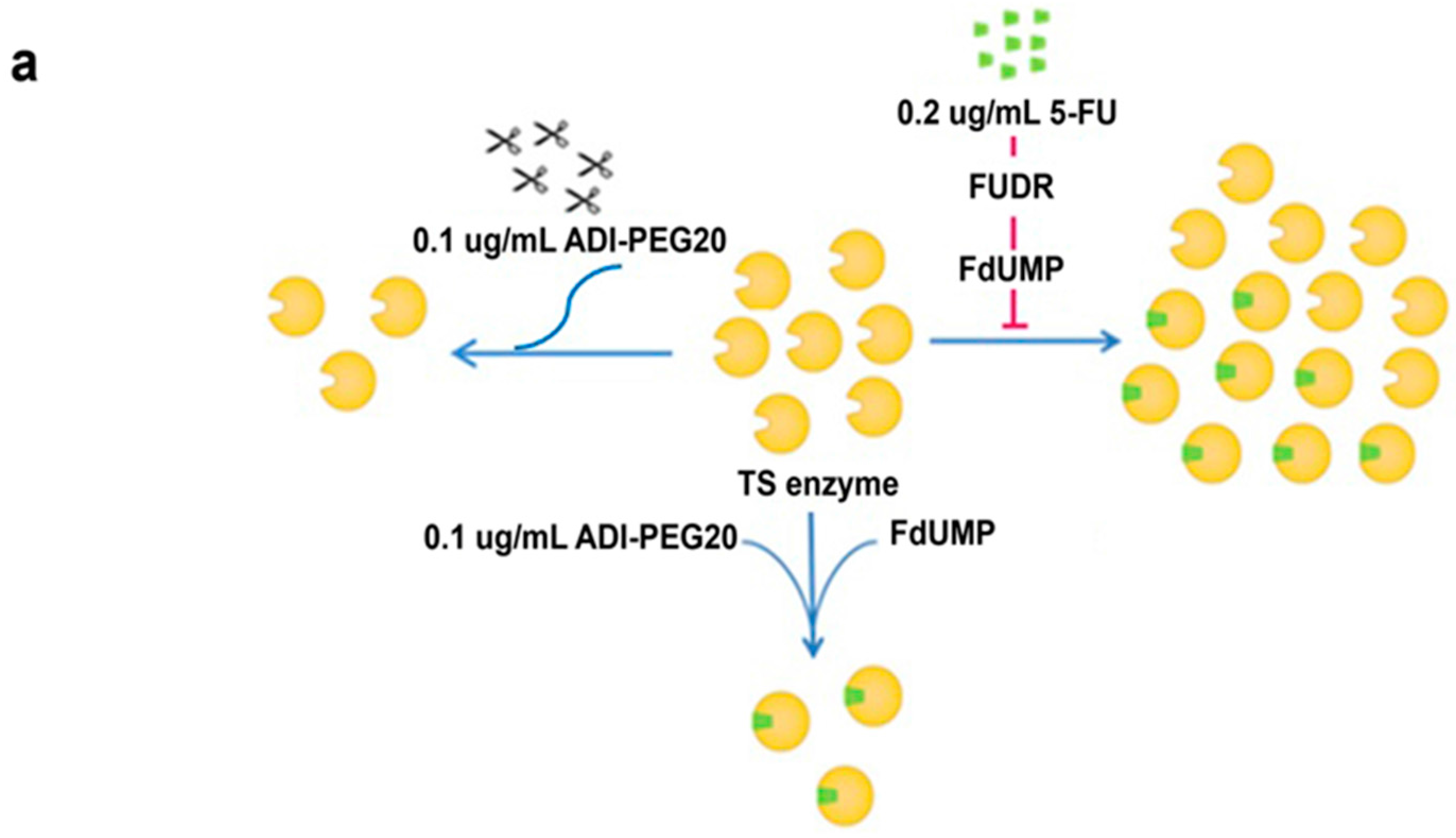
2.5. Effects of ADI-PEG20 on the Key Enzymes Involved in 5-FU Sensitivity
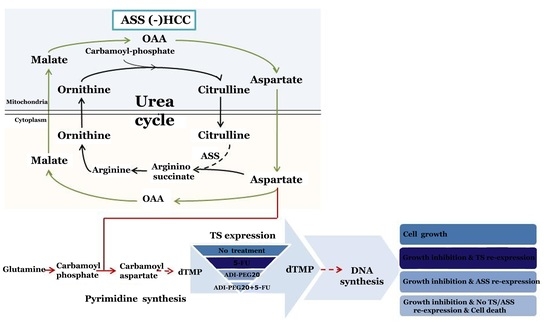
2.6. Reconstitution of ASS Expression Affect the Sensitivity to Combination Treatment with ADI-PEG20 Plus 5-FU Treatment as Well as the Enzyme Involved in the De Novo Pyrimidine Synthesis
2.7. ASS Expression in HCC Specimens from Thai Patients
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Culture
4.2. Reagents
4.3. Growth Inhibitory Assay
4.4. Reverse Transcription and Real Time PCR
4.5. Western Blotting
4.6. Apoptosis Assay
4.7. ASS Overexpression
4.8. Immunohistochemistry
4.9. Patient and Tissue Samples
4.10. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ADI-PEG20 | Pegylated arginine deiminase |
| dUMP | Deoxyuridine monophosphate |
| FDUMP | Fluorodeoxyuridine monophosphase |
| TS | Thymidylate synthase |
| DPYD | Dihydropyrimidine dehydrogenase |
| CPS I | Carbamoyl phosphate synthase I |
| OTC | Ornithine transcabamoylase |
| AAT | Aspartate aminotransferase |
| MDH 1 | Malate dehydrogenase 1 |
| MDH 2 | Malate dehydrogenase 2 |
| CAD | Carbamoyl-phosphate synthase 2, aspartate trancarbamylase and dihydrooratase complex |
| Ph-CAD | Phosphorylated-carbamoyl-phosphate synthase 2, aspartate trancarbamylase and dihydrooratase complex |
