Effects and Side Effects of Using Sorafenib and Sunitinib in the Treatment of Metastatic Renal Cell Carcinoma
Abstract
:1. Introduction
1.1. Renal Cell Carcinoma
1.2. Current Therapy
2. Anti-Angiogenic Therapy with Tyrosine Kinase Inhibitors
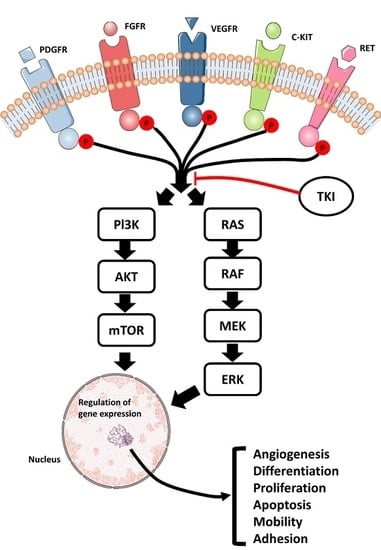
2.1. Overview of Tyrosine Kinase Inhibitors in General
2.2. Effects of Sorafenib and Sunitinib in the Treatment of Metastatic Renal Cell Carcinoma
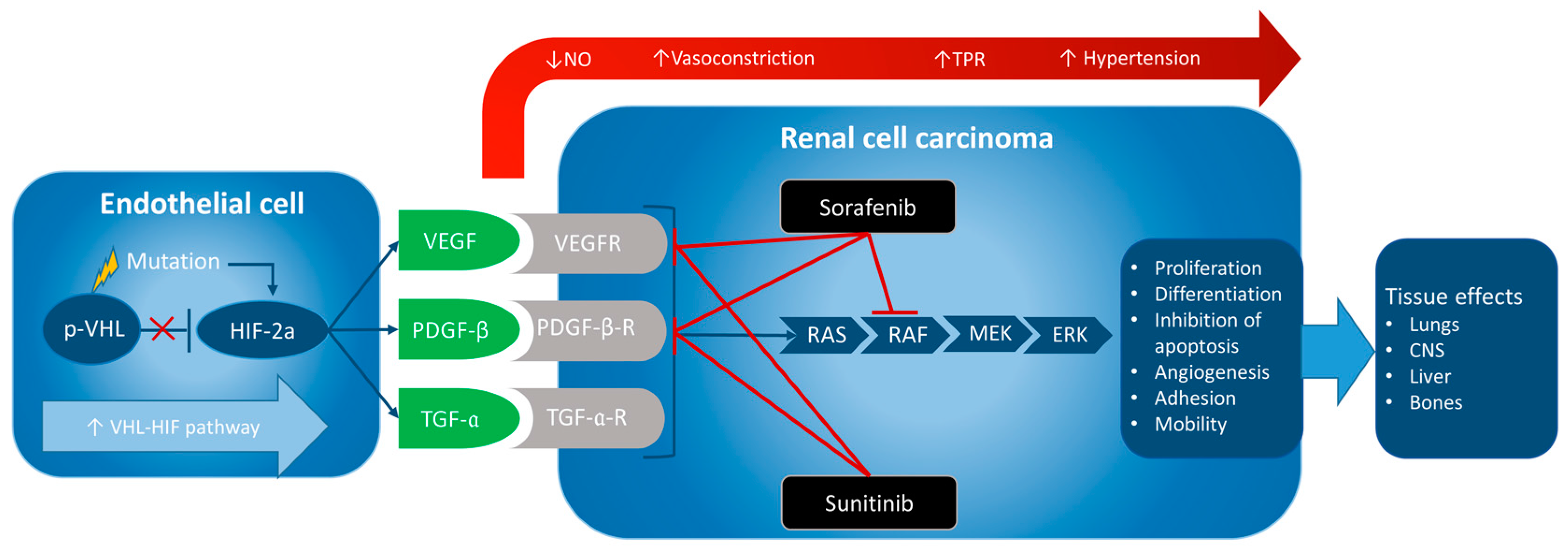
2.2.1. Sorafenib
2.2.2. Sunitinib
2.3. Treatment of mRCC Resistant to Sorafenib or Sunitinib
2.4. Adverse Effects of Sorafenib and Sunitinib
2.4.1. Sorafenib
2.4.2. Sunitinib
2.5. Management of Hypertension Induced by Sorafenib and Sunitinib
2.6. Biomarkers in RCC
3. Discussion
4. Conclusions and Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| 95% CI | 95% confidence interval |
| β-blockers | β-adrenoceptor antagonists/blockers |
| ACE | angiotensin converting enzyme |
| ATP | adenosine triphosphate |
| BMI | body mass index |
| BP | blood pressure |
| c-Kit | c-Kit protein |
| ccRCC | clear cell renal cell carcinoma |
| CYP | cytochrome P450 |
| CYPIP | cytohesin 1 interacting protein |
| CXCR4 | chemokine receptor type 4 |
| eGFR | estimated glomerular filtration rate |
| ERK | extracellular signal-regulated kinases |
| Flt-3 | FMS-like tyrosine kinase 3 |
| ESMO | European Society for Medical Oncology |
| HR | hazard ratio |
| HIF-2α | hypoxia-inducible factor-2α |
| IFN-α | interferon-α |
| IL-2 | interleukin-2 |
| MEK | mitogen-activated protein kinases |
| mmHg | millimetres of mercury |
| mRCC | metastatic renal cell carcinoma |
| mTOR | mammalian target of rapamycin |
| NP | not provided |
| OS | overall survival |
| PDE | phosphodiesterase |
| PDGF-β | platelet-derived growth factor-β |
| PDGFR-α | platelet-derived growth factor-α receptor |
| PDGFR-β | platelet-derived growth factor-β receptor |
| PFS | progression-free survival |
| PLC | phospholipase C |
| PI3K | phosphoinositide 3-kinase |
| PS | prognostic score |
| RCC | renal cell carcinoma |
| Raf | rapidly accelerated fibrosarcoma |
| Ras | Ras protein |
| TA | targeted agent |
| TKI | tyrosine kinase inhibitor |
| TKIs | tyrosine kinase inhibitors |
| TGF-α | transforming growth factor-α |
| VEGF | vascular endothelial growth factor |
| VEGFR | vascular endothelial growth factor receptor |
| VHL | von Hippel–Lindau |
| VHL-HIF | von Hippel–Lindau-hypoxia-inducible factor |
References
