The Lys-Asp-Tyr Triad within the Mite Allergen Der p 1 Propeptide Is a Critical Structural Element for the pH-Dependent Initiation of the Protease Maturation
Abstract
:1. Introduction
2. Results
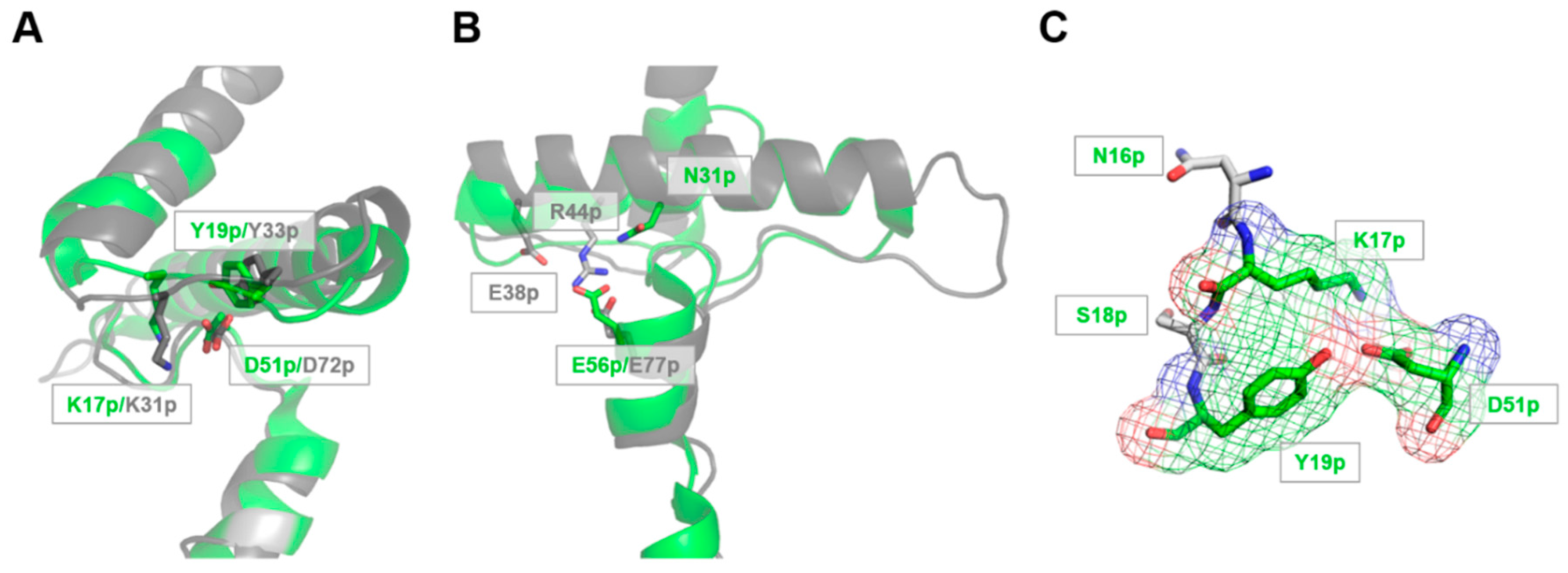
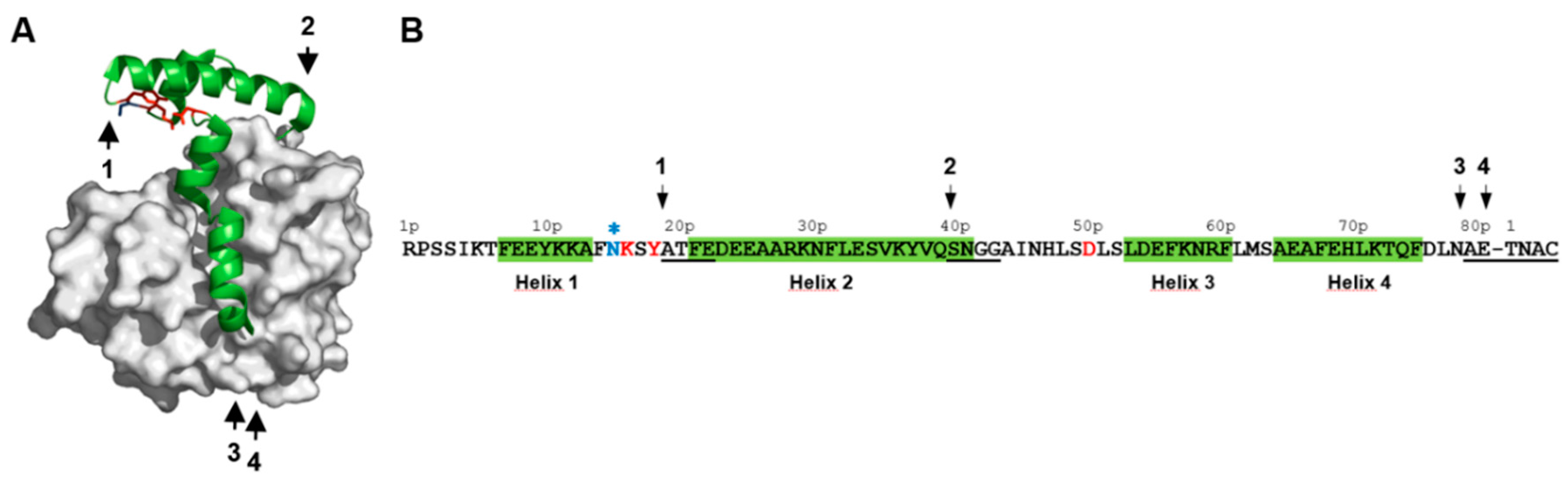
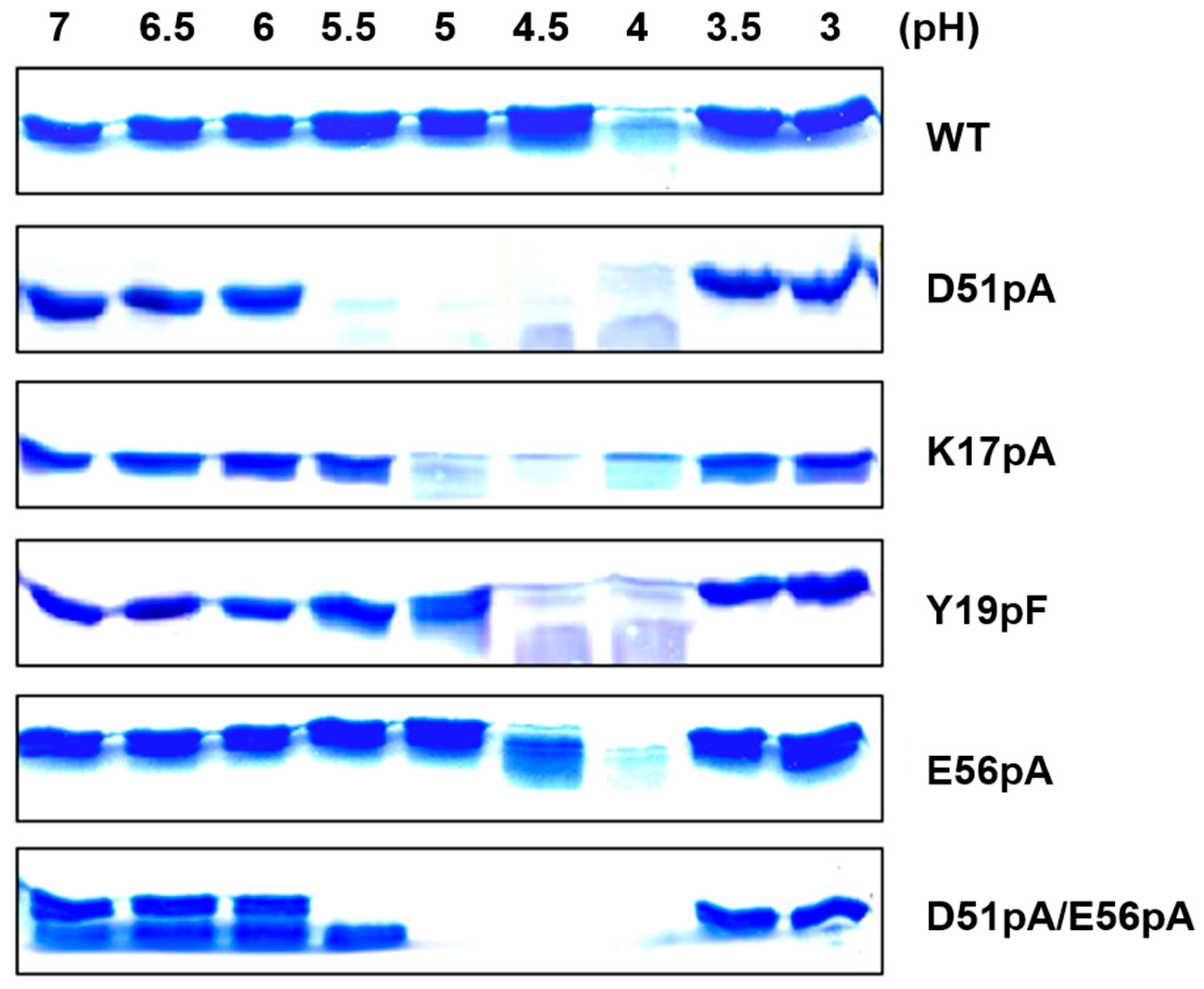
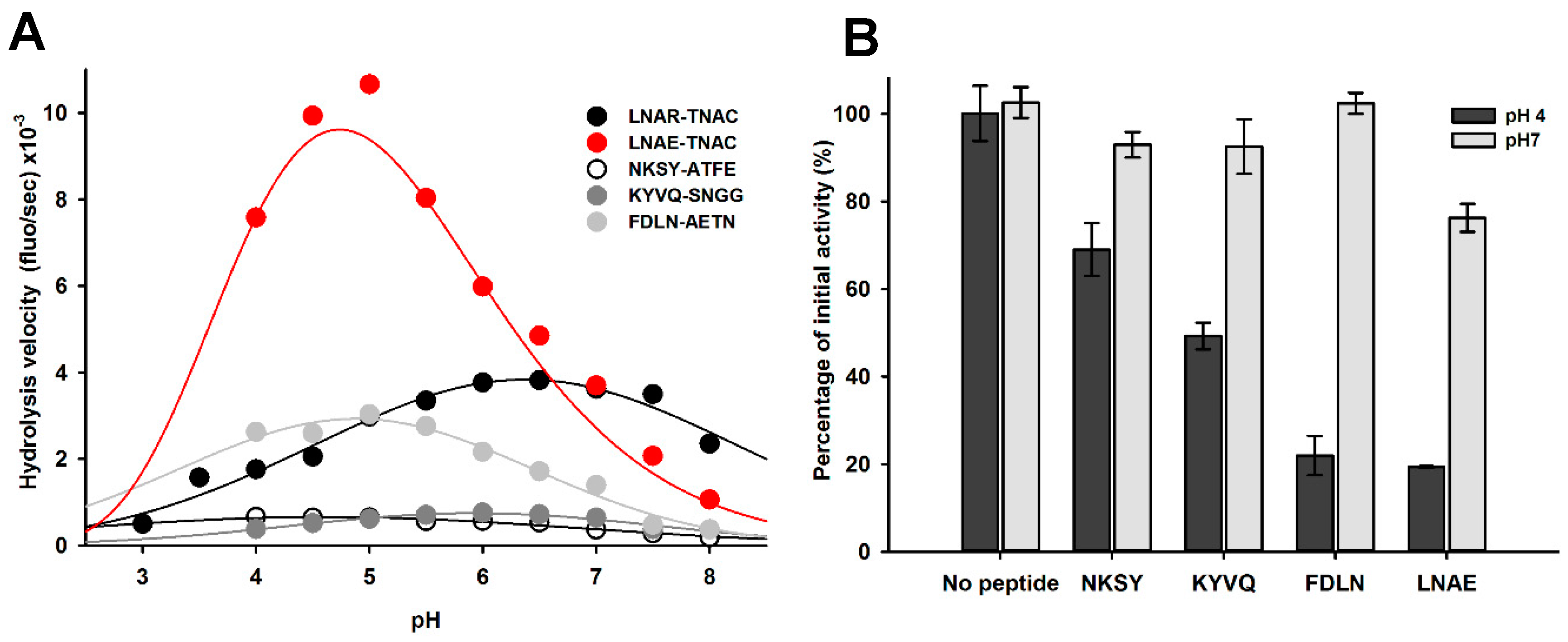
2.1. Identification of pH-Responsive Elements within the Der p 1 Propeptide N-Terminal Domain
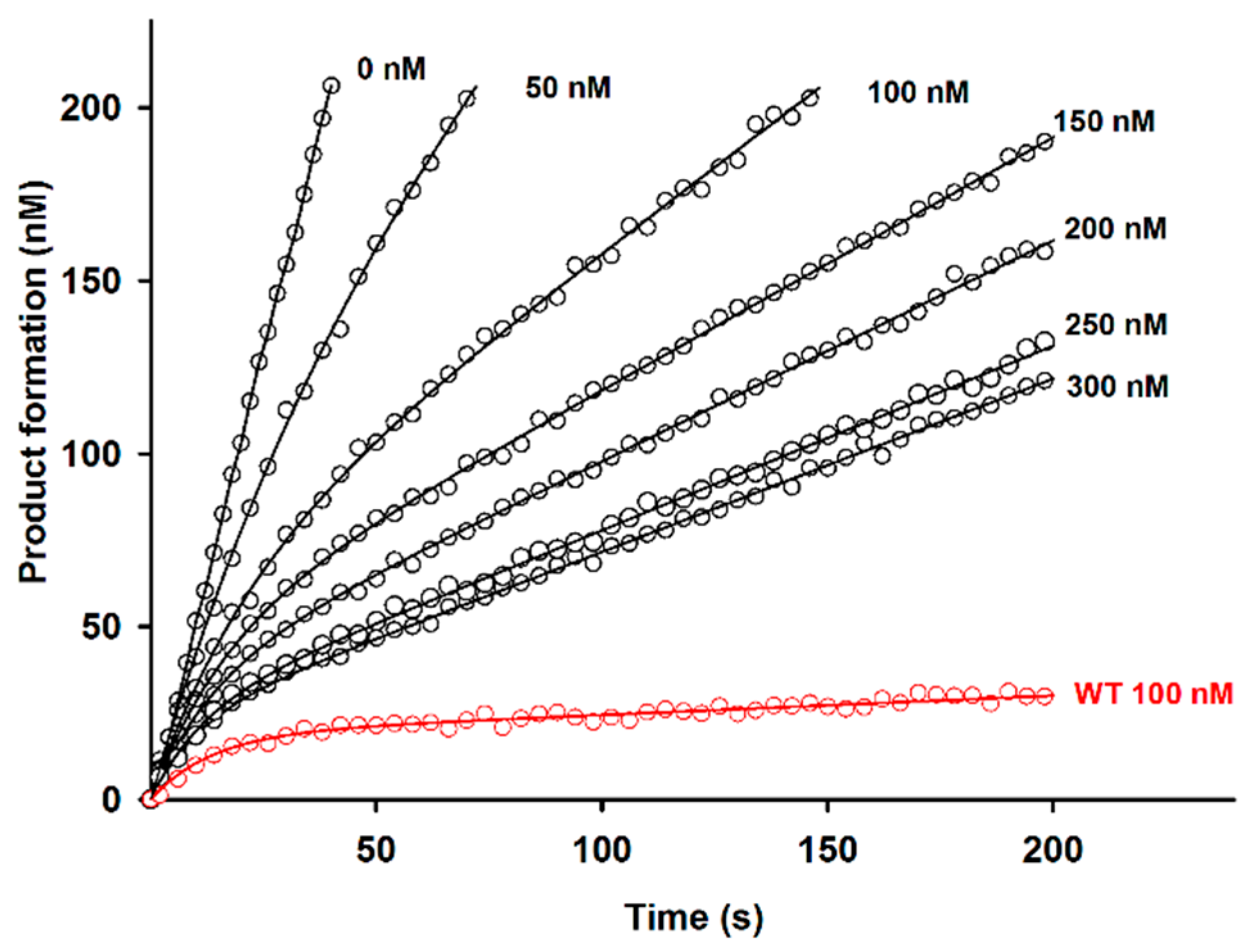
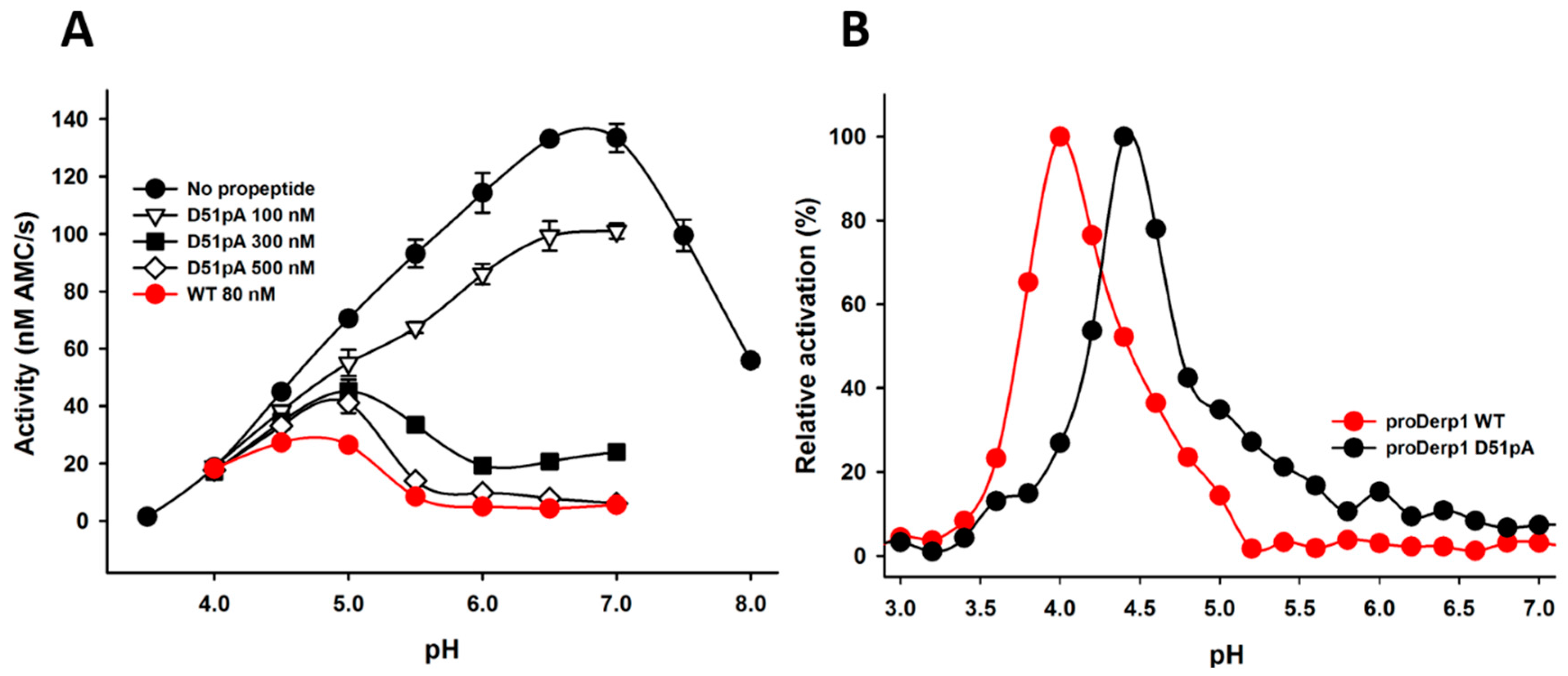
2.2. Effect of D51pA Mutation on the pH Dependence of Propeptide Inhibition and Zymogen Activation
2.3. Impact of Mutations on the Propeptide Proteolytic Degradation by Der p 1
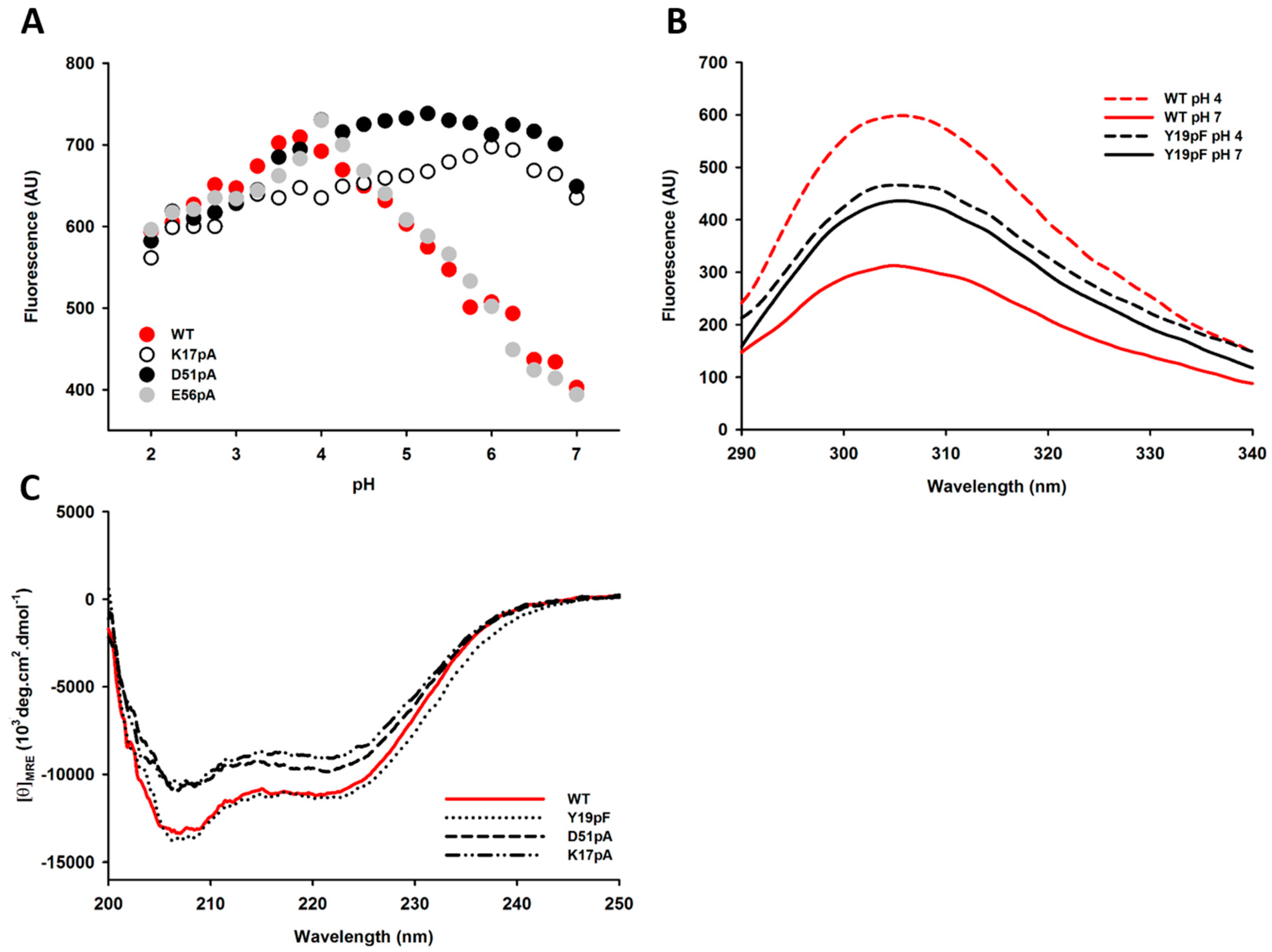
2.4. Impact of Mutations on the Tertiary and Secondary Structures of the Propeptide
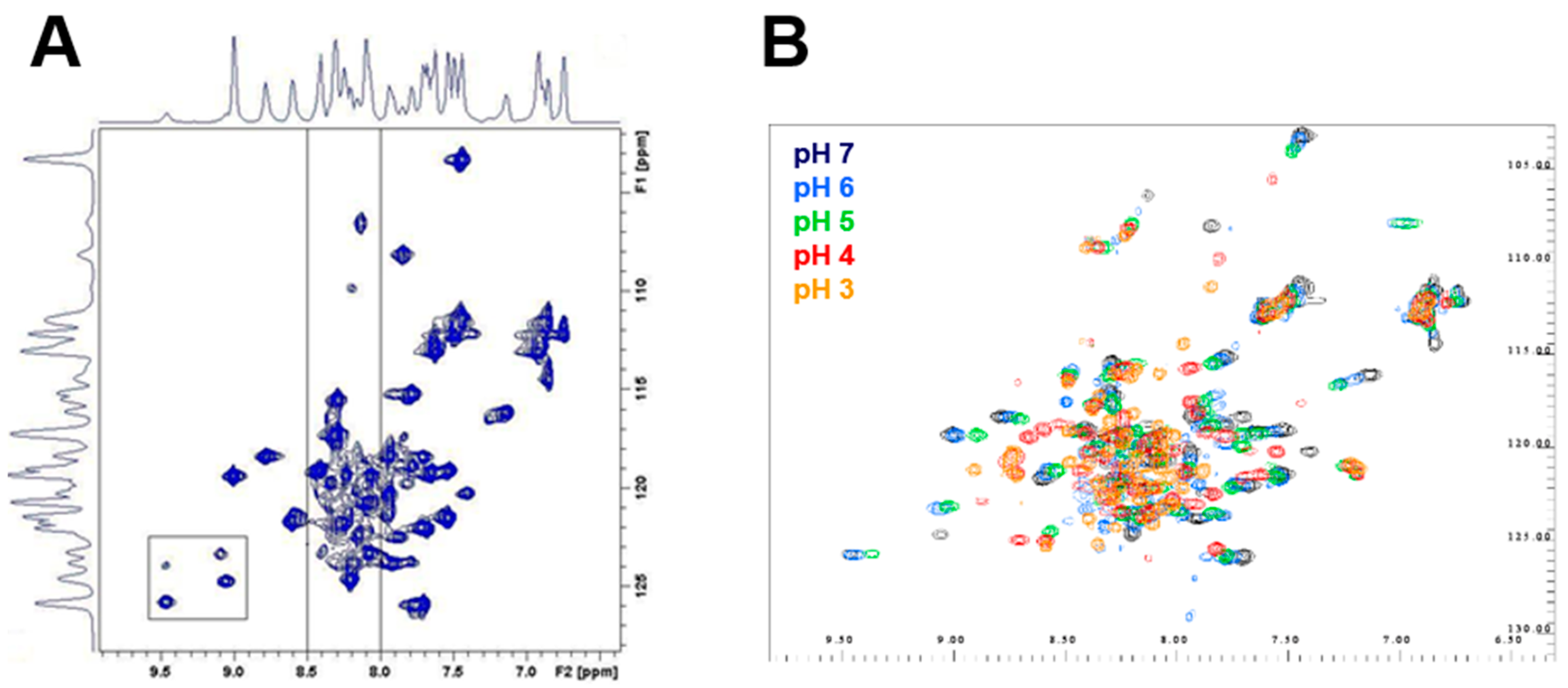
2.5. Differential Flexibility and Compactness of WT and D51pA Propeptides
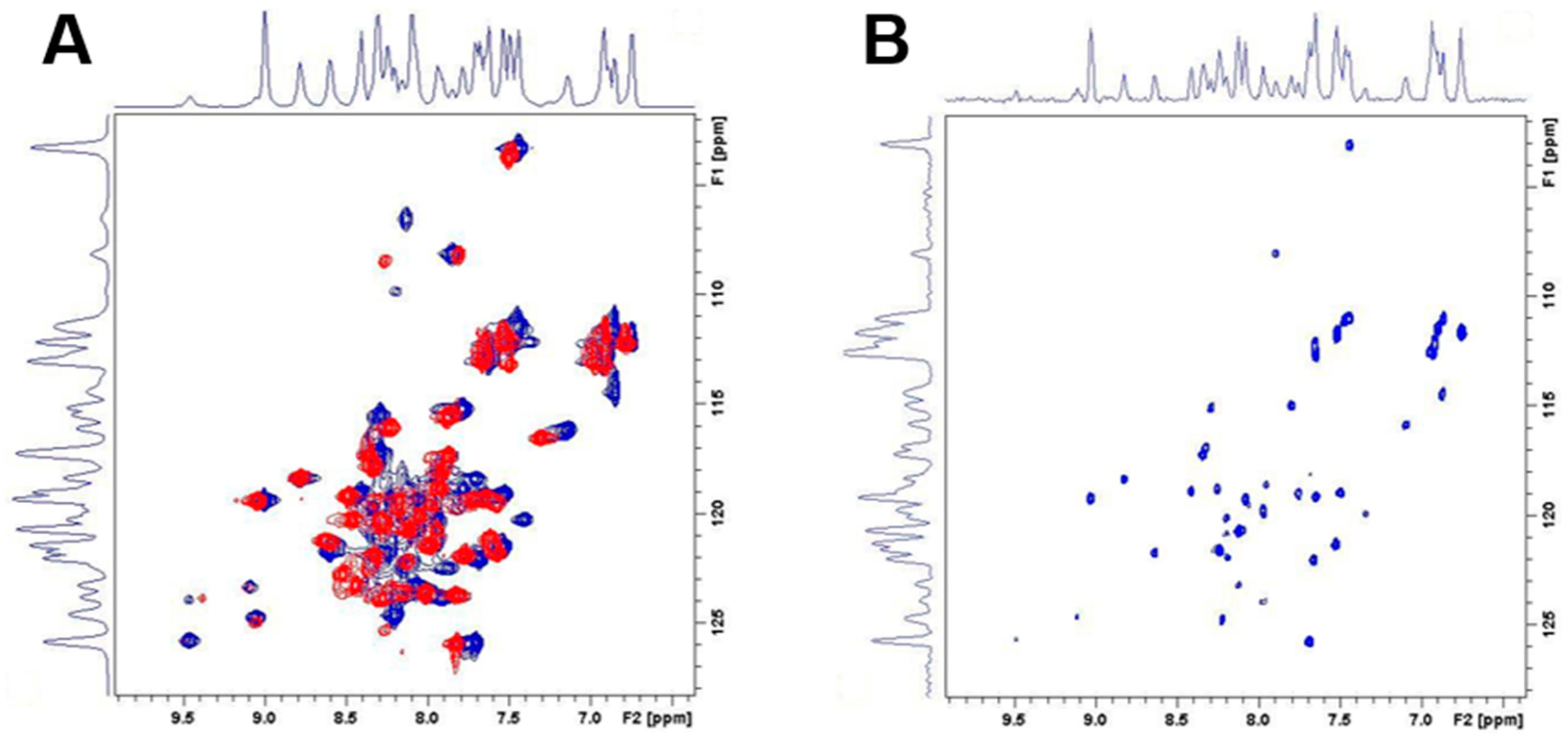
2.6. Impact of pH on proDer p 1 Activation Site Recognition and Proteolysis
3. Discussion
3.1. The Lys17p-Asp51p-Tyr19p Triad Is the Major Structural pH-Responsive Element in Der p 1 Propeptide
3.2. The Release and Docking of the Asn16p-Lys17p-Ser18p-Tyr19p Sequence Regulates the Activation Process
4. Materials and Methods
4.1. Expression of Der p 1 Propeptide Variants
4.2. Expression and Maturation of proDer p 1 Variants
4.3. Propeptide Degradation Assay
4.4. Inhibition of the Recombinant Der p 1 Enzymatic Activity by Propeptide Variants
4.5. Proteolytic Activity of Der p 1 towards proDer p 1 Activation Site-Based FRET Substrates and Inhibition of Der p 1 Activity by Tetrapeptides Mimicking the Intermediate Processing Sites
