A New Oleanolic Acid Derivative against CCl4-Induced Hepatic Fibrosis in Rats
Abstract
:1. Introduction
2. Results
2.1. Histological Assessment
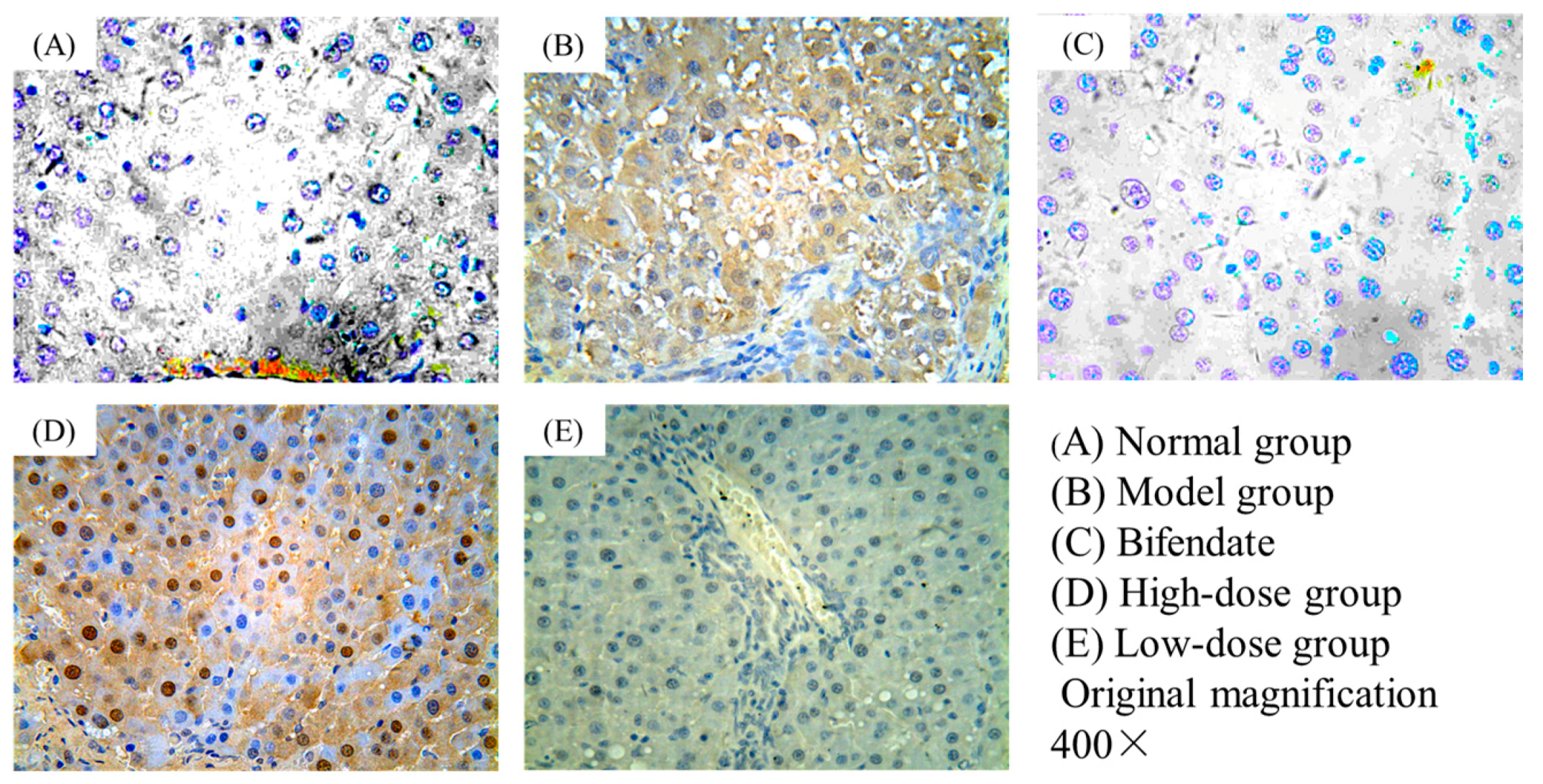
2.2. Immunohistochemical Staining
2.3. Effects of Oxy-Di-OA on Serum Biochemistry
2.4. The Effects of Oxy-Di-OA on Liver and Spleen Indices
2.5. Acute Toxicity Test In Vivo
2.5.1. Acute Toxicity Assay via Intraperitoneal Injection
2.5.2. Acute Toxicity Assay via Gavage
2.6. Pharmacokinetic Study
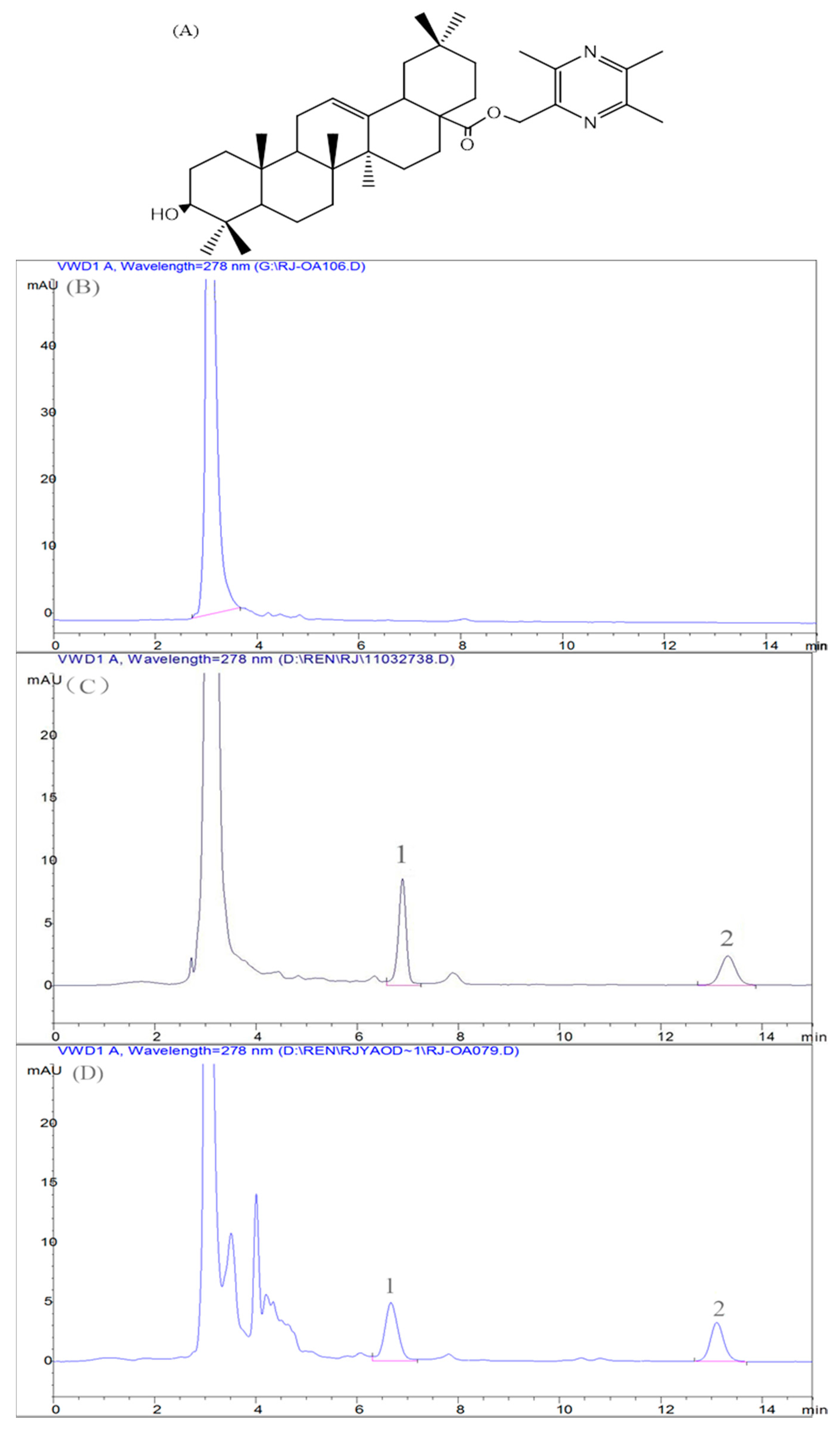
2.6.1. Method Validation
Specificity
Calibration Curve and Linearity
Precision, Limit of Detection, and Quantitation
Extraction Recovery and Stability
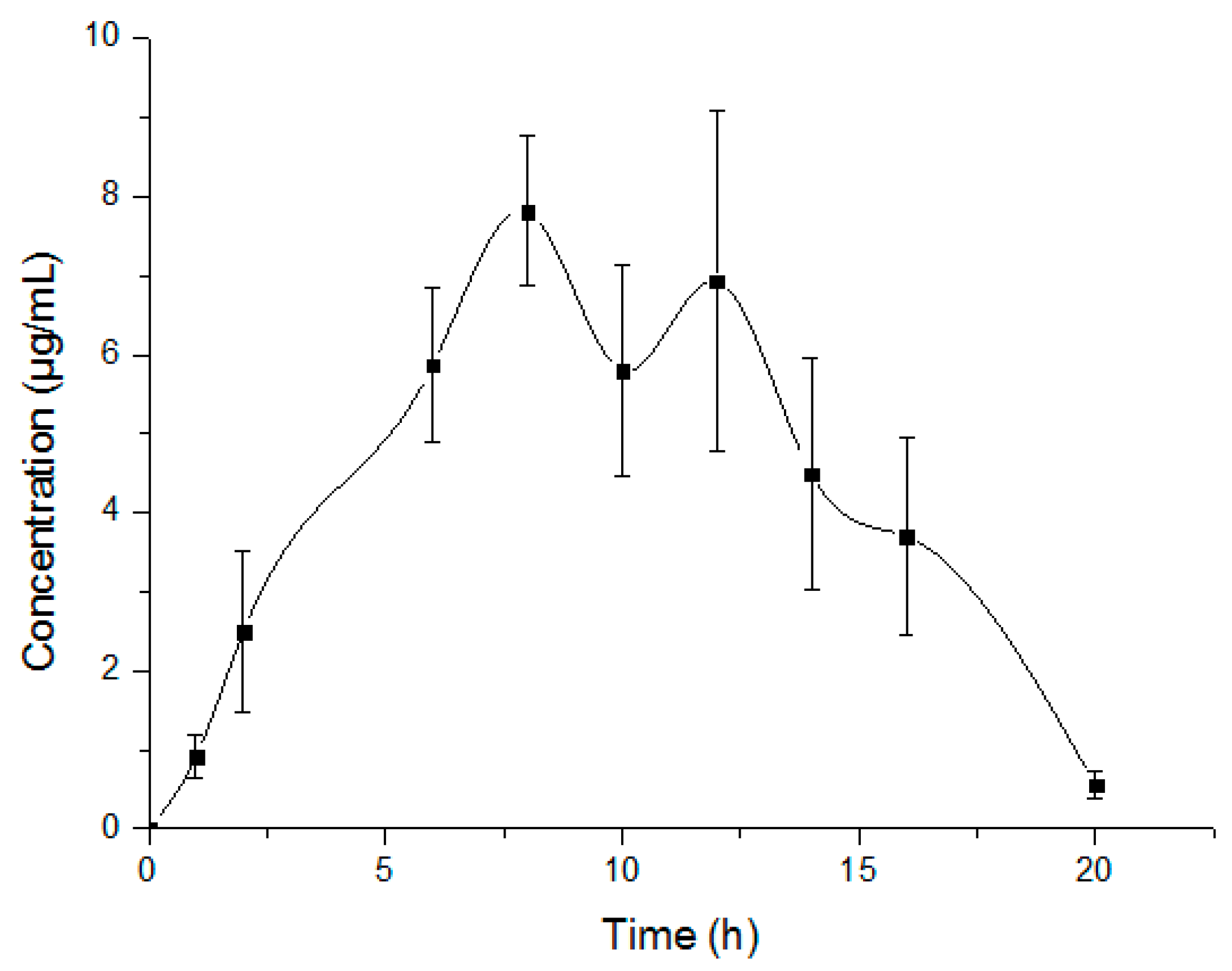
2.6.2. Pharmacokinetic Study and Data Analysis
3. Discussion
4. Materials and Methods
4.1. Animals and Housing Environment
4.2. Reagents
4.3. Experimental Procedure
4.4. Histological Assay
4.5. Immunohistochemical Staining
4.6. Measurement of Plasma Transaminase Activities
4.7. Liver and Spleen Indices
4.8. Acute Toxicity Assay
4.8.1. Acute Toxicity Assay via Intraperitoneal Injection
4.8.2. Acute Toxicity Assay via Gavage
4.9. Pharmacokinetic Study
4.9.1. Preparation of Standard Solution, Quality Control Working Solution, and Samples
4.9.2. Method Validation
Calibration Curve and Linearity
Precision, Limit of Detection, and Quantitation
Extraction Recovery and Stability
Pharmacokinetic Study and Data Analysis
4.10. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hernandez-Gea, V.; Friedman, S.L. Pathogenesis of liver fibrosis. Annu. Rev. Pathol. 2011, 6, 425–456. [Google Scholar] [CrossRef] [PubMed]
- Tsochatzis, E.A.; Bosch, J.; Burroughs, A.K. New therapeutic paradigm for patients with cirrhosis. Hepatology 2012, 56, 1983–1992. [Google Scholar] [CrossRef] [PubMed]