- Randall, J.M.; Millard, F.; Kurzrock, R. Molecular aberrations, targeted therapy, and renal cell carcinoma: Current state-of-the-art. Cancer Metastasis Rev. 2014, 33, 1109–1124. [Google Scholar] [CrossRef] [PubMed]
- Buonerba, C.; di Lorenzo, G.; Sonpavde, G. Combination therapy for metastatic renal cell carcinoma. Ann. Transl. Med. 2016, 4, 100. [Google Scholar] [CrossRef] [PubMed]
- Gollob, J.A.; Wilhelm, S.; Carter, C.; Kelley, S.L. Role of Raf kinase in cancer: Therapeutic potential of targeting the Raf/MEK/ERK signal transduction pathway. Semin. Oncol. 2006, 33, 392–406. [Google Scholar] [CrossRef] [PubMed]
- Escudier, B.; Eisen, T.; Stadler, W.M.; Szczylik, C.; Oudard, S.; Staehler, M.; Negrier, S.; Chevreau, C.; Desai, A.A.; Rolland, F.; et al. Sorafenib for treatment of renal cell carcinoma: Final efficacy and safety results of the phase III treatment approaches in renal cancer global evaluation trial. J. Clin. Oncol. 2009, 27, 3312–3318. [Google Scholar] [CrossRef] [PubMed]
- Escudier, B.; Porta, C.; Schmidinger, M.; Algaba, F.; Patard, J.J.; Khoo, V.; Eisen, T.; Horwich, A.; ESMO Guidelines Working Group. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2014, 25 (Suppl. 3), iii49–iii56. [Google Scholar] [CrossRef] [PubMed]
- Yagoda, A.; Petrylak, D.; Thompson, S. Cytotoxic chemotherapy for advanced renal cell carcinoma. Urol. Clin. N. Am. 1993, 20, 303–321. [Google Scholar]
- Schwandt, A.; Wood, L.S.; Rini, B.; Dreicer, R. Management of side effects associated with sunitinib therapy for patients with renal cell carcinoma. Onco Targets Ther. 2009, 2, 51–61. [Google Scholar] [PubMed]
- Larochelle, P.; Kollmannsberger, C.; Feldman, R.D.; Schiffrin, E.L.; Poirier, L.; Patenaude, F.; Ruether, D.; Myers, M.; Bjarnason, G. Hypertension management in patients with renal cell cancer treated with anti-angiogenic agents. Curr. Oncol. 2012, 19, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.M.; Carter, C.; Tang, L.; Wilkie, D.; McNabola, A.; Rong, H.; Chen, C.; Zhang, X.; Vincent, P.; McHugh, M.; et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the Raf/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004, 64, 7099–7109. [Google Scholar] [CrossRef] [PubMed]
- Wehland, M.; Bauer, J.; Magnusson, N.E.; Infanger, M.; Grimm, D. Biomarkers for anti-angiogenic therapy in cancer. Int. J. Mol. Sci. 2013, 14, 9338–9364. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Guo, G.; Li, X.; Zhang, C.; Huang, L.; Fang, D.; Song, Y.; Zhang, X.; Zhou, L. Retrospective analysis of the efficacy and safety of sorafenib in Chinese patients with metastatic renal cell carcinoma and prognostic factors related to overall survival. Medicine 2015, 94, e1361. [Google Scholar] [CrossRef] [PubMed]