References
- Alazawi, W.; Cunningham, M.; Dearden, J.; Foster, G.R. Systematic review: Outcome of compensated cirrhosis due to chronic hepatitis C infection. Aliment. Pharmacol. Ther. 2010, 32, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Shin, H.R.; Bray, F.; Forman, D.; Mathers, C.; Parkin, D.M. Estimates of worldwide burden of cancer in 2008: Globocan 2008. Int. J. Cancer 2010, 127, 2893–2917. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.X.; Seto, W.-K.; Lai, C.-L.; Yuen, M.-F. Epidemiology of hepatocellular carcinoma in the Asia-Pacific region. Gut Liver 2016, 10, 332–339. [Google Scholar] [CrossRef] [PubMed]
- International Atomic Energy Agency (IAEA). International and Practices in Diagnosis and Treatment of Hepatocellular carcinoma: Idea Human Health Series No. 10; IAEA Publishing Section: Vienna, Austria, 2010. [Google Scholar]
- McDonald, M.; Robin, P.; Susan, W.; Pitman, L. The Burden of Cancer in Asia; Pfizer Inc.: New York City, NY, USA, 2008; pp. 1–84. [Google Scholar]
- World Health Organization. Global Cancer Facts & Figures, 2nd ed.; American Cancer Society Inc.: Atlanta, GA, USA, 2011; pp. 1–52. [Google Scholar]
- Liver Cancer: Statistics. Available online: http://www.cancer.net/cancer-types/liver-cancer/statistics (accessed on 22 April 2017).
