1. Introduction
The use of unmodified nucleic acids as insecticides looks very promising, since they can work selectively, are subject to fast biodegradation in ecosystems (in contrast to the majority of conventional chemical insecticides), and the commercial synthesis of nucleic acids in vitro is becoming more affordable.
RNA interference (RNAi) and the use of double-stranded RNA (dsRNA) fragments is a viable possibility for use in the control of insect pests, particularly lepidopterans [
1,
2]. However, this approach has several apparent drawbacks. The efficacy of RNAi action in lepidopteran insects could be compromised because: (i) some insect genes are resistant to RNAi [
3], (ii) the high cost of RNA substrate synthesis, and (iii) the problem of target delivery under field conditions [
2]. Moreover, the dsRNAs in cells are cleaved into numerous, very short (21–23 nucleotide) siRNAs with abundant direct sequence matches among the genomes of non-target organisms, seriously lowering the selectivity of this approach [
4].
In 2008, we proposed the topical application of nucleic acids for control of phytophagous insects. Application of short pest-specific unmodified antisense DNA fragments (DNA insecticides) against the gypsy moth
Lymantria dispar, a major insect pest of hardwood trees, was followed by significant larval mortality [
5]. Three years later, Wang et al. [
6] topically applied dsRNA fragments specific for the Asian corn borer
Ostrinia furnacalis, which was also followed by significant larval mortality [
6]. Previously, it had been thought that oral administration was the only possible way to deliver double-stranded RNAs to target tissues, other than injection, as the insect midgut is not protected by chitin integument [
2]. The induction of insect mortality by topical application of single-stranded DNA or double-stranded RNA could be explained by their passage into interior tissues via the tracheal system, which is not covered by chitinous exoskeleton [
1] or via diffusion through the soft thin cuticle of young instars.
We see a number of advantages that make DNA insecticides a more promising approach against lepidopterans at the larval stage than elaborate RNA preparations. First, short (~18–20 nucleotides long) insect-specific DNA insecticides are more affordable compared to relatively long double-stranded RNA fragments. Second, gene silencing achieved by feeding or injection of double-stranded RNA requires high concentrations for success. This issue could be resolved with topical application of DNA insecticides based on short antisense DNA fragments acting in substantially lower concentrations. In most studies, the “standard” amount of double-stranded RNA injected to achieve high levels of RNAi (but not death) in insects varies between 1 and 100 µg per mg of biomass [
3]. For comparison, in experiments with DNA insecticides, we topically applied 3–30 pmol of viral 18-nucleotide-long DNA fragments per gypsy moth larva, corresponding to approximately 1.8–180 ng of DNA per mg of larval biomass. Therefore, single-stranded DNA insecticides work in substantially lower concentrations; accordingly, this approach may be cheaper for insect pest control compared with RNA preparations. It may be impossible to use DNA insecticides against secretive insects and adult beetles, because elytra could provide some protection from a contact insecticide. Nevertheless, DNA insecticides look very suitable for use in insect pest control of non-secretive lepidopteran pests at the larval stage, especially during early larval instars, when the insects’ exoskeletons are thin. Third, very short 18 nucleotides long pest-specific antisense DNA fragments (DNA insecticide) will not be cleaved in the cells of non-target organisms, unlike relatively long dsRNAs, which are diced into very short siRNAs that may silence non-target genes [
4]. Lastly, the presence of the 2′-OH group makes the hydrolysis of RNA much more facile than hydrolysis of DNA [
7]. Thus, DNA insecticides will be more stable than RNA preparations under natural conditions, and thus, can cause a greater insecticidal effect before they are degraded.