- Iacovelli, R.; Verri, E.; Cossu Rocca, M.; Aurilio, G.; Cullura, D.; Santoni, M.; de Cobelli, O.; Nole, F. Is there still a role for sorafenib in metastatic renal cell carcinoma? A systematic review and meta-analysis of the effectiveness of sorafenib over other targeted agents. Crit. Rev. Oncol. Hematol. 2016, 99, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Borregales, L.D.; Adibi, M.; Thomas, A.Z.; Wood, C.G.; Karam, J.A. The role of neoadjuvant therapy in the management of locally advanced renal cell carcinoma. Ther. Adv. Urol. 2016, 8, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.B.; Panchal, H.P.; Karanwal, A.B.; Parekh, B.B.; Shah, S.; Prasad, S. Sunitinib in metastatic renal cell carcinoma: Experience from single center study, efficacy and safety. Indian J. Cancer 2016, 53, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Noronha, V.; Joshi, A.; Bakshi, G.; Tongaonkar, H.; Prabhash, K. Current evidence and the evolving role of sunitinib in the management of renal cell carcinoma. Indian J. Cancer 2016, 53, 102–108. [Google Scholar] [PubMed]
- Motzer, R.J.; Hutson, T.E.; Glen, H.; Michaelson, M.D.; Molina, A.; Eisen, T.; Jassem, J.; Zolnierek, J.; Maroto, J.P.; Mellado, B.; et al. Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: A randomised, phase 2, open-label, multicentre trial. Lancet Oncol. 2015, 16, 1473–1482. [Google Scholar] [CrossRef]
- Motzer, R.J.; Hutson, T.E.; Ren, M.; Dutcus, C.; Larkin, J. Independent assessment of lenvatinib plus everolimus in patients with metastatic renal cell carcinoma. Lancet Oncol. 2016, 17, e4–e5. [Google Scholar] [CrossRef]
- Sikic, D.; Meidenbauer, N.; Lieb, V.; Keck, B. Side effect management of tyrosine kinase inhibitors in urology: Hypertension. Urol. A 2016, 55, 952–955. [Google Scholar] [CrossRef] [PubMed]
- Rini, B.I.; Quinn, D.I.; Baum, M.; Wood, L.S.; Tarazi, J.; Rosbrook, B.; Arruda, L.S.; Cisar, L.; Roberts, W.G.; Kim, S.; et al. Hypertension among patients with renal cell carcinoma receiving axitinib or sorafenib: Analysis from the randomized phase III AXIS trial. Target Oncol. 