4.6. Intrinsic Fluorescence Measurements of Der p 1 Propeptide
4.7. Far-UV Circular Dichroism Spectroscopy
4.8. Nuclear Magnetic Resonance Spectroscopy
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Thomas, W.R. Hierarchy and molecular properties of house dust mite allergens. Allergol. Int. 2015, 64, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Chapman, M.D.; Rowntree, S.; Mitchell, E.B.; di Prisco de Fuenmajor, M.C.; Platts-Mills, T.A. Quantitative assessments of IgG and IgE antibodies to inhalant allergens in patients with atopic dermatitis. J. Allergy Clin. Immunol. 1983, 72, 27–33. [Google Scholar] [CrossRef]
- Platts-Mills, T.A.; Chapman, M.D. Dust mites: Immunology, allergic disease, and environmental control. J. Allergy Clin. Immunol. 1987, 80, 755–775. [Google Scholar] [CrossRef]
- Shakib, F.; Schulz, O.; Sewell, H. A mite subversive: Cleavage of CD23 and CD25 by Der p 1 enhances allergenicity. Immunol. Today 1998, 19, 313–316. [Google Scholar] [CrossRef]
- Wan, H.; Winton, H.L.; Soeller, C.; Tovey, E.R.; Gruenert, D.C.; Thompson, P.J.; Stewart, G.A.; Taylor, G.W.; Garrod, D.R.; Cannell, M.B.; et al. Der p 1 facilitates transepithelial allergen delivery by disruption of tight junctions. J. Clin. Investig. 1999, 104, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, Y.; Takai, T.; Kuhara, T.; Ota, M.; Kato, T.; Hatanaka, H.; Ichikawa, S.; Tokura, T.; Akiba, H.; Mitsuishi, K.; et al. Crucial commitment of proteolytic activity of a purified recombinant major house dust mite allergen Der p1 to sensitization toward IgE and IgG responses. J. Immunol. 2006, 177, 1609–1617. [Google Scholar] [CrossRef] [PubMed]