According to results from our most recent research, unmodified antisense DNA oligonucleotides (oligoDNAs) of the LdMNPV (
Lymantria dispar multiple nucleopolyhedrovirus)
IAP-3 (inhibitor-of-apoptosis) gene have pronounced insecticidal effects on LdMNPV-free gypsy moths [
5,
8,
9]. Baculoviruses are insect pathogenic viruses used as biological control agents. Baculoviruses encode inhibitors-of-apoptosis (IAP) proteins, which are classified into 5 groups, IAP-1–5, based on their sequence homology. Most of the baculovirus IAPs with anti-apoptotic functions belong to the
IAP-3 group, with certain exceptions [
10]. All IAP genes isolated from different baculoviruses display two distinct structural features. The first is the presence of amino-terminal repeats of an amino acid sequence termed the baculovirus IAP repeat (BIR). The second is a zinc binding domain known as a RING (really interesting new gene) finger [
11]. BIR contains approximately 70 amino acids that coordinate with a zinc ion via histidine and cysteine residues [
12]. It has been shown that the BIR domain is necessary for the interaction of IAP proteins with diverse pro-apoptotic factors, including invertebrate death inducers, and vertebrate and invertebrate members of the caspase family of proteases [
10]. RING domains are characterized by the presence of 6 or 7 cysteines and 1 or 2 histidines that form a cross brace architecture and coordinate two zinc ions. RING domains often function as modules that confer ubiquitin protein ligase (E3) activity and, in conjunction with an ubiquitin activity enzyme (E1) and an ubiquitin conjugating enzyme (E2), catalyze the transfer of ubiquitin to target proteins. All known RING-containing IAPs have E3 activity; the range of substrates includes molecules involved in apoptosis and signaling, as well as the RING-containing IAP themselves in a homo- or heterotypic fashion [
12]. A typical animal IAP protein includes three homologous BIR domains and a RING domain near the C-terminus. Both sequence and phylogenetic analyses have revealed that, in the case in which the viral IAP has only one BIR domain, it is homologous to the third BIR domain of animal IAP. For example, in LdMNPV the
IAP-3 gene encodes a 155-amino acid long protein, where the region from the 6th to 71st amino acids belongs to the BIR domain, and the region from the 105th to 149th amino acids belongs to the RING domain [
13]. Thus, the presence of homologous IAP in
Lymantria dispar, as was supposed in our previous studies, is a good target for apoptosis induction after oligoDNAs application. Moreover, the baculovirus IAPs bear a striking resemblance to the cellular IAPs carried by the host insects that they infect [
14]. Cellular IAPs are a highly conserved family of survival factors that regulate developmental- and stress-induced apoptosis, as well as inflammation, the cell cycle, and some signaling processes [
12,
15].
Although the exact mechanism of action of DNA insecticides is currently under study, we have a lot of evidence that DNA insecticides work in a manner similar to unmodified [
16] and modified antisense oligonucleotides [
17] used in medicine, generating antisense effects through RNase H-dependent mechanism [
18,
19]. The mechanism of action of antisense oligoDNAs from IAP genes is thought to be the same as that of antisenseRNase H-dependent oligonucleotides. RNase H is a ubiquitous enzyme that hydrolyzes the RNA strand of an RNA/DNA duplex. Oligonucleotide-assisted RNase H-dependent reduction of targeted RNA expression can be quite efficient, reaching 80–95% down-regulation of protein and mRNA expression [
10,
16]. In the case of IAP targeting, down-regulation of the anti-apoptosis protein expression leads to increased apoptotic processes in cells and subsequent death of the insect. Historically, we have chosen RING as a target because the RING domain is a highly conserved segment of insect and baculovirus IAP genes. Using the GenBank NIH genetic sequence database, we searched for the conserved antisense segment inside the RING domain fragment in the LdMNPV
IAP-3 gene and decided to use the 5′-CGACGTGGTGGCACGGCG-3′ sequence. This fragment is found in all LdMNPV genomes represented in GenBank and is considered to be well conserved. We decided to use this conserved fragment as oligoDNA (oligoRING) for the treatments, and the presence of 4 CG motifs in oligoRING suggests us to use oligoCpG, a non-specific TLR-9 inducer [
20], as a control. In the same vein, DNA insecticides could resolve or improve upon an important insecticide resistance problem: use of short single-stranded fragments of highly conserved segments of insect host anti-apoptosis genes will result in slower development of resistance to these insecticides, because it is known that the potential mutations that change the target anti-apoptosis genes occur at a very low rate in the conserved parts of the genes.
It is important to note that the use of phosphodiester oligonucleotides in mammalian tissues is limited, as they are rapidly degraded by intracellular endonucleases and exonucleases [
16]. However, the activity of insect enzymes (e.g., esterases) is weaker than that of mammals [
21]. This paves the way for the prospective use of unmodified antisense oligonucleotides as a selective tool for insect pest control [
10,
19,
22].
Our particular attention will be devoted to LdMNPV-infected larvae, a group widely distributed among gypsy moth populations [
23,
24]. Baculovirus infections in lepidopterans may cause difficulties in silencing with nucleic acids, particularly dsRNA [
3,
25]. In this study we investigate further the insecticidal effect of the oligoRING fragment antisense to RING (really interesting new gene) domain of the LdMNPV
IAP-3 gene on both non-infected and LdMNPV-infected gypsy moth larvae, and show how to turn this possible weakness into a strength by applying this approach to insect pest management.