2015, 10, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Hamnvik, O.P.; Choueiri, T.K.; Turchin, A.; McKay, R.R.; Goyal, L.; Davis, M.; Kaymakcalan, M.D.; Williams, J.S. Clinical risk factors for the development of hypertension in patients treated with inhibitors of the VEGF signaling pathway. Cancer 2015, 121, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Velcade (Bortezomib) and Sorafenib in Unresected or Metastatic Renal Cell Carcinoma. Available online: https://clinicaltrials.gov/ct2/show/study/NCT01100242?term=sorafenib+AND+renal+cell+carcinoma&rslt=With&lup_s=01%2F01%2F2014&lup_e=10%2F05%2F2016&rank=2§=X430156 (accessed on 15 January 2017).
- Phase III Study of Sorafenib in Patients with Renal Cell Carcinoma (RCC). Available online: https://clinicaltrials.gov/ct2/show/NCT00586105?id=NCT00586105&rank=1 (accessed on 15 January 2017).
- Bevacizumab, Sorafenib Tosylate, and Temsirolimus in Treating Patients with Metastatic Kidney Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT00378703?id=NCT00378703&rank=1 (accessed on 15 January 2017).
- Safety and Toxicity Study of Sorafenib in Patients with Kidney Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT00854620?id=NCT00854620&rank=1 (accessed on 15 January 2017).
- Sorafenib Dose Escalation in Renal Cell Carcinoma. Available online: https://clinicaltrials.gov/ct2/show/NCT00618982?id=NCT00618982&rank=1 (accessed on 15 January 2017).
- Fishman, M.N.; Tomshine, J.; Fulp, W.J.; Foreman, P.K. A systematic review of the efficacy and safety experience reported for sorafenib in advanced renal cell carcinoma (RCC) in the post-approval setting. PLoS ONE 2015, 10, e0120877. [Google Scholar] [CrossRef] [PubMed]
- Sunitinib Malate in Patients with Non-Clear Cell Renal Cell Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT00465179?term=sunitinib+AND+renal+cell+carcinoma&rslt=With&lup_s=01%2F01%2F2015&lup_e=10%2F05%2F2016&rank=1 (accessed on 15 January 2017).
- Sunitinib in Treating Patients with Locally Recurrent or Metastatic Kidney Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT00459875?term=sunitinib+AND+renal+cell+carcinoma&rslt=With&lup_s=01%2F01%2F2015&lup_e=10%2F05%2F2016&rank=7 (accessed on 15 January 2017).
- Sunitinib and Surgery in Treating Patients with Localized or Metastatic Kidney Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT00849186?term=sunitinib+AND+renal+cell+carcinoma&rslt=With&lup_s=01%2F01%2F2015&lup_e=10%2F05%2F2016&rank=8 (accessed on 15 January 2017).
- Sutent Rechallenge in mRCC Patients (RESUME). Available online: https://clinicaltrials.gov/ct2/show/results/NCT01827254?term=sunitinib+AND+renal+cell+carcinoma&rslt=With&lup_s=01%2F01%2F2015&lup_e=10%2F05%2F2016&rank=15§=X40156#othr (accessed on 15 January 2017).