- Bismuth, H.; Chiche, L.; Adam, R.; Castaing, D.; Diamond, T.; Dennison, A. Liver resection versus transplantation for hepatocellular carcinoma in cirrhotic patients. Ann. Surg. 1993, 218, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Ryder, S. Guidelines for the diagnosis and treatment of hepatocellular carcinoma (HCC) in adults. Gut 2003, 52, 1–8. [Google Scholar] [CrossRef]
- Deng, L.; Ren, Z.; Jia, Q.; Wu, W.; Shen, H.; Wang, Y. Schedule-dependent antitumor effects of 5-fluorouracil combined with sorafenib in hepatocellular carcinoma. BMC Cancer 2013, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.-F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.-L.; Forner, A.; et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.D.; Luong, P.; Hudson, C.; Leyton, J.; Delage, B.; Ghazaly, E.; Cutts, R.; Yuan, M.; Syed, N.; Lo Nigro, C.; et al. Prognostic and therapeutic impact of argininosuccinate synthetase 1 control in bladder cancer as monitored longitudinally by pet imaging. Cancer Res. 2014, 74, 896–907. [Google Scholar] [CrossRef] [PubMed]
- Savaraj, N.; You, M.; Wu, C.; Wangpaichitr, M.; Kuo, M.T.; Feun, L.G. Arginine deprivation, autophagy, apoptosis (AAA) for the treatment of melanoma. Curr. Mol. Med. 2010, 10, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Feun, L.; You, M.; Wu, C.J.; Kuo, M.T.; Wangpaichitr, M.; Spector, S.; Savaraj, N. Arginine deprivation as a targeted therapy for cancer. Curr.Pharm. Des. 2008, 14, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Savaraj, N.; Wu, C.; Li, Y.Y.; Wangpaichitr, M.; You, M.; Bomalaski, J.; He, W.; Kuo, M.T.; Feun, L.G. Targeting argininosuccinate synthetase negative melanomas using combination of arginine degrading enzyme and cisplatin. Oncotarget 2015, 6, 6295–6309. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Li, L.; Meng, S.; Qi, R.; Mao, Z.; Lin, M. Expression of argininosuccinate synthetase in patients with hepatocellular carcinoma. J. Gastroenterol. Hepatol. 2013, 28, 365–368. [Google Scholar] [CrossRef] [PubMed]
- Feun, L.G.; Kuo, M.T.; Savaraj, N. Arginine deprivation in cancer therapy. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Savaraj, N.; Wu, C.; Kuo, M.T.; You, M.; Wangpaichitr, M.; Robles, C.; Spector, S.; Feun, L. The relationship of arginine deprivation, argininosuccinate synthetase and cell death in melanoma. Drug Target Insights 2007, 2, 119–128. [Google Scholar] [PubMed]
- Uchibori, K.; Kasamatsu, A.; Sunaga, M.; Yokota, S.; Sakurada, T.; Kobayashi, E.; Yoshikawa, M.; Uzawa, K.; Ueda, S.; Tanzawa, H.; et al. Establishment and characterization of two 5-fluorouracil-resistant hepatocellular carcinoma cell lines. Int. J. Oncol. 2012, 40, 1005–1010. [Google Scholar] [PubMed]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Kinouchi, M.; Ishida, K.; Fujibuchi, W.; Naitoh, T.; Ogawa, H.; Ando, T.; Yazaki, N.; Watanabe, K.; Haneda, S.; et al. 5-FU metabolism in cancer and orally-administrable 5-FU drugs. Cancers 2010, 2, 1717–1730. [Google Scholar] [CrossRef] [PubMed]