3. Discussion
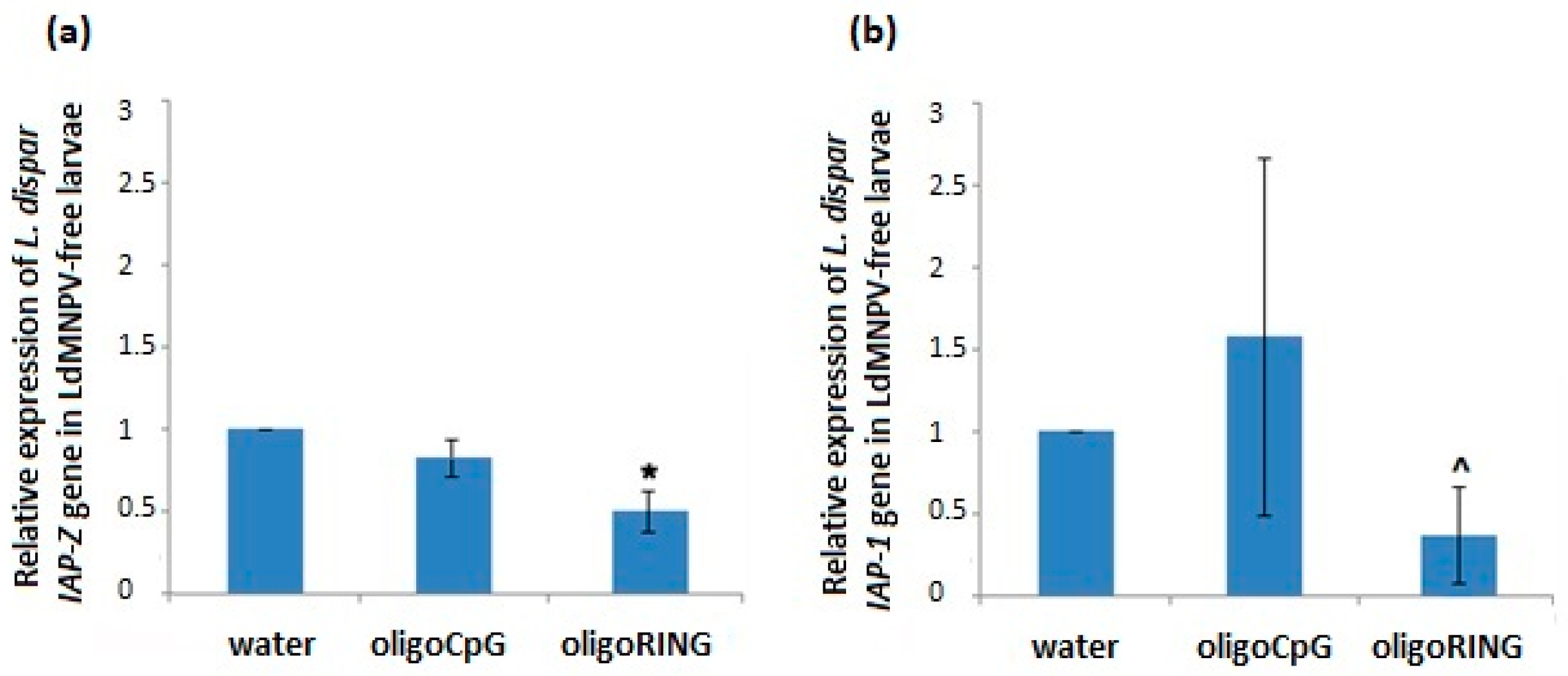
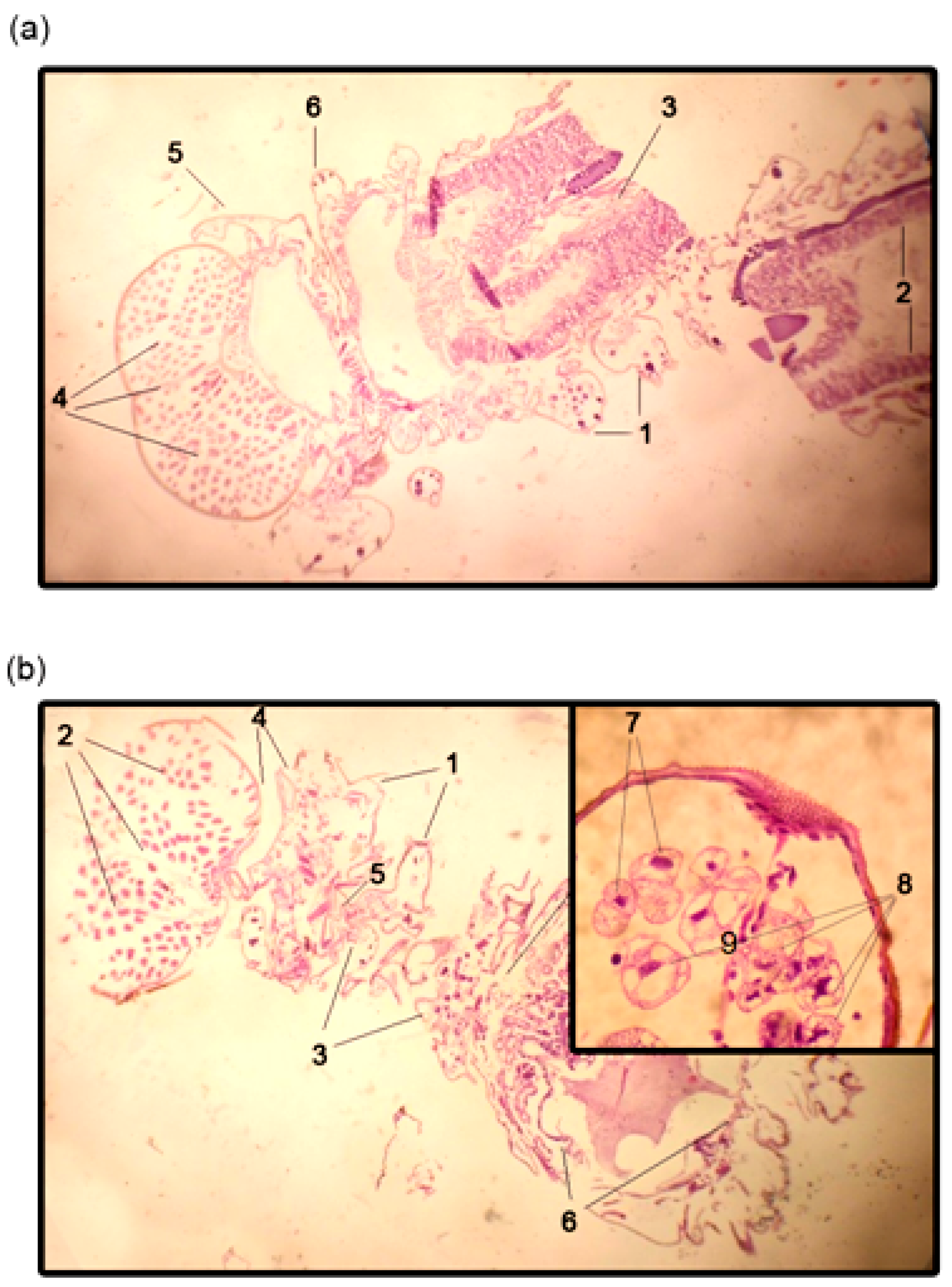
This study reports progress made both in basic knowledge and in applied pesticide research. The results obtained from the DNA sequencing lead us to believe that we have found a fragment of the mRNA belonging to an anti-apoptosis gene (
IAP-Z gene) of the gypsy moth. The oligoRING performed as a perfect complement to the base pairing on the fragment of gypsy moth cDNA and is an antisense molecule to the investigated mRNA. In this work, we provide evidence that the target gene, the
IAP-Z gene, is definitely expressed and that oligoRING fragment down-regulates it in LdMNPV-free larvae. Its actions as an antisenseRNase H-dependent oligonucleotide [
16] are accompanied by apoptotic patterns such as involution of cells, condensation (pyknosis), and fragmentation (karyorrhexis) of nuclear material. However, the observed effects may not have reached certain critical levels; the 2.04 ± 0.29-fold stronger down-regulation of host
IAP-Z gene we observed may not be enough to cause significant mortality among the treated insects. In our previous experiments, some groups of LdMNPV-free gypsy moth larvae reared under laboratory conditions also did not show significant sensitivity to treatment with oligoIAPs [
9]. This suggests that either insect populations may either possess different genetically based sensitivity to the specific oligonucleotide, or that an additional specific factor (or factors) might be necessary for apoptosis induction and synthesis of a sufficient amount of mRNA for a target anti-apoptosis gene silencing [
3]. According to our theory, when the oligoRING shows a significant insecticidal effect on LdMNPV-free gypsy moth larvae, it may interfere with the expression of the host
IAP-Z gene, which leads to apoptosis of the insect cells and subsequent death of larvae. Similarly, to observations of the RNAi in lepidopterans [
3], the obvious reason why the oligoRING does not always exert an insecticidal effect on healthy and LdMNPV-free gypsy moth larvae grown under lab conditions compared with those grown in a natural habitat, with exposure to concomitant stress factors [
8,
9], is that its action depends on the dynamics of synthesis and breakdown of the mRNA of the target host