- Eisen, T.; Loembe, A.B.; Shparyk, Y.; MacLeod, N.; Jones, R.J.; Mazurkiewicz, M.; Temple, G.; Dressler, H.; Bondarenko, I. A randomised, phase II study of nintedanib or sunitinib in previously untreated patients with advanced renal cell cancer: 3-Year results. Br. J. Cancer 2015, 113, 1140–1147. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Nosov, D.; Eisen, T.; Bondarenko, I.; Lesovoy, V.; Lipatov, O.; Tomczak, P.; Lyulko, O.; Alyasova, A.; Harza, M.; et al. Tivozanib versus sorafenib as initial targeted therapy for patients with metastatic renal cell carcinoma: Results from a phase III trial. J. Clin. Oncol. 2013, 31, 3791–3799. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, H.; Kondo, T.; Iida, S.; Takagi, T.; Tanabe, K. Treatment-related deterioration of renal function is associated with the antitumor efficacy of sunitinib in patients with metastatic renal cell carcinoma. Urol. Oncol. 2016, 8, 338.e1–338.e9. [Google Scholar] [CrossRef] [PubMed]
- Marcussen, N. Kap. 22: Patologived hypertension. In Patologi; FADL’s Forlazg: København, Denmark, 2013; pp. 711–716. [Google Scholar]
- PEG-Interferon α-2b and Sorafenib in Treating Patients with Unresectable or Metastatic Kidney Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT00589550?id=NCT00589550&rank=1 (accessed on 15 January 2017).
- Sunitinib in Treating Patients with Brain Metastases Caused by Kidney Cancer or Melanoma. Available online: https://clinicaltrials.gov/ct2/show/results/NCT00462982?term=sunitinib+AND+renal+cell+carcinoma&rslt=With&lup_s=01%2F01%2F2015&lup_e=10%2F05%2F2016&rank=10§=X340156#evnt (accessed on 15 January 2017).
- Sunitinib for Metastatic Renal Cell Cancer with Imaging Biomarker Assessments for the Early Prediction of Tumor Response. Available online: https://clinicaltrials.gov/ct2/show/results/NCT00694096?term=sunitinib+AND+renal+cell+carcinoma&rslt=With&lup_s=01%2F01%2F2015&lup_e=10%2F05%2F2016&rank=13§=X301256#evnt (accessed on 15 January 2017).
- Sorafenib Tosylate with or without Recombinant Interferon α-2b in Treating Patients with Metastatic Kidney Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT00126594?term=NCT00126594&rank=1 (accessed on 15 January 2017).
- Phase II Study of Afinitor vs. Sutent in Patients with Metastatic Non-Clear Cell Renal Cell Carcinoma (ASPEN). Available online: https://clinicaltrials.gov/ct2/show/NCT01108445?term=NCT01108445&rank=1 (accessed on 15 January 2017).
- Efficacy and Safety Comparison of RAD001 versus Sunitinib in the First-Line and Second-line Treatment of Patients with Metastatic Renal Cell Carcinoma (RECORD-3). Available online: https://clinicaltrials.gov/ct2/show/NCT00903175?term=NCT00903175&rank=1 (accessed on 15 January 2017).
- Pazopanib versus Sunitinib in the Treatment of Asian Subjects with Locally Advanced and/or Metastatic Renal Cell Carcinoma. Available online: https://clinicaltrials.gov/ct2/show/NCT01147822?term=NCT01147822&rank=1 (accessed on 15 January 2017).
- Pazopanib versus Sunitinib in the Treatment of Locally Advanced and/or Metastatic Renal Cell Carcinoma (COMPARZ). Available online: https://clinicaltrials.gov/ct2/show/NCT00720941?term=NCT00720941&rank=1 (accessed on 15 January 2017).
- Patient Preference Study of Pazopanib versus Sunitinib in Advanced or Metastatic Kidney Cancer (PISCES). Available online: https://clinicaltrials.gov/ct2/show/NCT01064310?term=NCT01064310&rank=1 (accessed on 15 January 2017).