- Dumez, M.E.; Herman, J.; Campizi, V.; Galleni, M.; Jacquet, A.; Chevigne, A. Orchestration of an uncommon maturation cascade of the house dust mite protease allergen quartet. Front. Immunol. 2014, 5, 138. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.; Thelen, N.; Smargiasso, N.; Mailleux, A.C.; Luxen, A.; Cloes, M.; de Pauw, E.; Chevigné, A.; Galleni, M.; Dumez, M.E. Der p 1 is the primary activator of Der p 3, Der p 6 and Der p 9 the proteolytic allergens produced by the house dust mite Dermatophagoides pteronyssinus. Biochim. Biophys. Acta 2014, 1840, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Dumez, M.E.; Teller, N.; Mercier, F.; Tanaka, T.; Vandenberghe, I.; Vandenbranden, M.; Devreese, B.; Luxen, A.; Frère, J.M.; Matagne, A.; et al. Activation mechanism of recombinant Der p 3 allergen zymogen: Contribution of cysteine protease Der p 1 and effect of propeptide glycosylation. J. Biol. Chem. 2008, 283, 30606–30617. [Google Scholar] [CrossRef] [PubMed]
- Gough, L.; Schulz, O.; Sewell, H.F.; Shakib, F. The cysteine protease activity of the major dust mite allergen Der p 1 selectively enhances the immunoglobulin E antibody response. J. Exp. Med. 1999, 190, 1897–1902. [Google Scholar] [CrossRef] [PubMed]
- Jacquet, A. The role of the house dust mite-induced innate immunity in development of allergic response. Int. Arch. Allergy Immunol. 2011, 155, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Jacquet, A. The role of innate immunity activation in house dust mite allergy. Trends Mol. Med. 2011, 17, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Chua, K.Y.; Stewart, G.A.; Thomas, W.R.; Simpson, R.J.; Dilworth, R.J.; Plozza, T.M.; Turner, K.J. Sequence analysis of cDNA coding