Synaptic Homeostasis and Its Immunological Disturbance in Neuromuscular Junction Disorders
Abstract
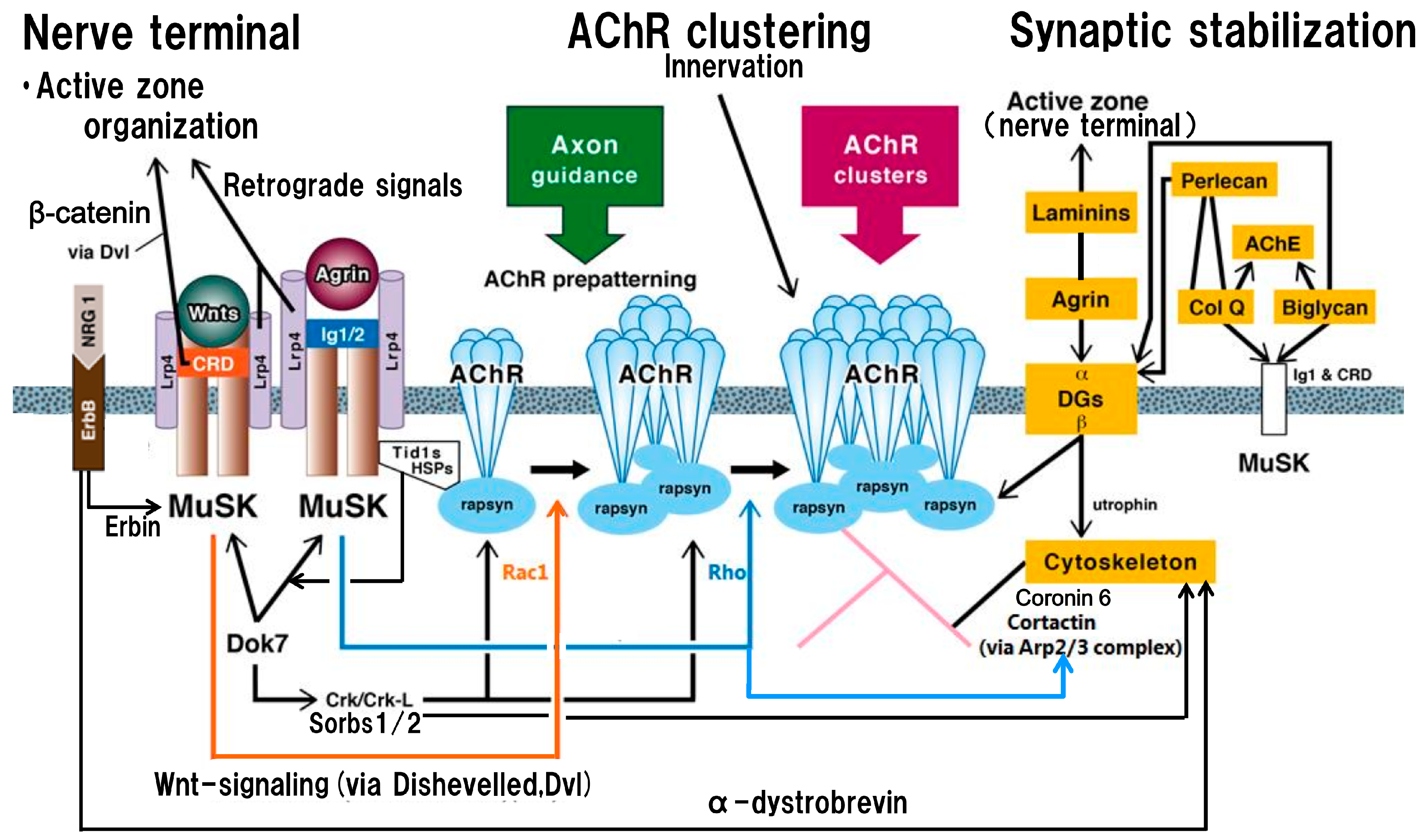
:1. Introduction
2. Postsynaptic Organizations, Centered on MuSK, Lrp4 and Synapse-Related Proteins
3. Trans-Synaptic Communication
4. Agrin, Cortaction and the Other Synapse-Related Proteins Contributing to the Modulation of Pre- and Postsynaptic Organizations
5. Conclusions
Conflicts of Interest
References
- Shi, L.; Fu, A.K.Y.; Ip, N.Y. Molecular mechanisms underlying maturation and maintenance of the vertebrate neuromuscular junction. Trends Neursci. 2012, 35, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Xiong, W.C.; Mei, L. To build a synapse: Signaling pathways in neuromuscular junction assembly. Development 2010, 137, 1017–1033. [Google Scholar] [CrossRef] [PubMed]
- Plomp, J.J.; van Kempen, G.T.; de Baets, M.B.; Graus, I.M.; Kuks, J.B.; Molenaar, P.C. Acetylcholine release in myasthenia gravis: Regulation at single end-plate level. Ann. Neurol. 1995, 37, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Klooster, R.; Plomp, J.J.; Huijbers, M.G.; Niks, E.H.; Straasheijm, K.R.; Detmers, F.J.; Hermans, P.W.; Sleijpens, A.; Verrips, A.; Losen, M.; et al. Muscle-specific kinase myasthenia gravis IgG4 autoantibodies cause severe neuromuscular junction dysfunction in mice. Brain 2012, 135 Pt 4, 1081–1101. [Google Scholar] [CrossRef] [PubMed]
- Viegas, S.; Jacobson, L.; Waters, P.; Cossins, J.; Jacob, S.; Leite, M.I.; Webster, R.; Vincent, A. Passive and active immunization models of MuSK-ab positive myasthenia: Electrophysiological evidence for pre and postsynaptic defects. Exp. Neurol. 2012, 234, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Koneczny, I.; Stevens, J.A.; de Rosa, A.; Huda, S.; Huijbers, M.G.; Saxena, A.; Maestri, M.; Lazaridis, S.K.; Zisimopoulou, P.; Tzartos, S.; et al. IgG4 autoantibodies against muscle-specific kinase undergo Fab-arm exchange in myasthenia gravis. J. Autoimmun. 2017, 77, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Hijbers, M.G.; Zhang, W.; Klooster, R.; Niks, E.H.; Friese, M.B.; Straasheijm, K.R.; Thijssen, P.E.; Vrolijk, Z.H.; Plomp, J.J.; Vogels, P.; et al. MuSK IgG4 autoantibodies cause myasthenia gravis by inhibiting binding between MuSK and Lrp4. Proc. Natl. Acad. Sci. USA 2013, 110, 20783–20788. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Lu, Y.; Zhang, B.; Figueiredo, D.; Bean, J.; Jung, J.; Wu, H.; Barik, A.; Yin, D.M.; Xiong, W.C. Antibodies against low-density lipoprotein receptor-related protein 4 induce myasthenia gravis. J. Clin. Investig. 2013, 123, 5190–5202. [Google Scholar] [CrossRef] [PubMed]
- Plomp, J.J.; Morsch, M.; Phillips, W.D.; Verschuuren, J.J. Electrophysiological analysis of neuromuscular synaptic function in myasthenia gravis patients and animal models. Exp. Neurol. 2015, 270, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Verschuuren, J.; Strijbos, E.; Vincent, A. Neuromuscular junction disorders. Handb. Clin. Neurol. 2016, 133, 447–466. [Google Scholar] [PubMed]
- Stiegler, A.L.; Burden, S.J.; Hubbard, S.R. Crystal structure of the frizzled-like cysteine domain of the receptor tyrosine kinase MuSK. J. Mol. Biol. 2009, 393, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Liang, C.; Bates, R.; Yin, Y.; Xiong, W.C.; Mei, L. Wnt proteins regulate acetylcholine receptor clustering in muscle cells. Mol. Brain 2012, 5, 7. [Google Scholar] [CrossRef] [PubMed]
- Koles, K.; Nunnari, J.; Korkut, C.; Barria, R.; Brewer, C.; Li, Y.; Leszyk, J.; Zhang, B.; Budnik, V. Mechanism of evenness interrupted (Evi)-exosome release at synaptic boutons. J. Biol. Chem. 2012, 287, 16820–16834. [Google Scholar] [CrossRef] [PubMed]