- Dillon, B.J.; Prieto, V.G.; Curley, S.A.; Ensor, C.M.; Holtsberg, F.W.; Bomalaski, J.S.; Clark, M.A. Incidence and distribution of argininosuccinate synthetase deficiency in human cancers: A method for identifying cancers sensitive to arginine deprivation. Cancer 2004, 100, 826–833. [Google Scholar] [CrossRef] [PubMed]
- McAlpine, J.A.; Lu, H.-T.; Wu, K.C.; Knowles, S.K.; Thomson, J.A. Down-regulation of argininosuccinate synthetase is associated with cisplatin resistance in hepatocellular carcinoma cell lines: Implications for pegylated arginine deiminase combination therapy. BMC Cancer 2014, 14, 621. [Google Scholar] [CrossRef] [PubMed]
- You, M.; Savaraj, N.; Wangpaichitr, M.; Wu, C.; Kuo, T.M.; Varona-Santos, J.; Nguyen, D.; Feun, L. The combination of ADI-PEG20 and trail effectively increases cell death in melanoma cell lines. Biochem. Biophys. Res. Commun. 2010, 394, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Koff, J.; Ramachandiran, S.; Bernal-Mizrachi, L. A time to kill: Targeting apoptosis in cancer. Int. J. Mol. Sci. 2015, 16, 2942. [Google Scholar] [CrossRef] [PubMed]
- Rabinovich, S.; Adler, L.; Yizhak, K.; Sarver, A.; Silberman, A.; Agron, S.; Stettner, N.; Sun, Q.; Brandis, A.; Helbling, D.; et al. Diversion of aspartate in ASS1-deficient tumours fosters de novo pyrimidine synthesis. Nature 2015, 527, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Diasio, R. The role of dihydropyrimidine dehydrogenase (DPD) modulation in 5-FU pharmacology. Oncology 1998, 12, 23–27. [Google Scholar] [PubMed]
- Noguchi, T.; Tanimoto, K.; Shimokuni, T.; Ukon, K.; Tsujimoto, H.; Fukushima, M.; Noguchi, T.; Kawahara, K.; Hiyama, K.; Nishiyama, M. Aberrant methylation of DPYD promoter, DPYD expression, and cellular sensitivity to 5-fluorouracil in cancer cells. Clin. Cancer Res. 2004, 10, 7100–7107. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Zhu, Z.G.; Ji, Y.B.; Zhang, Y.; Yu, Y.Y.; Liu, B.Y.; Yin, H.R.; Lin, Y.Z. Correlation of thymidylate synthase, thymidine phosphorylase and dihydropyrimidine dehydrogenase with sensitivity of gastrointestinal cancer cells to 5-fluorouracil and 5-fluoro-2′-deoxyuridine. World J. Gastroenterol. 2004, 10, 172–176. [Google Scholar] [PubMed]
- Diasio, R.B. Clinical implications of dihydropyrimidine dehydrogenase on 5-Fu pharmacology. Oncology 2001, 15, 21–26. [Google Scholar] [PubMed]
- Henricks, L.M.; Lunenburg, C.A.; Meulendijks, D.; Gelderblom, H.; Cats, A.; Swen, J.J.; Schellens, J.H.; Guchelaar, H.J. Translating DPYD genotype into DPD phenotype: Using the DPYD gene activity score. Pharmacogenomics 2015, 16, 1277–1286. [Google Scholar] [CrossRef] [PubMed]
- Salonga, D.; Danenberg, K.D.; Johnson, M.; Metzger, R.; Groshen, S.; Tsao-Wei, D.D.; Lenz, H.J.; Leichman, C.G.; Leichman, L.; Diasio, R.B.; et al. Colorectal tumors responding to 5-fluorouracil have low gene expression levels of dihydropyrimidine dehydrogenase, thymidylate synthase, and thymidine phosphorylase. Clin. Cancer Res. 2000, 6, 1322–1327. [Google Scholar] [PubMed]