for a major house dust mite allergen, Der p 1. Homology with cysteine proteases. J. Exp. Med. 1988, 167, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Takai, T.; Kato, T.; Yasueda, H.; Okumura, K.; Ogawa, H. Analysis of the structure and allergenicity of recombinant pro- and mature Der p 1 and Der f 1: Major conformational IgE epitopes blocked by prodomains. J. Allergy Clin. Immunol. 2005, 115, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Chevigne, A.; Barumandzadeh, R.; Groslambert, S.; Cloes, B.; Dehareng, D.; Filee, P.; Marx, J.C.; Frère, J.M.; Matagne, A.; Jacquet, A.; et al. Relationship between propeptide pH unfolding and inhibitory ability during ProDer p 1 activation mechanism. J. Mol. Biol. 2007, 374, 170–185. [Google Scholar] [CrossRef] [PubMed]
- Huete-Perez, J.A.; Engel, J.C.; Brinen, L.S.; Mottram, J.C.; McKerrow, J.H. Protease trafficking in two primitive eukaryotes is mediated by a prodomain protein motif. J. Biol. Chem. 1999, 274, 16249–16256. [Google Scholar] [CrossRef] [PubMed]
- Karrer, K.M.; Peiffer, S.L.; DiTomas, M.E. Two distinct gene subfamilies within the family of cysteine protease genes. Proc. Natl. Acad. Sci. USA 1993, 90, 3063–3067. [Google Scholar] [CrossRef] [PubMed]
- Cygler, M.; Mort, J.S. Proregion structure of members of the papain superfamily. Mode of inhibition of enzymatic activity. Biochimie 1997, 79, 645–652. [Google Scholar] [CrossRef]
- Groves, M.R.; Coulombe, R.; Jenkins, J.; Cygler, M. Structural basis for specificity of papain-like cysteine protease proregions toward their cognate enzymes. Proteins 1998, 32, 504–514. [Google Scholar] [CrossRef]