- Jing, L.; Lefebvre, J.L.; Gordon, L.R.; Granato, M. Wnt signals organize synaptic prepattern and axon guidance through the zebrafish unplugged/MuSK receptor. Neuron 2009, 61, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Henriquez, J.P.; Salinas, P.C. Dual roles for Wnt signaling during the formation of the vertebrate neuromuscular junction. Acta Physiol. 2012, 204, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Strochlic, L.; Falk, J.; Goillot, E.; Sigoillot, S.; Bourgeois, F.; Delers, P.; Rouviere, J.; Swain, A.; Castellani, V.; Schaeffer, L.; et al. Wnt4 participates in the formation of vertebrate neuromuscular junction. PLoS ONE 2012, 7, e29976. [Google Scholar] [CrossRef] [PubMed]
- Koles, K.; Budnik, V. Wnt signaling in neuromuscular junction development. Cold Spring Harb. Perspect. Biol. 2012, 4, a008045. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.G.; Wang, Q.; Zhou, J.Z.; Wang, J.; Luo, Z.; Liu, M.; He, X.; Wynshaw-Boris, A.; Xiong, W.C.; Lu, B.; et al. Regulation of AChR clustering by dishevelled interacting with MuSK and PAK1. Neuron 2002, 35, 489–505. [Google Scholar] [CrossRef]
- Stiegler, A.L.; Burden, S.J.; Hubbard, S.R. Crystal structure of the agrin-responsive immunoglobulin-like domains 1 and 2 of the receptor tyrosine kinase MuSK. J. Mol. Biol. 2006, 364, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Coldefy, A.S.; Hubbard, S.R.; Burden, S.J. Agrin binds to the N-terminal region of Lrp4 protein and stimulates association between Lrp4 and the first Ig-like domain in muscle-specific kinase (MuSK). J. Biol. Chem. 2011, 286, 40624–40630. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.; Zhang, B.; Gu, S.; Lee, K.; Zhou, J.; Yao, G.; Figueiredo, D.; Perry, K.; Mei, L.; Jin, R. Structural basis of agrin-Lrp4-MuSK signaling. Genes Dev. 2012, 26, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.; Jin, R. Structural mechanisms of the agrin-Lrp4-MuSK signaling pathway in neuromuscular junction differentiation. Cell. Mol. Life Sci. 2013, 70, 3077–3088. [Google Scholar] [CrossRef] [PubMed]
- Linnoila, J.; Wang, Y.; Yao, Y.; Wang, Z.Z. A mammalian homolog of Drosophila tumorous imaginal discs, Tid1, mediates agrin signaling at the neuromuscular junction. Neuron 2008, 60, 625–641. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Zang, B.; Dong, X.P.; Tao, Y.; Ting, A.; Zhou, Z.; Meixiong, J.; Luo, J.; Chiu, F.C.; Mei, L. HSP90β regulates rapsyn turnover and subsequent AChR cluster formation and maintenance. Neuron 2008, 60, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Weatherbee, S.D.; Anderson, K.V.; Niswander, L.A. LDL-receptor-related protein 4 is crucial for formation of the neuromuscular junction. Development 2006, 133, 4993–5000. [Google Scholar] [CrossRef] [PubMed]
- Zang, B.; Luo, S.; Wang, Q.; Suzuki, T.; Xiong, W.C.; Mei, L. Lrp4 serves as a coreceptor for agrin. Neuron 2008, 60, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Stiegler, A.L.; Cameron, T.O.; Hallock, P.T.; Gomez, A.M.; Huang, J.H.; Hubbard, S.R.; Dustin, M.L.; Burden, S.J. Lrp4 is a receptor for agrin and forms a complex with MuSK. Cell 2008, 135, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Barik, A.; Zhang, B.; Sohal, G.S.; Xiong, W.C.; Mei, L. Crosstalk between agrin and Wnt signaling pathways in development of vertebrate neuromuscular junction. Dev. Neurobiol. 2014, 74, 828–838. [Google Scholar] [CrossRef] [PubMed]
- Stamatakou, E.; Salinas, P.C. Postsynaptic assembly: A role for Wnt signaling. Dev. Neurobiol. 2014, 74, 818–827. [Google Scholar] [CrossRef] [PubMed]
- Ohkawara, B.; Cabrera-Serrano, M.; Nakata, T.; Milon, M.; Asai, N.; Ito, K.; Ito, M.; Masuda, A.; Ito, Y.; Engel, A.G.; et al. Lrp4 third β-propeller domain mutations cause novel congenital myasthenia by compromising agrin-mediated MuSK signaling in a position-specific manner. Human Mol. Genet. 2014, 23, 1856–1868. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jing, Z.; Zhang, L.; Zhou, G.; Braun, J.; Yao, Y.; Wang, Z.Z. Regulation of acetylcholine receptor clustering by the tumor suppressor APC. Nat. Neurosci. 2003, 6, 1017–1018. [Google Scholar] [CrossRef] [PubMed]