- Liu, H.; Dong, H.; Robertson, K.; Liu, C. DNA methylation suppresses expression of the urea cycle enzyme carbamoyl phosphate synthetase 1 (CPS1) in human hepatocellular carcinoma. Am. J. Pathol. 2011, 178, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Feun, L.G.; Marini, A.; Walker, G.; Elgart, G.; Moffat, F.; Rodgers, S.E.; Wu, C.J.; You, M.; Wangpaichitr, M.; Kuo, M.T.; et al. Negative argininosuccinate synthetase expression in melanoma tumours may predict clinical benefit from arginine-depleting therapy with pegylated arginine deiminase. Br. J. Cancer 2012, 106, 1481–1485. [Google Scholar] [CrossRef] [PubMed]
- Ascierto, P.A.; Scala, S.; Castello, G.; Daponte, A.; Simeone, E.; Ottaiano, A.; Beneduce, G.; de Rosa, V.; Izzo, F.; Melucci, M.T.; et al. Pegylated arginine deiminase treatment of patients with metastatic melanoma: Results from phase I and II studies. J. Clin. Oncol. 2005, 23, 7660–7668. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.S.; Lu, S.N.; Chao, Y.; Sheen, I.S.; Lin, C.C.; Wang, T.E.; Chen, S.C.; Wang, J.H.; Liao, L.Y.; Thomson, J.A.; et al. A randomised phase II study of pegylated arginine deiminase (ADI-PEG20) in asian advanced hepatocellular carcinoma patients. Br. J. Cancer 2010, 103, 954–960. [Google Scholar] [CrossRef] [PubMed]
- Feun, L.G.; Savaraj, N.; Wu, C.; You, M.; Wangpaichitr, M.; Marini, A.; Levi, D.; Bomalaski, J.; Kuo, M.T. Clinical and pharmacologic study of ADI-PEG20 in hepatocellular carcinoma. J. Clin. Oncol. 2008, 26, 15584. [Google Scholar] [CrossRef]
- Tsai, W.B.; Aiba, I.; Lee, S.Y.; Feun, L.; Savaraj, N.; Kuo, M.T. Resistance to arginine deiminase treatment in melanoma cells is associated with induced argininosuccinate synthetase expression involving c-myc/HIF-1α/SP4. Mol. Cancer Ther. 2009, 8, 3223–3233. [Google Scholar] [CrossRef] [PubMed]
- Ben-Sahra, I.; Howell, J.J.; Asara, J.M.; Manning, B.D. Stimulation of de novo pyrimidine synthesis by growth signaling through mtor and S6K1. Science 2013, 339, 1323–1328. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.U.A.; Lin, M.; Xiong, F.X.; Yang, Y.U.; Nie, X.I.U.; Zhou, R.L. Reduced expression of ass is closely related to clinicopathological features and post-resectional survival of hepatocellular carcinoma. Oncol. Lett. 2010, 1, 31–36. [Google Scholar] [PubMed]
- Yoon, C.Y.; Shim, Y.J.; Kim, E.H.; Lee, J.H.; Won, N.H.; Kim, J.H.; Park, I.S.; Yoon, D.K.; Min, B.H. Renal cell carcinoma does not express argininosuccinate synthetase and is highly sensitive to arginine deprivation via arginine deiminase. Int. J. Cancer 2007, 120, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.S.; Lim, K.H.; Tan, H.T.; Khoo, M.L.; Tan, S.H.; Toh, H.C.; Ching Ming Chung, M. Novel proteomic biomarker panel for prediction of aggressive metastatic hepatocellular carcinoma relapse in surgically resectable patients. J. Proteome Res. 2014, 13, 4833–4846. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.Y.; Wu, W.R.; Wang, Y.H.; Wang, J.W.; Fang, F.M.; Tsai, J.W.; Li, S.H.; Hung, H.C.; Yu, S.C.; Lan, J.; et al. ASS1 as a novel tumor suppressor gene in myxofibrosarcomas: Aberrant loss via epigenetic DNA methylation confers aggressive phenotypes, negative prognostic impact, and therapeutic relevance. Clin. Cancer Res. 2013, 19, 2861–2872. [Google Scholar] [CrossRef] [PubMed]