- Groves, M.R.; Taylor, M.A.; Scott, M.; Cummings, N.J.; Pickersgill, R.W.; Jenkins, J.A. The prosequence of procaricain forms an alpha-helical domain that prevents access to the substrate-binding cleft. Structure 1996, 4, 1193–1203. [Google Scholar] [CrossRef]
- Guay, J.; Falgueyret, J.P.; Ducret, A.; Percival, M.D.; Mancini, J.A. Potency and selectivity of inhibition of cathepsin K, L and S by their respective propeptides. Eur. J. Biochem. 2000, 267, 6311–6318. [Google Scholar] [CrossRef] [PubMed]
- Meno, K.; Thorsted, P.B.; Ipsen, H.; Kristensen, O.; Larsen, J.N.; Spangfort, M.D.; Gajhede, M.; Lund, K. The crystal structure of recombinant proDer p 1, a major house dust mite proteolytic allergen. J. Immunol. 2005, 175, 3835–3845. [Google Scholar] [CrossRef] [PubMed]
- McQueney, M.S.; Amegadzie, B.Y.; D’Alessio, K.; Hanning, C.R.; McLaughlin, M.M.; McNulty, D.; Carr, S.A.; Ijames, C.; Kurdyla, J.; Jones, C.S. Autocatalytic activation of human cathepsin K. J. Biol. Chem. 1997, 272, 13955–13960. [Google Scholar] [CrossRef] [PubMed]
- Menard, R.; Carmona, E.; Takebe, S.; Dufour, E.; Plouffe, C.; Mason, P.; Mort, J.S. Autocatalytic processing of recombinant human procathepsin L. Contribution of both intermolecular and unimolecular events in the processing of procathepsin L in vitro. J. Biol. Chem. 1998, 273, 4478–4484. [Google Scholar] [CrossRef] [PubMed]
- Takai, T.; Mineki, R.; Nakazawa, T.; Takaoka, M.; Yasueda, H.; Murayama, K.; Okumura, K.; Ogawa, H. Maturation of the activities of recombinant mite allergens Der p 1 and Der f 1, and its implication in the blockade of proteolytic activity. FEBS Lett. 2002, 531, 265–272. [Google Scholar] [CrossRef]