- Constantin, B. Dystrophin complex functions as a scaffold for signaling proteins. Biochem. Biophys. Acta 2014, 1838, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Hochenester, E.; Yurchenco, P.D. Laminins in basement membrane assembly. Cell Adh. Migr. 2013, 7, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Yurchenco, P.D. Basement membranes: Cell scaffoldings and signaling platforms. Cold Spring Harb. Perspect. Biol. 2011, 3, a004911. [Google Scholar] [CrossRef] [PubMed]
- Singhal, N.; Martin, P.T. Role of extracellular matrix proteins and their receptors in the development of the vertebrate neuromuscular junction. Dev. Neurobiol. 2011, 71, 982–1005. [Google Scholar] [CrossRef] [PubMed]
- Pilgram, G.S.K.; Potikanond, S.; Baines, R.A.; Fradkin, L.G.; Noordemeer, J.N. The roles of the dystrophin-associated glycoprotein complex at the synapse. Mol. Neurobiol. 2010, 41, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Karmouch, J.; Dobbertin, A.; Sigoillot, S.; Legay, C. Developmental consequences of the Col Q/MuSK interactions. Chem. Biol. Interact. 2013, 203, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Amenta, A.R.; Creely, H.E.; Mercado, M.L.; Hagiwara, H.; McKechnie, B.A.; Lechner, B.E.; Rossi, S.G.; Wang, Q.; Owens, R.T.; Marrero, E.; et al. Biglycan is an extracellular MuSK binding protein important for synapse stability. J. Neurosci. 2012, 32, 2324–2334. [Google Scholar] [CrossRef] [PubMed]
- Nastase, M.V.; Young, M.F.; Schaefer, L. Biglycan: A multivalent proteoglycan providing structure and signals. J. Histochem. Cytochem. 2012, 60, 963–975. [Google Scholar] [CrossRef] [PubMed]
- Madhavan, R.; Gong, Z.L.; Ma, J.J.; Chan, A.W.S.; Peng, H.B. The function of cortactin in the clustering of acetylcholine receptors at the vertebrate neuromuscular junction. PLoS ONE 2009, 4, e8478. [Google Scholar] [CrossRef] [PubMed]
- Carson, S.S.; Valdez, G.; Sanes, J.R. Presynaptic calcium channels and α3-integrins are complexed with synaptic cleft laminins, cytoskeletal elements and active zone components. J. Neurochem. 2010, 115, 654–666. [Google Scholar] [CrossRef] [PubMed]
- Nishimune, H. Molecular mechanism of active zone organization at vertebrate neuromuscular junctions. Mol. Neurobiol. 2012, 45, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Samuel, M.A.; Valdez, G.; Tapia, J.C.; Lichtman, J.W.; Sanes, J.R. Agrin and synaptic laminin are required to maintain adult neuromuscular junctions. PLoS ONE 2012, 7, e46663. [Google Scholar] [CrossRef] [PubMed]
- Rogers, R.S.; Nishimune, H. The role of laminins in the organization and function of neuromuscular junctions. Matrix Biol. 2017, 57, 86–105. [Google Scholar] [CrossRef] [PubMed]
- Simeone, L.; Straubinger, M.; Khan, M.A.; Nalleweg, N.; Cheusova, T.; Hashemolhosseini, S. Identification of Erbin interlinking MuSK and ErbB2 and its impact on acetylcholine receptor aggregation at the neuromuscular junction. J. Neurosci. 2012, 30, 6620–6634. [Google Scholar] [CrossRef] [PubMed]
- Ngo, S.T.; Cole, R.N.; Sunn, N.; Phillips, W.D.; Noakes, P.G. Neuregulin-1 potentiates agrin-induced acetylcholine receptor clustering through muscle-specific kinase phosphorylation. J. Cell Sci. 2012, 125 Pt 6, 1531–1543. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, N.; Akaaboune, M.; Gajendran, N.; Martinez-Pena y Valenzuela, I.; Wakefield, S.; Thurnheer, R.; Brenner, H.R. Neuregulin/ErbB regulate neuromuscular junction development by phosphorylation of α-dystrobrevin. J. Cell Biol. 2011, 195, 1171–1184. [Google Scholar] [CrossRef] [PubMed]
- Okada, K.; Inoue, A.; Okada, M.; Murata, Y.; Kakuta, S.; Jigami, T.; Kubo, S.; Shiraishi, H.; Eguchi, K.; Motomura, M.; et al. The muscle protein Dok-7 is essential for neuromuscular synaptogenesis. Science 2006, 312, 1802–1805. [Google Scholar] [CrossRef] [PubMed]