- El-Khoueiry, A.B.; Sangro, B.; Yau, T.; Crocenzi, T.S.; Kudo, M.; Hsu, C.; Kim, T.Y.; Choo, S.P.; Trojan, J.; Welling, T.H.; et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017. [Google Scholar] [CrossRef]
- Edmondson, H.A.; Steiner, P.E. Primary carcinoma of the liver: A study of 100 cases among 48,900 necropsies. Cancer 1954, 7, 462–503. [Google Scholar] [CrossRef]






| Cancer | Level of ASS Expression (Arbitrary Unit) | ||
|---|---|---|---|
| Normal Liver | HCC | Ratio (Tumor/Normal) | |
| HCC | 57.07 ± 1.47 a | 43.94 ± 1.46 * | 0.79 ± 0.03 |
| (N = 40) | 56.48 (39.38 − 83.44) b | 43.88 (18.21 − 75.23) | 0.81 (0.27 − 1.13) |
| Variables | Patients | ASS Expression Ratio a | p-Value b | |
|---|---|---|---|---|
| Low | High | |||
| All case | 40 | 18 | 22 | - |
| Gender | - | - | - | 0.673 |
| Male | 37 | 17 | 20 | - |
| Female | 3 | 1 | 2 | - |
| Age (years) | - | - | - | 0.251 |
| <60 | 25 | 13 | 12 | - |
| ≥60 | 15 | 5 | 10 | - |
| Viral status (Hepatitis B or C) | - | - | - | 0.165 |
| Yes | 29 | 15 | 14 | - |
| No | 11 | 3 | 8 | - |
| Tumor differentiation | - | - | - | 0.032 |
| Grade 1 | 7 | 1 | 6 | - |
| Grade 2 | 14 | 4 | 10 | - |
| Grade 3 | 14 | 9 | 5 | - |
| Grade 4 | 5 | 4 | 1 | - |
| Lympho-vascular invasion | - | - | - | 0.533 |
| Yes | 6 | 2 | 4 | - |
| No | 34 | 16 | 18 | - |
| Lymph node metastasis | - | - | - | 0.386 |
| Yes | 14 | 5 | 9 | - |
| No | 26 | 13 | 13 | - |
| TNM stage | - | - | - | 0.072 |
| I–II | 14 | 9 | 5 | - |
| III–IV | 26 | 9 | 17 | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thongkum, A.; Wu, C.; Li, Y.-Y.; Wangpaichitr, M.; Navasumrit, P.; Parnlob, V.; Sricharunrat, T.; Bhudhisawasdi, V.; Ruchirawat, M.; Savaraj, N. The Combination of Arginine Deprivation and 5-Fluorouracil Improves Therapeutic Efficacy in Argininosuccinate Synthetase Negative Hepatocellular Carcinoma. Int. J. Mol. Sci. 2017, 18, 1175. https://doi.org/10.3390/ijms18061175
Thongkum A, Wu C, Li Y-Y, Wangpaichitr M, Navasumrit P, Parnlob V, Sricharunrat T, Bhudhisawasdi V, Ruchirawat M, Savaraj N. The Combination of Arginine Deprivation and 5-Fluorouracil Improves Therapeutic Efficacy in Argininosuccinate Synthetase Negative Hepatocellular Carcinoma. International Journal of Molecular Sciences. 2017; 18(6):1175. https://doi.org/10.3390/ijms18061175
Chicago/Turabian StyleThongkum, Angkana, Chunjing Wu, Ying-Ying Li, Medhi Wangpaichitr, Panida Navasumrit, Varabhorn Parnlob, Thaniya Sricharunrat, Vajarabhongsa Bhudhisawasdi, Mathuros Ruchirawat, and Niramol Savaraj. 2017. "The Combination of Arginine Deprivation and 5-Fluorouracil Improves Therapeutic Efficacy in Argininosuccinate Synthetase Negative Hepatocellular Carcinoma" International Journal of Molecular Sciences 18, no. 6: 1175. https://doi.org/10.3390/ijms18061175
APA StyleThongkum, A., Wu, C., Li, Y.-Y., Wangpaichitr, M., Navasumrit, P., Parnlob, V., Sricharunrat, T., Bhudhisawasdi, V., Ruchirawat, M., & Savaraj, N. (2017). The Combination of Arginine Deprivation and 5-Fluorouracil Improves Therapeutic Efficacy in Argininosuccinate Synthetase Negative Hepatocellular Carcinoma. International Journal of Molecular Sciences, 18(6), 1175. https://doi.org/10.3390/ijms18061175