- Choueiri, T.K.; Fay, A.P.; Gagnon, R.; Lin, Y.; Bahamon, B.; Brown, V.; Rosenberg, J.E.; Hutson, T.E.; Baker-Neblett, K.L.; Carpenter, C.; et al. The role of aberrant VHL/HIF pathway elements in predicting clinical outcome to pazopanib therapy in patients with metastatic clear-cell renal cell carcinoma. Clin. Cancer Res. 2013, 19, 5218–5226. [Google Scholar] [CrossRef] [PubMed]
- Choueiri, T.K.; Vaziri, S.A.J.; Jaeger, E.; Elson, P.; Wood, L.; Bhalla, I.P.; Small, E.J.; Weinberg, V.; Sein, N.; Simko, J.; et al. Von Hippel–Lindau gene status and response to vascular endothelial growth factor targeted therapy for metastatic clear cell renal cell carcinoma. J. Urol. 2008, 180, 860–866. [Google Scholar] [CrossRef] [PubMed]
- Heng, D.Y.C.; Xie, W.; Regan, M.M.; Harshman, L.C.; Bjarnason, G.A.; Vaishampayan, U.N.; Mackenzie, M.; Wood, L.; Donskov, F.; Tan, M.-H.; et al. External validation and comparison with other models of the International Metastatic Renal-Cell Carcinoma Database Consortium prognostic model: A population-based study. Lancet Oncol. 2013, 14, 141–148. [Google Scholar] [CrossRef]
- Rodriguez-Vida, A.; Strijbos, M.; Hutson, T. Predictive and prognostic biomarkers of targeted agents and modern immunotherapy in renal cell carcinoma. ESMO Open 2016, 1, e000013. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Mazumdar, M.; Bacik, J.; Berg, W.; Amsterdam, A.; Ferrara, J. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J. Clin. Oncol. 2016, 17, 2530–2540. [Google Scholar]
- Ljungberg, B.; Bensalah, K.; Canfield, S.; Dabestani, S.; Hofmann, F.; Hora, M.; Kuczyk, M.A.; Lam, T.; Marconi, L.; Merseburger, A.S.; et al. EAU guidelines on renal cell carcinoma: 2014 update. Eur. Urol. 2015, 67, 913–924. [Google Scholar] [CrossRef] [PubMed]
- NCCN Clinical Practice Guidelines in Oncology. Available online: https://www.nccn.org/professionals/physician_gls/f_guidelines.asp (accessed on 10 January 2017).
- Rini, B.I.; Halabi, S.; Rosenberg, J.E.; Stadler, W.M.; Vaena, D.A.; Archer, L.; Atkins, J.N.; Picus, J.; Czaykowski, P.; Dutcher, J.; et al. Phase III trial of bevacizumab plus interferon α versus interferon α monotherapy in patients with metastatic renal cell carcinoma: Final results of CALGB. J. Clin. Oncol. 2010, 28, 2137–2143. [Google Scholar] [CrossRef] [PubMed]
- Rini, B.I.; Cohen, D.P.; Lu, D.R.; Chen, I.; Hariharan, S.; Gore, M.E.; Figlin, R.A.; Baum, M.S.; Motzer, R.J. Hypertension as a biomarker of efficacy in patients with metastatic renal cell carcinoma treated with sunitinib. J. Natl. Cancer Inst. 2011, 103, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Gunnarsson, O.; Pfanzelter, N.R.; Cohen, R.B.; Keefe, S.M. Evaluating the safety and efficacy of axitinib in the treatment of advanced renal cell carcinoma. Cancer Manag. Res. 2015, 7, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Nosov, D.A.; Esteves, B.; Lipatov, O.N.; Lyulko, A.A.; Anischenko, A.A.; Chacko, R.T.; Doval, D.C.; Strahs, A.; Slichenmyer, W.J.; Bhargava, P. Antitumor activity and safety of tivozanib (AV-951) in a phase II randomized discontinuation trial in patients with renal cell carcinoma. J. Clin. Oncol. 2012, 30, 1678–1685. [Google Scholar] [CrossRef] [PubMed]