- Kisseleva, T.; Brenner, D.A. Anti-fibrogenic strategies and the regression of fibrosis. Best Pract. Res. Clin. Gastroenterol. 2011, 25, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Naftaly, M.; Friedman, S.L. Current status of novel antifibrotic therapies in patients with chronic liver disease. Ther. Adv. Gastroenterol. 2011, 4, 391–417. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.M.; Wu, X.X.; Sun, Y.; Kong, X.W.; Zhang, Y.H.; Xu, Q. A novel synthetic oleanolic acid derivative (CPU-II2) attenuates liver fibrosis in mice through regulating the function of hepatic stellate cells. J. Biomed. Sci. 2008, 15, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Chu, F.H.; Li, G.L.; Xu, X.; Wang, P.L.; Song, J.X.; Zhou, S.; Lei, H.M. Oleanolic acid synthetic oligoglycosides: A review on recent progress in biological activities. Pharmzie 2014, 69, 483–495. [Google Scholar]
- Gao, D.W.; Li, Q.W.; Li, Y.; Liu, Z.H.; Fan, Y.S.; Liu, Z.W.; Zhao, H.W.; Li, J.; Han, Z.S. Antidiabetic and antioxidant effects of oleanolic acid from Ligustrum lucidum Ait in alloxan-induced diabetic rats. Phytother. Res. 2009, 23, 1257–1262. [Google Scholar] [CrossRef] [PubMed]
- Maia, J.L.; Lima-Junior, R.C.P.; Melo, C.M.; David, J.P.; David, J.M.; Campos, A.R.; Santos, F.A.; Rao, V.S. Oleanolic acid, a pentacyclic triterpene attenuates capsaicin-induced nociception in mice: Possible mechanisms. Pharmacol. Res. 2006, 54, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.J.; Sun, W.Z.; Peng, W.B.; Yu, R.L.; Li, G.Q.; Jiang, T. Pharmacokinetics in vitro and in vivo of two novel prodrugs of oleanolic acid in rats and its hepatoprotective effects against liver injury induced by CCl4. Mol. Pharm. 2016, 13, 1699–1710. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Zhang, C.; Li, B.; Xu, X.; Liang, M.; Gu, S.; Chu, F.; Xu, B.; Rem, J.; Wang, P.; et al. A Series of oleanolic acid derivatives as anti-hepatitis B virus agents: Design, synthesis, and in vitro and in vivo biological evaluation. Molecules 2016, 21, 402. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.C.; Ma, L.N.; Li, Y.F.; Liu, X.Y.; Zhang, X.; Liu, J.Y.; Sheng, Y.J.; Zhang, D.Z.; Hu, H.D.; Ren, H. Association between serum platelet-derived growth factor BB and degree of liver damage, fibrosis and hepatitis B e antigen (HBeAg) status in CHB patients. Hepato Gastroenterol. 2012, 59, 2357–2360. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.L.; Wong, G.L.; Wong, V.W. A review of the natural history of chronic hepatitis B in the era of transient elastography. Antivir. Ther. 2009, 14, 489–499. [Google Scholar] [PubMed]
- Pinzani, M. Pathophysiology of liver fibrosis. Dig. Dis. 2015, 33, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Ilan, E.; Burakova, T.; Dagan, S.; Nussbaum, S.; Lubin, I.; Eren, R.; Ben-Moshe, O.; Arazi, J.; Berr, S.; Neville, L. The hepatitis B virus-trimera mouse: A model for human HBV infection and evaluation of anti-HBV therapeutic agents. Hepatology 1999, 29, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Dorner, M.; Horwitz, J.A.; Robbins, J.B.; Barry, W.T.; Feng, Q.; Mu, K.; Jones, C.T.; Schoggins, J.W.; Catanese, M.T.; Burton, D.R.; et al. A genetically humanized mouse model for hepatitis C virus infection. Nature 2011, 474, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Sentíes-Gómez, M.D.; Gálvez-Gastélum, F.J.; Meza-García, E.; Armendáriz-Borunda, J. Hepatic fibrosis: Role of matrix metalloproteases and TGF-β. Gac. Med. Mex. 2005, 141, 315–322. [Google Scholar] [PubMed]