- Jacquet, A.; Magi, M.; Petry, H.; Bollen, A. High-level expression of recombinant house dust mite allergen Der p 1 in Pichia pastoris. Clin. Exp. Allergy 2002, 32, 1048–1053. [Google Scholar] [CrossRef] [PubMed]
- Van Oort, E.; de Heer, P.G.; van Leeuwen, W.A.; Derksen, N.I.; Muller, M.; Huveneers, S.; Aalberse, R.C.; van Ree, R. Maturation of Pichia pastoris-derived recombinant pro-Der p 1 induced by deglycosylation and by the natural cysteine protease Der p 1 from house dust mite. Eur. J. Biochem. 2002, 269, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Takai, T.; Mizuuchi, E.; Kikuchi, Y.; Nagamune, T.; Okumura, K.; Ogawa, H. Glycosylation of recombinant proforms of major house dust mite allergens Der p 1 and Der f 1 decelerates the speed of maturation. Int. Arch. Allergy Immunol. 2006, 139, 181–187. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, G.F.; Godbold, G.D.; Erickson, A.H. The pH-dependent membrane association of procathepsin L is mediated by a 9-residue sequence within the propeptide. J. Biol. Chem. 1994, 269, 567–572. [Google Scholar] [PubMed]
- Jerala, R.; Zerovnik, E.; Kidric, J.; Turk, V. pH-induced conformational transitions of the propeptide of human cathepsin L. A role for a molten globule state in zymogen activation. J. Biol. Chem. 1998, 273, 11498–11504. [Google Scholar] [CrossRef] [PubMed]
- Bromme, D.; Nallaseth, F.S.; Turk, B. Production and activation of recombinant papain-like cysteine proteases. Methods 2004, 32, 199–206. [Google Scholar] [CrossRef]
- Ishidoh, K.; Kominami, E. Procathepsin L degrades extracellular matrix proteins in the presence of glycosaminoglycans in vitro. Biochem. Biophys. Res. Commun. 