- Zurita, A.J.; Jonasch, E.; Wang, X.; Khajavi, M.; Yan, S.; Du, D.Z.; Xu, L.; Herynk, M.H.; McKee, K.S.; Tran, H.T.; et al. A cytokine and angiogenic factor (CAF) analysis in plasma for selection of sorafenib therapy in patients with metastatic renal cell carcinoma. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2012, 23, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.T.; Liu, Y.; Zurita, A.J.; Lin, Y.; Baker-Neblett, K.L.; Martin, A.-M.; Figlin, R.A.; Hutson, T.E.; Sternberg, C.N.; Amado, R.G.; et al. Prognostic or predictive plasma cytokines and angiogenic factors for patients treated with pazopanib for metastatic renal-cell cancer: A retrospective analysis of phase 2 and phase 3 trials. Lancet Oncol. 2012, 13, 827–837. [Google Scholar] [CrossRef]
- Hutson, T.E.; Lesovoy, V.; Al-Shukri, S.; Stus, V.P.; Lipatov, O.N.; Bair, A.H.; Rosbrook, B.; Chen, C.; Kim, S.; Vogelzang, N.J. Axitinib versus sorafenib as first-line therapy in patients with metastatic renal-cell carcinoma: A randomised open-label phase 3 trial. Lancet Oncol. 2013, 14, 1287–1294. [Google Scholar] [CrossRef]
- Negrier, S.; Gravis, G.; Perol, D.; Chevreau, C.; Delva, R.; Bay, J.O.; Blanc, E.; Ferlay, C.; Geoffrois, L.; Rolland, F.; et al. Temsirolimus and bevacizumab, or sunitinib, or interferon α and bevacizumab for patients with advanced renal cell carcinoma (TORAVA): A randomised phase 2 trial. Lancet Oncol. 2011, 12, 673–680. [Google Scholar] [CrossRef]
- Motzer, R.J.; Hutson, T.E.; Cella, D.; Reeves, J.; Hawkins, R.; Guo, J.; Nathan, P.; Staehler, M.; de Souza, P.; Merchan, J.R.; et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N. Engl. J. Med. 2013, 369, 722–731. [Google Scholar] [CrossRef] [PubMed]
- Escudier, B.; Porta, C.; Bono, P.; Powles, T.; Eisen, T.; Sternberg, C.N.; Gschwend, J.E.; de Giorgi, U.; Parikh, O.; Hawkins, R.; et al. Randomized, controlled, double-blind, cross-over trial assessing treatment preference for pazopanib versus sunitinib in patients with metastatic renal cell carcinoma: PISCES study. J. Clin. Oncol. 2014, 32, 1412–1418. [Google Scholar] [CrossRef] [PubMed]
| Degree of Hypertension | Definition |
|---|---|
| 1 | Prehypertension |
| Systolic BP: 120–139 mmHg and/or | |
| diastolic BP: 80–89 mmHg | |
| 2 | Systolic BP: 140–159 mmHg and/or |
| diastolic BP: 90–99 mmHg | |
| 3 | Systolic BP >160 mmHg and/or |
| diastolic BP >100 mmHg | |
| 4 | Life-threatening hypertension (neurological outcomes) |
| Drug(s) | Diagnosis | No. of Patients | Phase | Design | Frequency of Hypertension | Clinical Trials. Gov | Ref. |
|---|---|---|---|---|---|---|---|
| Velcade (bortezomib) and sorafenib | Metastatic renal cell carcinoma | 17 | II | Interventional, non-randomized | 35.3% | NCT01100242 | [21] |
| Sorafenib | Metastatic renal cell carcinoma | 39 | III | Interventional, non-randomized | 18% | NCT00586105 | [22] |
| Sorafenib + PEG-interferon α-2b | Kidney cancer | 1 | NP | Interventional, single group assignment | Not yet reported | NCT00589550 | [35] |
| Bevacizumab, sorafenib tosylate, and temsirolimus | Metastatic kidney cancer | 331 | NP | Interventional, randomized | Hypertension: Bevacizumab + sorafenib (46.7%) and temsirolimus + sorafenib (37.4%) | NCT00378703 | [23] |