IAP-Z gene. Although down-regulation of host IAP genes does not always lead to a significant mortality rate among LdMNPV-free larvae grown under laboratory conditions, in our experiments this approach always worked on early instar larvae insects of a younger larval age collected from a forest where the presence of many natural stress factors (baculovirus and bacterial infections, UV radiation, air pollution, etc.) may activate an apoptosis–anti-apoptosis system. For example, in this study we show that in LdMNPV-infected larvae 14 days post-infection, the total expression of host
IAP-Z and LdMNPV
IAP-3 genes is 3.65 ± 0.39-fold more strongly up-regulated than the host
IAP-Z gene in LdMNPV-free larvae (
p < 0.05). This indicates the presence of a higher concentration of target mRNA molecules and increases the probability of interaction with the antisense oligoRING, leading to the down-regulation of expression of target anti-apoptosis proteins. Since gypsy moth control will mainly take place in forests, it is hypothesized that this approach will be of higher efficiency under natural conditions.
In the absence of stress factors (e.g., viral infection), concentration of the target anti-apoptosis mRNA would be low, and a pronounced insecticidal effect should not be generated by the oligoRING. In the case of LdMNPV-infected larvae, we think that the oligoRING obviously efficiently blocks mainly LdMNPV
IAP-3 mRNA and triggers higher levels of apoptosis in the infected cells, leading to the subsequent death of the insect. The high efficiency of baculovirus infection is explained in part by the ability of the virus to suppress the host defense machinery connected with the apoptosis pathway, an ability that accounts for baculovirus inhibitor-of-apoptosis genes (vIAPs). Indeed, in this study, LdMNPV-infected gypsy moth larvae were treated with an antisense oligoRING fragment specific to the viral
IAP-3 and host
IAP-Z genes, and the oligoRING fragment caused significant total down-regulation of host
IAP-Z and baculovirus
IAP-3 genes, which resulted in increased mortality of the insects and development of apoptotic DNA fragmentation. Thus, baculovirus infection in gypsy moths did not cause difficulties by silencing the nucleic acids described in [
25], when we used an antisense oligonucleotide complementary to the virus