- Bergamin, E.; Hallock, P.T.; Burden, S.J.; Hubbard, S.R. The cytoplasmic adaptor protein Dok7 activates the receptor tyrosine kinase MuSK via dimerization. Mol. Cell 2010, 39, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Hallock, P.T.; Xu, C. F.; Park, T.J.; Neubert, T.A.; Curran, T.; Burden, S.J. Dok7 regulates neuromuscular synapse formation by recruiting Crk and Crk-L. Genes Dev. 2010, 24, 2451–2461. [Google Scholar] [CrossRef] [PubMed]
- Hallock, P.T.; Chin, S.; Blais, S.; Neubert, T.A.; Glass, D.J. Sorbs 1 and 2 interact with Crk-L and are required for acetylcholine receptor cluster formation. Mol. Cell. Biol. 2015, 36, 262–270. [Google Scholar] [PubMed]
- Wu, H.; Lu, Y.; Barik, A.; Joseph, A.; Taketo, M.M.; Xiong, W.C.; Mei, L. β-catenin gain of function in muscles impairs neuromuscular junction formation. Development 2012, 139, 2392–2404. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Barik, A.; Lu, Y.; Shen, C.; Bowman, A.; Li, L.; Sathyamurthy, A.; Lin, T.W.; Xiong, W.C.; Mei, L. Slit2 as a β-catenin/Ctnnb1-dependent retrograde signal for presynaptic differentiation. eLife 2015, 4, e07266. [Google Scholar] [CrossRef] [PubMed]
- Salinas, P.C. Retrograte signaling at the synapse: A role for Wnt proteins. Biochem. Soc. Trans. 2005, 33 Pt 6, 1295–1298. [Google Scholar] [CrossRef] [PubMed]
- Südhof, T.C. The presynaptic active zone. Neuron 2012, 75, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Wu, C. Active zone stability: Insights from fly neuromuscular junction. Neural Regen. Res. 2015, 10, 677–680. [Google Scholar] [PubMed]
- Jung, J.H.; Szule, J.A.; Marshall, R.M.; McMahan, U.J. Variable priming of a docked synaptic vesicle. Proc. Natl. Acad. Sci. USA 2016, 113, E1098–E1107. [Google Scholar] [CrossRef] [PubMed]
- Körber, C.; Kuner, T. Molecular machines regulating the release probability of synaptic vesicles at the active zone. Front. Synaptic Neurosci. 2016, 8, 5. [Google Scholar] [CrossRef] [PubMed]
- Harris, K.P.; Littleton, J.T. Transmission, development, and plasticity of synapses. Genetics 2015, 201, 345–375. [Google Scholar] [CrossRef] [PubMed]
- Yumoto, N.; Kim, N.; Burden, S.J. Lrp4 is a retrograde signal for presynaptic differentiation at neuromuscular synapses. Nature 2012, 489, 438–442. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Lu, Y.; Shen, C.; Patel, N.; Gan, L.; Xiong, W.C.; Mei, L. Distinct roles of muscle and motoneuron Lrp4 in neuromuscular junction formation. Neuron 2012, 75, 94–107. [Google Scholar] [CrossRef] [PubMed]
- Takamori, M. Lambert-Eaton myasthenic syndrome: Search for alternative autoimmune targets and possible compensatory mechanisms based on presynaptic calcium homeostasis. J. Neuroimmunol. 2008, 201–202, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Jones, R.T.; Whippen, J.M.; Davis, G.W. α2δ-3 is required for rapid transsynaptic homeostatic signaling. Cell Rep. 2016, 16, 2875–2888. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Dominguez, B.; de Winter, F.; Gould, T.W.; Eriksson, J.E.; Lee, K.F. Nestin negatively regulates postsynaptic differentiation of the neuromuscular synapse. Nat. Neurosci. 2011, 14, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Takamori, M.; Nakamura, T.; Motomura, M. Antibodies against Wnt receptor of muscle-specific tyrosine kinase in myasthenia gravis. J. Neuroimmunol. 2013, 254, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Huijbers, M.G.; Vink, A.F.D.; Niks, E.H.; Westhuis, R.H.; van Zwet, E.W.; de Meel, R.H.; Rojas-Garcia, R.; Diaz-Manera, J.; Kuks, J.B.; Klooster, R.; et al. Longitudinal epitope mapping in MuSK myasthenia gravis: Implications for disease severity. J. Neuroimmunol. 2016, 291, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, K.; Ito, M.; Ohkawara, B.; Masuda, B.; Kawakami, Y.; Sahashi, K.; Nishida, H.; Mabuchi, N.; Takano, A.; Engel, A.G.; et al. Collagen Q and anti-MuSK autoantibody competitively suppress agrin/Lrp4/MuSK signaling. Sci. Rep. 2015, 5, 13928–13939. [Google Scholar] [CrossRef] [PubMed]