- Nitou, M.; Ishikawa, K.; Shiojiri, N. Immunohistochemical analysis of development of desmin-positive hepaticstellate cells in mouse liver. J. Anat. 2000, 197, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Lee, S.J.; Park, H.J.; Chung, J.P.; Han, K.H.; Chon, C.Y.; Lee, S.I.; Moon, Y.M. Oxidative stress effect on the activation of hepatic stellate cells. Yonsei Med. J. 2001, 42, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.M.; Shi, B.; Wei, H.S.; Ma, X.H.; He, X.Y.; Feng, K. γ-Aminobutyric acid Breceptor improves carbon tetrachloride-induced liver fibrosis in rats. Dig. Dis. Sci. 2013, 58, 1909–1915. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.Q.; Zhang, S.R.; Yu, F.J.; Li, H.Q.; Guo, C.Y.; Fan, X.M. Ghrelin attenuates liver fibrosis through regulation of TGF-β1 expression and autophagy. Mol. Sci. 2015, 16, 21911–21930. [Google Scholar] [CrossRef] [PubMed]
- Kotaro, S.; Satoshi, E.; Mitsuko, H.; Hara, M.; Imoto, M.; Kojima, S. Neovessel formation promotes liver fibrosis via providing latent transforming growth factor-β. Biochem. Biophys. Res. Commun. 2014, 443, 950–956. [Google Scholar]
- Wu, J.Y.; Guo, C.Y.; Liu, J.; Xuan, X.F. Expression of integrin in hepatic fibrosis and intervention of resveratrol. Front. Med. China 2009, 3, 100–107. [Google Scholar] [CrossRef]
- Lee, C.; Sintich, S.M.; Mathews, E.P.; Shah, A.H.; Kundu, S.D.; Perry, K.T.; Cho, J.S.; Ilio, K.Y.; Cronauer, M.V.; Janulis, L.; et al. Transforming growth factor-β in benign and malignant prosetate. Prosetate 1999, 39, 285–290. [Google Scholar] [CrossRef]
- Gressner, A.M.; Weiskirchen, R. Modern pathogenetic concepts of liver fibrosis suggest stellate cells and TGF-β as major players and therapeutic targets. Cell. Mol. Med. 2006, 10, 76–99. [Google Scholar] [CrossRef]
- Lamireau, T.; Desmouliere, A.; Bioulac-Sage, P. Mechanisms of hepatic fibrogenesis. Arch. Pediatr. 2002, 9, 392–405. [Google Scholar] [CrossRef]