1995, 217, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Kihara, M.; Kakegawa, H.; Matano, Y.; Murata, E.; Tsuge, H.; Kido, H.; Katunuma, N. Chondroitin sulfate proteoglycan is a potent enhancer in the processing of procathepsin L. Biol. Chem. 2002, 383, 1925–1929. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.; Mason, D.E.; Li, J.; Burdick, K.W.; Backes, B.J.; Chen, T.; Shipway, A.; van Heeke, G.; Gough, L.; Ghaemmaghami, A.; et al. Activity profile of dust mite allergen extract using substrate libraries and functional proteomic microarrays. Chem. Biol. 2004, 11, 1361–1372. [Google Scholar] [CrossRef] [PubMed]
- Chevigne, A.; Dumez, M.E.; Dumoulin, M.; Matagne, A.; Jacquet, A.; Galleni, M. Comparative study of mature and zymogen mite cysteine protease stability and pH unfolding. Biochim. Biophys. Acta 2010, 1800, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Tovey, E.R.; Baldo, B.A. Localization of antigens and allergens in thin sections of the house dust mite, Dermatophagoides pteronyssinus (Acari: Pyroglyphidae). J. Med. Entomol. 1990, 27, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Erban, T.; Hubert, J. Determination of pH in regions of the midguts of acaridid mites. J. Insect Sci. 2010, 10, 42. [Google Scholar] [CrossRef] [PubMed]
| Constants | WT | D51pA | K17pA | Y19pF | E56pA | D51pA/E56pA |
|---|---|---|---|---|---|---|
| kon (M−1·s−1 × 104) | 110 ± 2 | 44 ± 7 | 46 ± 4 | 120 ± 4 | 150 ± 5 | ND |
| koff (s−1 × 10−3) | 7.8 ± 1 | 17 ± 2 | 7 ± 1 | 7 ± 2 | 7 ± 3 | ND |
| KD Global (M × 10−9) | 7 ± 1 | 37 ± 5 | 15 ± 3 | 6 ± 2 | 5 ± 2 | 130 ± 6 |
| Cleavage Site | FRET Substrate | kcat/Km (min−1·mM−1) * |
|---|---|---|
| NKSY19p–A20pTFE | Dabcyl-NKSY↓ATFE-EDANS | 1221.7 ± 142.3 |
| KYVQ40p–S41pNGG | Dabcyl-KYVQ↓SNGG-EDANS | 2722.4 ± 119.7 |