- Messéant, J.; Dobbertin, A.; Girard, E.; Delers, P.; Manuel, M.; Mangione, F.; Schmitt, A.; Le Denmat, D.; Molgó, J.; Zytnichi, D.; et al. MuSK Frizzled-like domain is critical for mammalian neuromuscular junction formation and maintenance. J. Neurosci. 2015, 35, 4926–4941. [Google Scholar] [CrossRef] [PubMed]
- Remédio, L.; Gribble, K.D.; Lee, J.K.; Kim, N.; Hallock, P.T.; Delestrée, N.; Mentis, G.Z.; Froemke, R.C.; Granato, M.; Burden, S.J. Diverging roles for Lrp4 and Wnt signaling in neuromuscular synapse development during evolution. Genes Dev. 2016, 30, 1058–1069. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, Y.; Ito, M.; Hirayama, M.; Sahashi, K.; Ohkawara, B.; Masuda, A.; Nishida, H.; Mabuchi, N.; Engel, A.G.; Ohno, K. Anti-MuSK autoantibodies block binding of collagen Q to MuSK. Neurology 2011, 77, 1819–1826. [Google Scholar] [CrossRef] [PubMed]
- Cartaudo, A.; Strochlic, L.; Guerra, M.; Blanchard, B.; Lambergeon, M.; Krejci, E.; Cartaud, J.; Legay, C. MuSK is required for anchoring acetylcholinesterase at the neuromuscular junction. J. Cell Biol. 2004, 165, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Padgett, D.; Takahashi, M.; Li, H.; Sayeed, A.; Teichert, R.W.; Olivera, B.M.; McArdle, J.J.; Green, W.N.; Lin, W. Essential roles of the acetylcholine receptor γ-subunit in neuromuscular synaptic patterning. Development 2008, 135, 1957–1967. [Google Scholar] [CrossRef] [PubMed]
- Hacohen, Y.; Jacobson, L.W.; Byrne, S.; Norwood, F.; Lall, A.; Robb, S.; Dilena, R.; Fumagalli, M.; Born, A.P.; Clarke, D.; et al. Fetal acetylcholine receptor inactivation syndrome. A myopathy due to maternal antibodies. Neurol. Neuroimmunol. Neuroinflamm. 2015, 2, e57. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Liu, Y.; Sugiura, Y.; Allen, P.D.; Gregg, R.G.; Lin, W. Neuromuscular synaptic patterning requires the function of skeletal muscle dihydropyridine receptors. Nat. Neurosci. 2011, 14, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Maruta, T.; Yoshikawa, H.; Fukasawa, S.; Umeshita, S.; Inaoka, Y.; Edahiro, S.; Kado, H.; Motozaki, Y.; Iwasa, K.; Yamada, M. Autoantibody to dihydropyridine receptor in myasthenia gravis. J. Neuroimmunol. 2009, 208, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Niks, E.H.; Kuks, J.B.; Wokke, J.H.; Verdman, H.; Bakker, E.; Vershuuren, J.J.; Plomp, J.J. Pre- and postsynaptic neuromuscular junction abnormalities in MuSK myasthenia. Muscle Nerve 2010, 42, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Davis, G.W.; Müller, M. Homeostatic control of presynaptic neurotransmitter release. Annu. Rev. Physiol. 2015, 77, 251–270. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Pinter, M.J.; Rich, M.M. Reversible recruitment of a homeostatic reserve pool of synaptic vesicles underlies rapid homeostatic plasticity of quantal content. J. Neurosci. 2016, 36, 828–836. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.; Timóteo, M.A.; Correia-de-Sá, P. Modulation by adenosine of both muscarinic M1-facilitation and M2-inhibition of [3H]-acetylcholine release from the rat motor nerve terminals. Eur. J. Neurosci. 2002, 15, 1728–1736. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.; Correia-de-Sá, P. Protein kinase A and Cav1 (L-Type) channels are common targets to facilitatory adenosine A2A and muscarinic M1 receptors on rat motoneurons. Neurosignals 2005, 14, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.; Timóteo, M.A.; Correira-de-Sá, P. Negative crosstalk between M1 and M2 muscarinic autoreceptors involves endogeneous adenosine activating A1 receptors at the rat motor endplate. Neurosci. Lett. 2009, 459, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Correira-de-Sá, P.; Timóteo, M.A.; Ribeiro, J.A. Presynaptic A1 inhibitory/A2A facilitatory adenosine receptor activation balance depends on motor nerve stimulation paradigm at the rat hemidiaphragm. J. Neurophysiol. 1996, 76, 3910–3919. [Google Scholar]