- Wei, X.L.; Fang, R.T.; Yang, Y.H.; Bi, X.Y.; Ren, G.X.; Luo, A.L.; Zhao, M.; Zang, W.J. Protective effects of extracts from pomegranate peels and seeds on liver fibrosis induced by carbon tetrachloride in rats. BMC Complement. Altern. Med. 2015, 15, 389. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.W.; Kim, Y.H.; Kim, H.H.; Ji, H.Y.; Yoo, S.D.; Choi, W.R.; Lee, S.M.; Han, C.K.; Lee, H.S. Dose-linear pharmacokinetics of oleanolic acid after intravenous and oral administration in rats. Biopharm. Drug Dispos. 2007, 28, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Batts, K.P.; Ludwig, J. An update on terminology and reporting. Am. J. Surg. Pathol. 1995, 19, 1409–1417. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Hu, Z.; Li, W.; Hu, M.; Ran, J.; Hu, M.; Ran, J.; Chen, P.; Sun, Q. Establishment of a standardized liver fibrosis model with different pathological stages in rats. Gastroenterol. Res. Pract. 2012, 2012, 560345. [Google Scholar] [CrossRef] [PubMed]
- Ishak, K.; Baptista, A.; Bianchi, L.; Callea, F.; De Groote, J.; Gudat, F.; Denk, H.; Desmet, V.; Korb, G.; MacSween, R.N.M.; et al. Histological grading and staging of chronic hepatitis. J. Hepatol. 1995, 22, 696–699. [Google Scholar] [CrossRef]
- Wang, P.L.; Zhang, Y.Z.; An, Y.W.; Xu, K.; Xu, X.; Fu, C.; Lin, J.X.; Xu, S.X.; Li, Q.; Lei, H.M. Protection of a new heptapeptide from Carapax trionycis against carbon tetrachloride-induced acute liver injury in mice. Chem. Pharm. Bull. 2013, 61, 1130–1135. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.L.; She, G.M.; Yang, Y.N.; Li, Q.; Zhang, H.G.; Liu, J.; Cao, Y.Q.; Xu, X.; Lei, H.M. Synthesis and biological evaluation of new ligustrazine derivatives as anti-tumor agents. Molecules 2012, 17, 4972–4985. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhong, D.; Duan, X.; Chen, X. Liquid chromatography/negative ion electrospray tandem mass spectrometry method for the quantification of rosuvastatin in human plasma: Application to a pharmacokinetic study. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2007, 856, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.Y.; Wang, F.; Li, Y.M.; Li, Y.; Wang, M.Q.; Sun, D.J.; Sun, C.H. Pharmacokinetic study of mangiferin in human plasma after oral administration. Biopharm. Drug Dispos. 2012, 132, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, H.N.; Wang, X.L.; Wu, J.F.; Jiang, J.; Wang, Y.Q. Pharmacokinetic study of a novel stroke therapeutic, 2-[[(1,1-dimethylethyl) oxidoimino] methyl]-3,5,6-trimethylpyrazine, by a simple HPLC-UV method in rats. Eur. J. Drug Metab. Pharmacokinet. 2011, 36, 95–101. [Google Scholar] [CrossRef] [PubMed]


| Groups | Dose (mg/kg) | n | ALT (U/L) | AST (U/L) |
|---|---|---|---|---|
| Normal | - | 12 | 50.04 ± 11.59 | 130.80 ± 45.67 |
| Model | - | 12 | 99.78 ± 55.68 * | 209.83 ± 111.08 * |
| Bifendate | 10.5 | 9 | 54.54 ± 21.40 # | 122.56 ± 39.18 ## |
| Oxy-Di-OA | 14 | 11 | 77.58 ± 39.58 * | 122.00 ± 52.19 ## |
| Oxy-Di-OA | 28 | 11 | 67.06 ± 51.59 # | 141.89 ± 71.57 # |
| Groups | Dose (mg/kg) | n | Liver Index (%) | Spleen Index (%) |
|---|---|---|---|---|
| Normal | - | 12 | 2.70 ± 0.18 | 0.17 ± 0.03 |
| Model | - | 12 | 3.72 ± 0.50 * | 0.28 ± 0.09 * |
| Bifendate | 10.5 | 9 | 3.57 ± 0.34 * | 0.24 ± 0.04 * |
| Oxy-Di-OA | 14 | 11 | 3.46 ± 0.38 * | 0.24 ± 0.06 * |
| Oxy-Di-OA | 28 | 11 | 3.15 ± 0.36 *,# | 0.26 ± 0.05 * |
| Group | Mice Number Start/End | Dose (mg/kg) | Death Rate (%) | LD50 (mg/kg) | 95% CIs (mg/kg) |
|---|---|---|---|---|---|
| 1 | 6/6 | 178 | 0 | 714.83 | 639.73–798.73 |
| 2 | 6/6 | 422 | 0 | ||
| 3 | 6/6 | 600 | 0 | ||
| 4 | 6/2 | 750 | 67 | ||
| 5 | 6/1 | 900 | 83 |
| Concentration Added (μg/mL) | Concentration Observed (μg/mL) | Precision (RSD %) | ||
|---|---|---|---|---|
| Intra-Day | Inter-Day | Intra-Day | Inter-Day | |
| 1.02 | 1.0156 ± 0.0246 | 1.0351 ± 0.0183 | 2.43 | 1.76 |
| 5.10 | 4.9083 ± 0.0839 | 4.7791 ± 0.2039 | 1.71 | 4.24 |