IAP-3 gene to avoid possible physiological barriers. To our knowledge, this is the first case of a recorded insecticidal effect of a virus-specific antisense oligoDNAs on LdMNPV-infected gypsy moth larvae, which could be of potential significance in insect pest management. We describe the newly discovered phenomenon using the term the VOVA (Virus before Oligonucleotide—Vent to Apoptosis) effect.
It is important to note that most of the baculovirus IAPs belong to the
IAP-3 group [
28]. To achieve efficient infection rates, baculoviruses induce a pro-apoptotic DNA Damage Response (DDR) [
31,
32]. Induction of the DDR by viral replication increases virus yields up to 100-fold [
31]. The DDR induces depletion of host inhibitor-of-apoptosis genes (hIAP), and thus, promotes cell death [
33]. To overcome the consequences of DDR activation, baculoviruses employ special anti-apoptotic proteins (vIAPs) [
10,
12]. These proteins either interfere directly with the cellular apoptotic proteins, or alter the activity of cellular genes, leading to an anti-apoptotic state [
34]. It is noteworthy that, despite the evolutionary relatedness of the vIAPs and hIAPs, there is an important structural distinction between them. In contrast to the hIAPs, which possess a specific N-terminal domain and are negatively regulated by signal-induced N-terminal degrons upon virus infection, the vIAPs do not have an equivalent N-terminal domain [
14,
33]. This makes vIAPs more stable and active as apoptosis inhibitors [
14]. For the
Autographa californica multicapsid nuclear polyhedrosis virus, it was found that by 12 h post infection, the viral transcripts comprised 38% of total cellular mRNA [
35]. Thus, in the presence of LdMNPV infection, it is better to rely on the alteration of expression of functionally important virus genes (for example, anti-apoptosis genes) that could have an insecticidal effect on gypsy moths. According to data discussed here, the oligoRING fragment is among the antisense oligonucleotides most appropriate for triggering higher mortality among LdMNPV-infected gypsy moth larvae. By expanding our study from non-infected
Lymantria dispar larvae to include LdMNPV-infected
Lymantria dispar larvae, we were able to investigate the variants also to be found in nature, thus expanding the proofs of DNA insecticide effectiveness.