| FDLN78p–A79pETN | Dabcyl-FDLN↓AETN-EDANS | 3863.0 ± 722.0 |
| LNAE80p–T1NAC | Dabcyl-LNAE↓TNAC-EDANS | 6614.2 ± 204.0 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chevigné, A.; Campizi, V.; Szpakowska, M.; Bourry, D.; Dumez, M.-E.; Martins, J.C.; Matagne, A.; Galleni, M.; Jacquet, A. The Lys-Asp-Tyr Triad within the Mite Allergen Der p 1 Propeptide Is a Critical Structural Element for the pH-Dependent Initiation of the Protease Maturation. Int. J. Mol. Sci. 2017, 18, 1087. https://doi.org/10.3390/ijms18051087
Chevigné A, Campizi V, Szpakowska M, Bourry D, Dumez M-E, Martins JC, Matagne A, Galleni M, Jacquet A. The Lys-Asp-Tyr Triad within the Mite Allergen Der p 1 Propeptide Is a Critical Structural Element for the pH-Dependent Initiation of the Protease Maturation. International Journal of Molecular Sciences. 2017; 18(5):1087. https://doi.org/10.3390/ijms18051087
Chicago/Turabian StyleChevigné, Andy, Vincenzo Campizi, Martyna Szpakowska, David Bourry, Marie-Eve Dumez, José C. Martins, André Matagne, Moreno Galleni, and Alain Jacquet. 2017. "The Lys-Asp-Tyr Triad within the Mite Allergen Der p 1 Propeptide Is a Critical Structural Element for the pH-Dependent Initiation of the Protease Maturation" International Journal of Molecular Sciences 18, no. 5: 1087. https://doi.org/10.3390/ijms18051087
APA StyleChevigné, A., Campizi, V., Szpakowska, M., Bourry, D., Dumez, M.-E., Martins, J. C., Matagne, A., Galleni, M., & Jacquet, A. (2017). The Lys-Asp-Tyr Triad within the Mite Allergen Der p 1 Propeptide Is a Critical Structural Element for the pH-Dependent Initiation of the Protease Maturation. International Journal of Molecular Sciences, 18(5), 1087. https://doi.org/10.3390/ijms18051087