| 10.2 | 10.0974 ± 0.0548 | 9.7475 ± 0.3434 | 0.54 | 3.52 |
| Concentration Added (μg/mL) | Relative Recovery | Absolute Recovery | ||
|---|---|---|---|---|
| Co/Ct % | RSD % | Ao/At % | RSD % | |
| 1.02 | 100.17 ± 0.90 | 0.90 | 97.17 ± 4.25 | 4.38 |
| 5.10 | 97.46 ± 0.76 | 0.78 | 99.68 ± 3.48 | 3.49 |
| 10.2 | 99.76 ± 1.06 | 1.07 | 96.36 ± 3.14 | 3.26 |
| Pharmacokinetic Parameters | Units | Mean ± SD |
|---|---|---|
| AUClast | μg·h/mL | 90.21 ± 15.68 |
| CL | mL/h/kg | 5575.84 ± 992.65 |
| Vz | mL/kg | 18,290.17 ± 9453.59 |
| t1/2 | H | 2.19 ± 0.71 |
| Cmax | μg/mL | 8.18 ± 0.66 |
| Tmax | H | 10.00 ± 2.19 |
| MRT | H | 9.48 ± 0.48 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, H.; Han, Y.; Zhang, Y.; Yan, W.; Xu, B.; Chu, F.; Xie, T.; Jia, M.; Yan, M.; Zhao, R.; et al. A New Oleanolic Acid Derivative against CCl4-Induced Hepatic Fibrosis in Rats. Int. J. Mol. Sci. 2017, 18, 553. https://doi.org/10.3390/ijms18030553
Xiang H, Han Y, Zhang Y, Yan W, Xu B, Chu F, Xie T, Jia M, Yan M, Zhao R, et al. A New Oleanolic Acid Derivative against CCl4-Induced Hepatic Fibrosis in Rats. International Journal of Molecular Sciences. 2017; 18(3):553. https://doi.org/10.3390/ijms18030553
Chicago/Turabian StyleXiang, Hongjun, Yaotian Han, Yuzhong Zhang, Wenqiang Yan, Bing Xu, Fuhao Chu, Tianxin Xie, Menglu Jia, Mengmeng Yan, Rui Zhao, and et al. 2017. "A New Oleanolic Acid Derivative against CCl4-Induced Hepatic Fibrosis in Rats" International Journal of Molecular Sciences 18, no. 3: 553. https://doi.org/10.3390/ijms18030553
APA StyleXiang, H., Han, Y., Zhang, Y., Yan, W., Xu, B., Chu, F., Xie, T., Jia, M., Yan, M., Zhao, R., Wang, P., & Lei, H. (2017). A New Oleanolic Acid Derivative against CCl4-Induced Hepatic Fibrosis in Rats. International Journal of Molecular Sciences, 18(3), 553. https://doi.org/10.3390/ijms18030553