4. Materials and Methods
4.1. Origin of L. dispar Larvae
Egg masses of the gypsy moth Lymantria dispar (Lepidoptera: Erebidae) were identified and collected in November 2014 from forested wilderness located near Opolznevoye on the Crimean peninsula (lat. 44.3971, long. 33.9290, alt. 27.4 m).
4.2. Insect Rearing
Gypsy moth larvae were grown in Petri dishes under standard conditions with wheat germ-based medium at a temperature of 25 °C used for treatments [
9,
36,
37,
38]. Laboratory scales (Axis BTU210; Axis, Gdańsk, Poland) with 1 mg discreteness were used to weigh larvae.
4.3. Sequences of the Applied oligoDNAs Fragments
We designed an antisense RING domain fragment according to the LdMNPV genome sequenced by Kuzio et al. [
13] found in ICTVdb database (
http://www.ictvonline.org). The sequence of the RING domain fragment of the LdMNPV
IAP-3 gene is as follows: 5′-CGACGTGGTGGCACGGCG-3′ (antisense strand; experimental group; oligoRING). The sequences of oligoDNAs used as a control were as follows: 5′-CGCGCGCGCGCGCGCGCG-3′ (oligoCpG). OligoDNAs were synthesized by Evrogen (Moscow, Russia) [
8,
36].
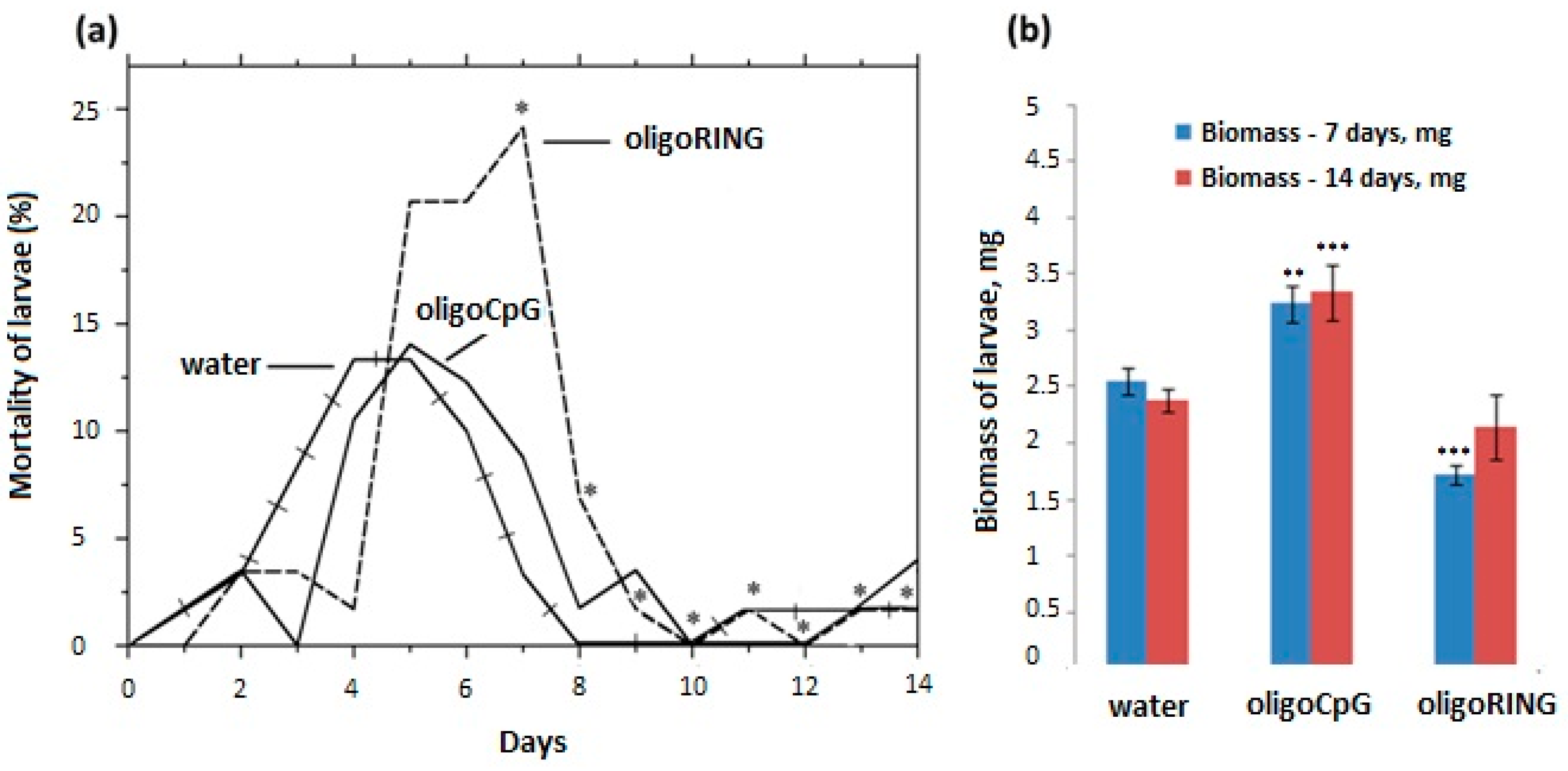
4.4. OligoDNA Treatment of Lymantria Dispar Larvae
Before the experiment, larvae were randomized into groups and weighed to achieve the same starting point for measuring biomass for each group; the difference in biomass among different groups was within 3%. On average, 20–25 1st instar larvae were used per each control and experimental group for the treatment with oligoDNAsfragments. Each experiment was performed three times. A water solution with an ssoligoDNAsfragment (10 pmol/µL, either oligoRING or control oligoCpG sequence) was applied to larvae topically using a hand-held sprayer. We collected small drops of solution from the surface of 10 larvae and found approximately 