| Sorafenib | Renal cell carcinoma | 9 | II | Interventional, single group assignment | 77.8% | NCT00854620 | [24] |
| Sorafenib | Renal cell carcinoma | 83 | II | Interventional, single group assignment | 48% | NCT00618982 | [25] |
| Sunitinib | Advanced non-clear cell carcinoma | 57 | II | Interventional, single group assignment | 61.4% | NCT00465179 | [27] |
| Sunitinib | Kidney cancer (locally or metastatic) | 24 | II | Interventional, single group assignment | 8.7% | NCT00459875 | [28] |
| Sunitinib | Kidney cancer (locally or metastatic) | 26 | NP | Interventional, single group assignment | 25% | NCT00849186 | [29] |
| Sunitinib | Metastatic kidney cancer and melanoma | 8 | II | Interventional, single group assignment | Not reported | NCT00462982 | [36] |
| Sunitinib | Renal cell cancer | 25 | I | Interventional, single group assignment | Not reported | NCT00694096 | [37] |
| Sunitinib | Metastatic renal cell carcinoma | 61 | NP | Retrospective observational cohort | 3.9% | NCT01827254 | [30] |
| Sunitinib | Advanced renal cell cancer | 32 | II | Interventional, randomised, controlled trial | 15.6% | From Pubmed.gov | [31] |
| Sorafenib tosylate with or without recombinant interferon α-2b | Metastatic renal cell carcinoma | Sorafenib tosylate with recombinant interferon α-2b: 40 Sorafenib tosylate without recombinant interferon α-2b: 40 | II | Interventional | Sorafenib tosylate with recombinant interferon α-2b: 25% Sorafenib tosylate without recombinant interferon α-2b: 40% | NCT00126594 | [38] |
| Sunitinib | Metastatic non-clear cell renal cell carcinoma | 51 | II | Interventional | 45.1% | NCT01108445 | [39] |
| Sunitinib + everolimus | Renal cell carcinoma | Everolimus 1. line/sunitinib 2. line: 238 Sunitinib 1. line/everolimus 2. line: 231 | II | Interventional | Everolimus 1. line/sunitinib 2. line: 25.6% Sunitinib 1. line/everolimus 2. line: 36.4% | NCT00903175 | [40] |
| Sunitinib | Renal cell carcinoma | 179 | II | Interventional | 53.11% | NCT01147822 | [41] |
| Sunitinib | Renal cell carcinoma | 553 | III | Interventional | 40.51% | NCT00720941 | [42] |
| Sunitinib | Renal cell carcinoma | 148 | III | Interventional | 24.32% | NCT01064310 | [43] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Randrup Hansen, C.; Grimm, D.; Bauer, J.; Wehland, M.; Magnusson, N.E. Effects and Side Effects of Using Sorafenib and Sunitinib in the Treatment of Metastatic Renal Cell Carcinoma. Int. J. Mol. Sci. 2017, 18, 461. https://doi.org/10.3390/ijms18020461
Randrup Hansen C, Grimm D, Bauer J, Wehland M, Magnusson NE. Effects and Side Effects of Using Sorafenib and Sunitinib in the Treatment of Metastatic Renal Cell Carcinoma. International Journal of Molecular Sciences. 2017; 18(2):461. https://doi.org/10.3390/ijms18020461
Chicago/Turabian StyleRandrup Hansen, Caroline, Daniela Grimm, Johann Bauer, Markus Wehland, and Nils E. Magnusson. 2017. "Effects and Side Effects of Using Sorafenib and Sunitinib in the Treatment of Metastatic Renal Cell Carcinoma" International Journal of Molecular Sciences 18, no. 2: 461. https://doi.org/10.3390/ijms18020461