- Oliveira, L.; Timóteo, M.A.; Correia-de-Sá, P. Tetanic depression is overcome by tonic adenosine A2A receptor facilitation of L-type Ca2+ influx into rat motor nerve terminals. J. Physiol. 2004, 560 Pt 1, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.; Costa, A.C.; Noronha-Matos, J.B.; Silva, I.; Cavalcante, W.L.; Timóteo, M.A.; Corrado, A.P.; del Belo, C.A.; Ambiel, C.R.; Alves-do-Prado, W.; et al. Amplification of neuromuscular transmission by methylprednisolone involves activation of presynaptic facilitatory adenosine A2A receptors and redistribution of synaptic vesicles. Neuropharmacology 2015, 89, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Noronha-Matos, J.B.; Morais, T.; Trigo, D.; Timóteo, M.A.; Magalhăes-Cardoso, M.T.; Oliveira, L.; Correia-de-Sá, P. Tetanic failure due to decreased endogeneous adenosine A2A tonus operating neuronal Cav1 (L-Type) influx in myasthenia gravis. J. Neurochem. 2011, 117, 797–811. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.; Correia, A.; Cristina-Costa, A.; Guerra-Gomes, S.; Ferreirinha, F.; Magalhăes-Cardoso, M.T.; Vilanova, M.; Correia-de-Sá, P. Deficits in endogeneous adenosine formation by ecto-5′-nucleotidase/CD73 impair neuromuscular transmission and immune competence in experimental autoimmune myasthenia gravis. Mediators Inflamm. 2015, 2015, 460610. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Shen, C.; Bealmear, B.; Ragheb, S.; Xiong, W.C.; Lewis, R.A.; Lisak, R.P.; Mei, L. Autoantibodies to agrin in myasthenia gravis patients. PLoS ONE 2014, 9, e91816. [Google Scholar] [CrossRef] [PubMed]
- Gasperi, C.; Meles, A.; Schoser, B.; Zhang, Y.; Meltoranta, J.; Risson, V.; Schaeffer, L.; Schalke, B.; Kröger, S. Anti-agrin autoantibodies in myastyhenia gravis. Neurology 2014, 82, 1976–1983. [Google Scholar] [CrossRef] [PubMed]
- Gesemann, M.; Denzer, A.J.; Ruegg, M.A. Acetylcholine receptor-aggregating activity of agrin isoforms and mapping of the active state. J. Cell Biol. 1995, 128, 625–636. [Google Scholar] [CrossRef] [PubMed]
- Scotton, P.; Bleckmann, D.; Stebler, M.; Sciandra, F.; Brancaccio, A.; Meier, T.; Stetefeld, J.; Ruegg, M.A. Activation of muscle-specific receptor tyrosine kinase and binding to dystroglycan are regulated by alternative mRNA splicing of agrin. J. Biol. Chem. 2006, 281, 36835–36845. [Google Scholar] [CrossRef] [PubMed]
- Tezuka, T.; Inoue, A.; Hoshi, T.; Wheatherbee, S.D.; Buraes, R.W.; Ueta, R. The MuSK activator agrin has a separate role essential for postnatal maintenance of neuromuscular synapses. Proc. Natl. Acad. Sci. USA 2014, 111, 16556–16561. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.G.; Quigley, A.F.; Bourke, J.L.; Nowell, C.J.; Myers, D.E.; Choong, P.F.; Kapsa, R.M. Combination of agrin and laminin increase acetylcholine receptor clustering and enhance functional neuromuscular junction formation in vitro. Dev. Neurobiol. 2015, 76, 551–565. [Google Scholar] [CrossRef] [PubMed]
- Patel, T.R.; Butler, G.; McFarlane, A.; Xie, I.; Overall, C.M.; Stetefeld, J. Site specific cleavage mediated by MMPs regulates function of agrin. PLoS ONE 2012, 7, e43669. [Google Scholar] [CrossRef] [PubMed]
- Luckman, S.P.; Gilhus, N.E.; Romi, F. Matrix metalloproteinase-3 in myasthenia gravis compared to other neurological disorders and healthy controls. Autoimmune Dis. 2011, 2011, 151258. [Google Scholar] [CrossRef] [PubMed]
- Molin, C.J.; Weaterberg, E.; Punga, A.R. Profile of upregulated inflammatory proteins in sera of myasthenia gravis patients. Sci. Rep. 2017, 7, 39716. [Google Scholar] [CrossRef] [PubMed]
- Todd, K.; Auld, D.S.; Robitaille, R. Neurotrophins modulate neuron-glia interactions at a vertebrate synapse. Eur. J. Neurosci. 2007, 25, 1287–1296. [Google Scholar] [CrossRef] [PubMed]
- Fenz, Z.; Ko, C.P. Schwann cells promote synaptogenesis at the neuromuscular junction via transforming growth factor-β1. J. Neurosci. 2008, 28, 9599–9609. [Google Scholar]