0.2–0.3 µL solution on each larvae after pulverization (2–3 pmol of ssoligoDNAs per larvae). On the 7th and 14th day after the treatment, the biomass of live larvae was measured. To exclude possible LdMNPV infection, prior to experiments with non-infected gypsy moth larvae, we used PCR with two oligonucleotide primers specific to the LdMNPV capsid gene p39: 5′-ACGTTCTCGTTGAACGTGCTG-3′ (forward primer) and 5′-CTGGTGAACCACAAAACCCTG-3′ (reverse primer) [
9].
4.5. Infection of Lymantria Dispar Larvae with LdMNPV
To infect the gypsy moth larvae with LdMNPV, the baculovirus preparation ‘Pinkvir’ (Pushkino, Russia) was used. After 1-day starvation, the insects were allowed to feed on the virus polyhedra-containing wheat germ-based medium for 2 days (10,000 virus polyhedra per 1 mg of medium). The larvae were then transferred to non-infected medium. Phase contrast microscopy and Goryayev’s chamber were used to count viral polyhedra in the ‘Pinkvir’ preparation. The larvae were treated with water, oligoRING, or control (oligoCpG) ssoligoDNAsfragment after 48 h infection with the virus.
4.6. Detection of the LdMNPV Infection in L. dispar by PCR
Specific PCR conditions and primers for the LdMNPV p39 capsid protein gene were used for detection of LdMNPV infection in gypsy moth larvae [
9]. DNA was extracted using the DNA-sorb-AM kit (AmpliSens, Moscow, Russia) and PCR reactions were performed using the AmpliSens-200-1amplification kit (AmpliSens), following the manufacturer’s protocols. DNA was initially denatured for 3 min at 94 °C, followed by 5 cycles of 1 min denaturation at 94 °C, 1 min hybridization at 61 °C and 1 min elongation at 72 °C, followed by 30 cycles of 0.75 min denaturation at 94 °C, 0.75 min hybridization at 61 °C, and 0.75 min elongation at 72 °C, followed by a final elongation step at 72 °C for 5 min.
4.7. Search for L. dispar mRNA Homologous to the LdMNPV IAP-3 Gene
Total mRNA was extracted from non-infected gypsy moth larvae using an RNA Extract kit (Evrogen), following the manufacturer’s protocols. First strand cDNA synthesis was performed using a MMLV RT kit (Evrogen), following the manufacturer’s protocols. Primers oligoBIR (5′-GCCGGCGGAACTGGCCCA-3′, sense strand) and oligoRING (5′-CGACGTGGTGGCACGGCG-3′, antisense strand; used as a DNA insecticide in this experiment) from the LdMNPV
IAP-3 gene were applied to detect homologous anti-apoptosis mRNA in the gypsy moth using its first cDNA strand. PCR reactions were performed on 2 µL (10 ng/µL) of DNA using 0.75 units of Goldstar polymerase (Eurogentec, Angers, France), and 0.2 mM dNTP, 1.5 mM MgCl
2, and 50 pmol of each primer. DNA was initially denatured for 4 min at 95 °C, followed by 30 cycles of 1 min of denaturation at 94 °C, 1 min of hybridization at 60 °C, and 1 min of elongation at 72 °C, followed by a final elongation step at 72 °C for 7 min. PCR products from the larvae were purified using the NucleoSpin Extract II Kit (Macherey-Nagel, Hoerdt, France) and the sequencing polymerase reaction was performed with Big Dye Terminator v3.1 RR-100 Mix (Applied Biosystems, Saint Aubin, France). Polymerase reactions were performed with 5 µL purified DNA and 0.35 µL of primers (10 pmol/µL). DNA was initially denatured for 2 min at 96 °C, followed by 30 cycles of 10 s of denaturation at 96 °C, 15 s of hybridization at 50 °C, and 4 min of elongation at 60 °C. Amplicons were sequenced in both directions with a capillary DNA sequencer (ABI PRISM 3100, Applied Biosystems). DNA sequences were analyzed using BLAST [
39] and ClustalW 2.0.3 programs [
40].
4.8. Quantification of Lymantria Dispar IAP-Z and IAP-1 Gene Expression
RNA extraction was carried out using a PureLink® RNA Mini Kit (Ambion, Life Technologies, Waltham, MA, USA), according to the manufacturer’s instructions. For extraction, gypsy moth larvae were ground using a pestle in liquid nitrogen in 1.5 mL tube. Three independent extractions, each with two larvae, were carried out to produce replicates for each condition. The quality of the extracted total RNA was assessed by loading 5 µL of the eluted volume onto a 1.5% agarose gel and running the gel in TBE (Tris-borate-EDTA) buffer (10 V/cm) for 30 min. The quantity, intensity, and pattern of RNA bands were equal in all experimental groups, confirming the quality and reproducibility of RNA extraction from the insect material. For reverse transcription, the total RNA (5 µg) was annealed with oligo(dT)18 primer and analyzed using aRevertAid H Minus Reverse Transcriptase kit (Thermo Scientific, Waltham, MA, USA), according to the manufacturer’s instructions. The reaction was conducted at 42 °C for 60 min in Thermostat Termite (DNA Technology, Moscow, Russia).
For quantitative real time PCR studies and amplification with gene specific primers, an aliquot of obtained cDNA (0.5 µL) was used for each sample, with addition of the following primers for quantification of the Lymantria dispar IAP-1 gene: forward 5′-CGCTGCAAGTAATGCTGAGG-3′, reverse 5′-GCACACGCAACTACATGTCC-3′ and IAP-Z gene, forward 5′-AGGCCCGTGTCGCCGGTC-3′ (oligoIAP-Z), reverse 5′-CGACGTGGTGGCACGGCG-3′ (oligoRING). The qPCRmix-HS SYBR (Evrogen) master mix was used according to the manufacturer’s instructions. A LightCycler® 96 instrument (Roche, Basel, Switzerland) was used to set up amplification according to the following procedure: 10 min of initial denaturation at 95 °C, followed by 40 cycles with 10 s of denaturation at 95 °C, 20 s of annealing at 60 °C, and 16 s of elongation at 72 °C. As a final step, all PCR products were melted to estimate the specificity of amplification and presence of additional products.
4.9. Histological Studies
Histological slides were made using non-infected gypsy moth larvae 14 days after treatment with oligonucleotides. Live larvae were fixed in 10% formaldehyde for 2 days. After that, larvae were dried in isopropyl alcohol and then placed in paraffin blocks. Sections (4 µm) were made with a microtome (Rotmik, Moscow, Russia), stained with hematoxylin and eosin (BioVitrum, Sankt-Peterburg, Russia), and fixed for analysis according to the manufacturer’s instructions (Blik, Sankt-Peterburg, Russia).
4.10. Apoptosis Detection
A Quick Apoptotic DNA LadderDetectionKit (Life Technologies) was used according to the manufacturer’s instructions to investigate the level of apoptotic DNA fragmentation in live LdMNPV-infected gypsy moth larvae.
4.11. Statistical Analyses
We used the non-parametric Pearson’s chi-squared test (χ2) with Yates’s correction and the Mann–Whitney test to evaluate the significant difference between the groups’ means (STATISTICA 7 software, Palo Alto, CA, USA).