- Amaral, M.D.; Pozzo-Miller, L. Intracellular Ca2+ stores and Ca2+ influx are both required for BDNF to rapidly increase quantal vesicular transmitter release. Neural. Plast. 2012, 2012, 203536. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.; Hu, J.; Quian, J.; Hackam, A.S. Expression of brain-derived neurotrophic factor is regulated by the Wnt signaling pathway. Neuroreports 2012, 23, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Bartus, R.T.; Bétourné, A.; Basile, A.; Peterson, B.L.; Glass, J.; Boulís, N.M. β2-adrenoceptor agonists as novel, safe and potentially effective therapies for amyotrophic lateral sclerosis (ALS). Neurobiol. Dis. 2015, 85, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Ghazanfari, N.; Morsch, M.; Tse, N.; Reddel, S.W.; Phillips, W.D. Effects of the β2-adrenoceptor agonist, alubuterol, in a mouse model of anti-MuSK myasthenia gravis. PLoS ONE 2014, 9, e87840. [Google Scholar] [CrossRef] [PubMed]
- Allen, N.M.; Hacohen, Y.; Palace, J.; Beeson, D.; Vincent, A.; Jungbluth, H. Salbutamol-responsive fetal acetylcholine receptor inactivation syndrome. Neurology 2016, 86, 692–694. [Google Scholar] [CrossRef] [PubMed]
- Lipka, A.F.; Vrinten, C.; van Zwet, E.W.; Schimmel, K.J.; Cornel, M.C.; Kuijpers, M.R.; Hekster, Y.A.; Weinreich, S.S.; Verschuuren, J.J. Ephedrine treatment for autoimmune myasthenia gravis. Neuromuscul. Disord. 2017, 27, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Engel, A.G.; Shen, X.M.; Selcen, D.; Sine, S.M. Congenital myasthenic syndromes: Pathogenesis, diagnosis, and treatment. Lancet Neurol. 2015, 144, 420–434. [Google Scholar] [CrossRef]
- Beeson, D. Congenital myasthenic syndromes: Recent advances. Curr. Opin. Neurol. 2016, 29, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Gallenmüller, C.; Müller-Felber, W.; Dusl, M.; Stucka, R.; Guergueltcheva, V.; Blaschek, A.; von der Hagen, M.; Huebner, A.; Müller, J.S.; Lochmüller, H.; et al. Sulbutamol-responsive limb-girdle congenital myasthenic syndrome due to a novel missense mutation and heteroallelic deletion in MuSK. Neuromuscul. Disord. 2014, 24, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Selcen, D.; Ohkawara, B.; Shen, X.M.; McEvoy, K.; Ohno, K.; Engel, A.G. Impaired synaptic development, maintenance, and neuromuscular transmission in Lrp4-related myasthenia. JAMA Neurol. 2015, 72, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, E.; Martínez-Hernández, E.; Titulaer, M.J.; Huijbers, M.G.; Martínez, M.A.; Ramos, A.; Querol, L.; Díaz-Manera, J.; Rojas-García, R.; Hayworth, C.R.; et al. Cortactin autoantibodies in myasthenia gravis. Autoimmun. Rev. 2014, 13, 1003–1007. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Vicente, E.; Gallardo, E.; Martínez, M.Á.; Díaz-Manera, J.; Querol, L.; Rojas-Gracía, R.; Illa, I. Clinical characteristics of patients with double-seronegative myasthenia gravis and antibodies to cortactin. JAMA Neurol. 2016, 73, 1099–1104. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.B.; Xie, H.; Dai, Z. Association of cortactin with developing neuromuscular specializations. J. Neurocytol. 1997, 26, 637–650. [Google Scholar] [CrossRef] [PubMed]
- Alicea, D.; Perez, M.; Maodonado, C.; Dominicci-Cotto, C.; Marie, B. Cortactin is a regulator of activity-dependent synaptic plasticity controlled by Wingless. J. Neurosci. 2017, 37, 2203–2215. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ip, F.C.F.; Shi, L.; Zhang, Z.; Tang, H.; Ng, Y.P.; Ye, W.C.; Fu, A.K.; Ip, N.Y. Coronin 6 regulates acetylcholine receptor clustering through modulating receptor anchorage to actin cytoskeleton. J. Neurosci. 2014, 34, 2413–2421. [Google Scholar] [CrossRef] [PubMed]
- Hezel, M.; de Groat, W.C.; Galbiati, F. Caveolin-3 promotes nicotinic acetylcholine receptor clustering and regulates neuromuscular junction activity. Mol. Biol. Cell 2010, 21, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Bourgeois, F.; Messéant, J.; Kordeli, E.; Petit, J.M.; Delers, P.; Bahi-Buisson, N.; Bernard, V.; Sigoillot, S.M.; Gitiaux, C.; Stouffer, M.; et al. A critical and previously unsuspected role for doublecortin at the neuromuscular junction in mouse and human. Neurmuscul. Disord. 2015, 25, 461–473. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, H.; Ohkawara, B.; Ishigaki, S.; Fukudome, T.; Ito, K.; Tsushima, M.; Konishi, H.; Okuno, T.; Yoshimura, T.; Ito, M.; et al. R-spondin 2 promotes acetylcholine receptor clustering at the neuromuscular junction via Lrg5. Sci. Rep. 2016, 6, 28512. [Google Scholar] [CrossRef] [PubMed]
- Vieira, G.C.; Chockalingam, S.; Melegh, Z.; Greenhough, A.; Malik, S.; Szemes, M.; Park, J.H.; Kaidi, A.; Zhou, L.; Catchpoole, D.; et al. LGR5 regulates pro-survival MEK/ERK and proliferative Wnt/β-catenin signalling in neuroblastoma. Oncotarget 2015, 6, 40053–40067. [Google Scholar] [PubMed]
- Akaaboune, M.; Allinguant, B.; Farza, H.; Roy, K.; Magoul, R.; Fiszman, M.; Festoff, B.W.; Hantaї, D. Developmental regulation of amyloid precursor protein at the neuromuscular junction in mouse skeletal muscle. Mol. Cell. Neurosci. 2000, 15, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, B.; Yang, L.; Guo, Q.; Aithmitti, N.; Songyang, Z.; Zheng, H. Pre- and postsynaptic interaction of the amyloid precursor protein promotes peripheral and central synaptogenesis. J. Neurosci. 2009, 29, 10788–10801. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.Y.; Liu, Y.; Tennert, C.; Sugiura, Y.; Karakatsani, A.; Kröger, S.; Johnson, E.B.; Hammer, R.E.; Lin, W.; Herz, J. APP interacts with Lrp4 and agrin to coordinate the development of the neuromuscular junction in mice. eLife 2013, 2, e00220. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, J.H.; Klevanski, M.; Saar, M.; Müller, U.C. Roles of the amyloid precursor protein family in the peripheral nervous system. Mech. Dev. 2013, 130, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Stanga, S.; Zanou, N.; Audouard, E.; Tasiaux, B.; Contino, S.; Vandermeulen, G.; René, F.; Loeffler, J.P.; Clotman, F.; Gailly, P.; et al. APP-dependent glial cell line-derived neurotrophic factor gene expression drives neuromuscular junction. FASEB J. 2016, 30, 1696–1711. [Google Scholar] [CrossRef] [PubMed]
- Baudet, C.; Pozas, E.; Adameyko, I.; Andersson, E.; Ericson, J.; Ernfors, P. Retrograde signaling onto Ret during motor nerve terminal maturation. J. Neurosci. 2008, 28, 963–975. [Google Scholar] [CrossRef] [PubMed]
- Südhof, T.C. Neuroligins and neurexins link synaptic function to cognitive disease. Nature 2008, 455, 903–911. [Google Scholar] [CrossRef] [PubMed]
| Groups (Number of Patients) | 10 Patients | 23 Patients |
|---|---|---|
| Antibodies against recombinant segments | Anti-Ig1/2-positive Anti-CRD-positive | Anti-Ig1/2-positive Anti-CRD-negative |
| Age at onset | Age (years): 22–75 | Age (years): 6–80 |
| Gender | Female: 8/Male: 2 | Female: 15/Male: 8 |
| MuSK antibody titers determined by standard RIA (control, <0.05 nmol/L) | 6.08–131.40 | 5.32–45.75 |
| MG severity (MGFA grades) | ||
| IIa | 0 | 3 |
| IIb | 0 | 7 |
| IIIa | 0 | 2 |
| IIIb | 4 | 1 |
| IVa | 0 | 0 |
| IVb | 0 | 0 |
| V | 6 | 10 |
| Immunoblots of purified recombinant proteins of human MuSK extracellular segments |  |  |
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takamori, M. Synaptic Homeostasis and Its Immunological Disturbance in Neuromuscular Junction Disorders. Int. J. Mol. Sci. 2017, 18, 896. https://doi.org/10.3390/ijms18040896
Takamori M. Synaptic Homeostasis and Its Immunological Disturbance in Neuromuscular Junction Disorders. International Journal of Molecular Sciences. 2017; 18(4):896. https://doi.org/10.3390/ijms18040896
Chicago/Turabian StyleTakamori, Masaharu. 2017. "Synaptic Homeostasis and Its Immunological Disturbance in Neuromuscular Junction Disorders" International Journal of Molecular Sciences 18, no. 4: 896. https://doi.org/10.3390/ijms18040896
APA StyleTakamori, M. (2017). Synaptic Homeostasis and Its Immunological Disturbance in Neuromuscular Junction Disorders. International Journal of Molecular Sciences, 18(4), 896. https://doi.org/10.3390/ijms18040896






