Using Next-Generation Sequencing to Detect Differential Expression Genes in Bradysia odoriphaga after Exposure to Insecticides
Abstract
:1. Introduction
2. Results
2.1. RNA-Seq Sequencing and Assembly
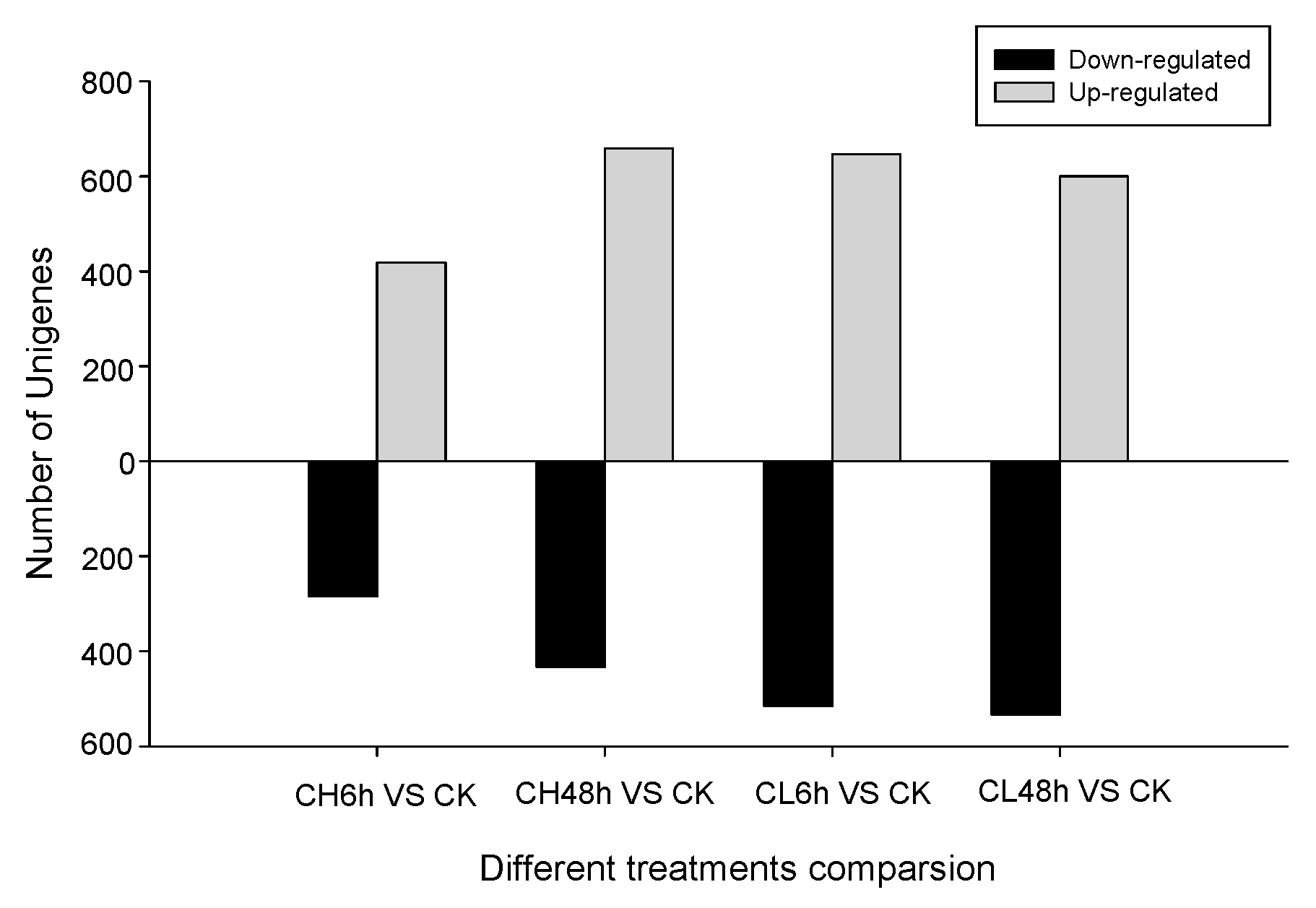
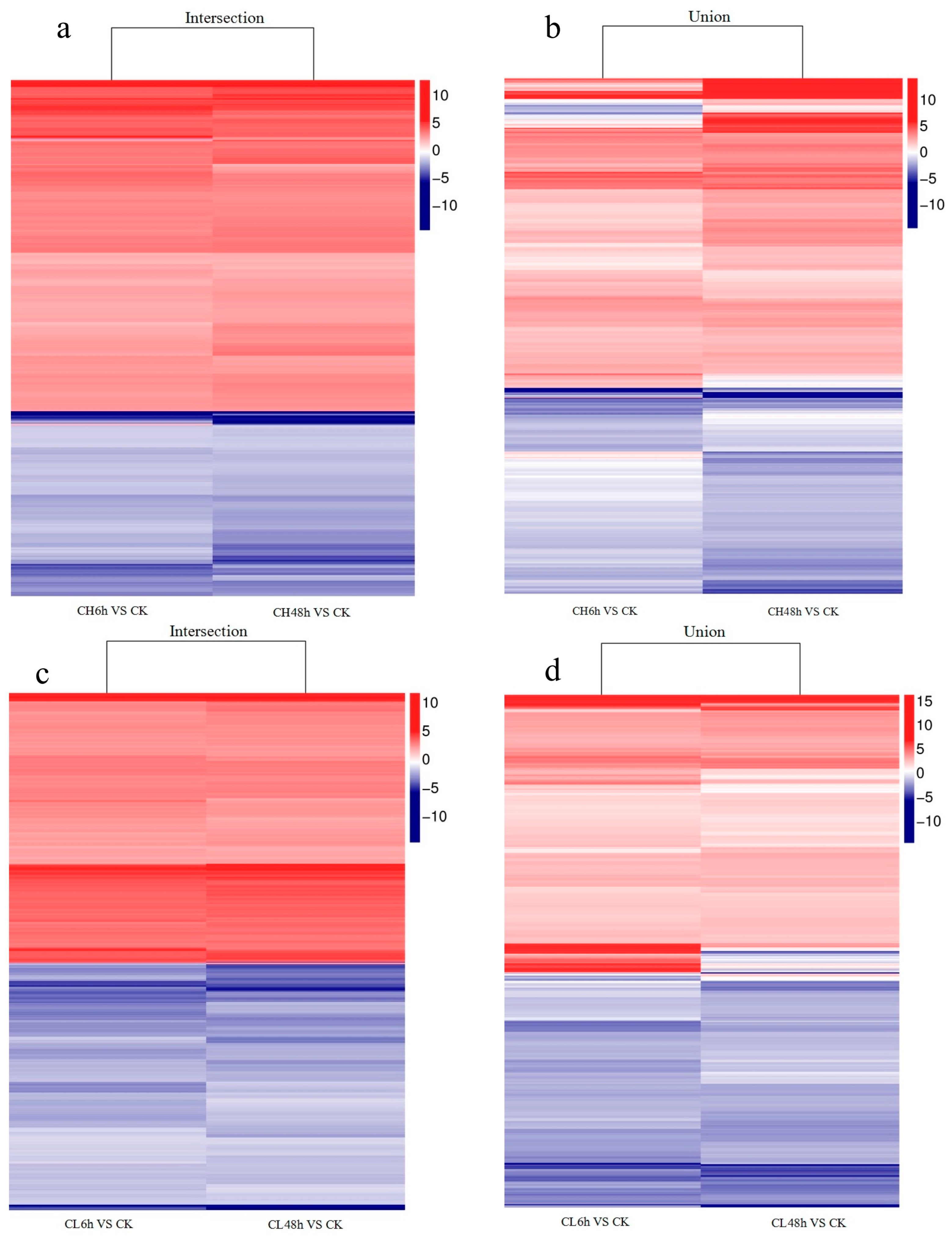
2.2. Unigene Expression Analysis for Insecticide Treatment
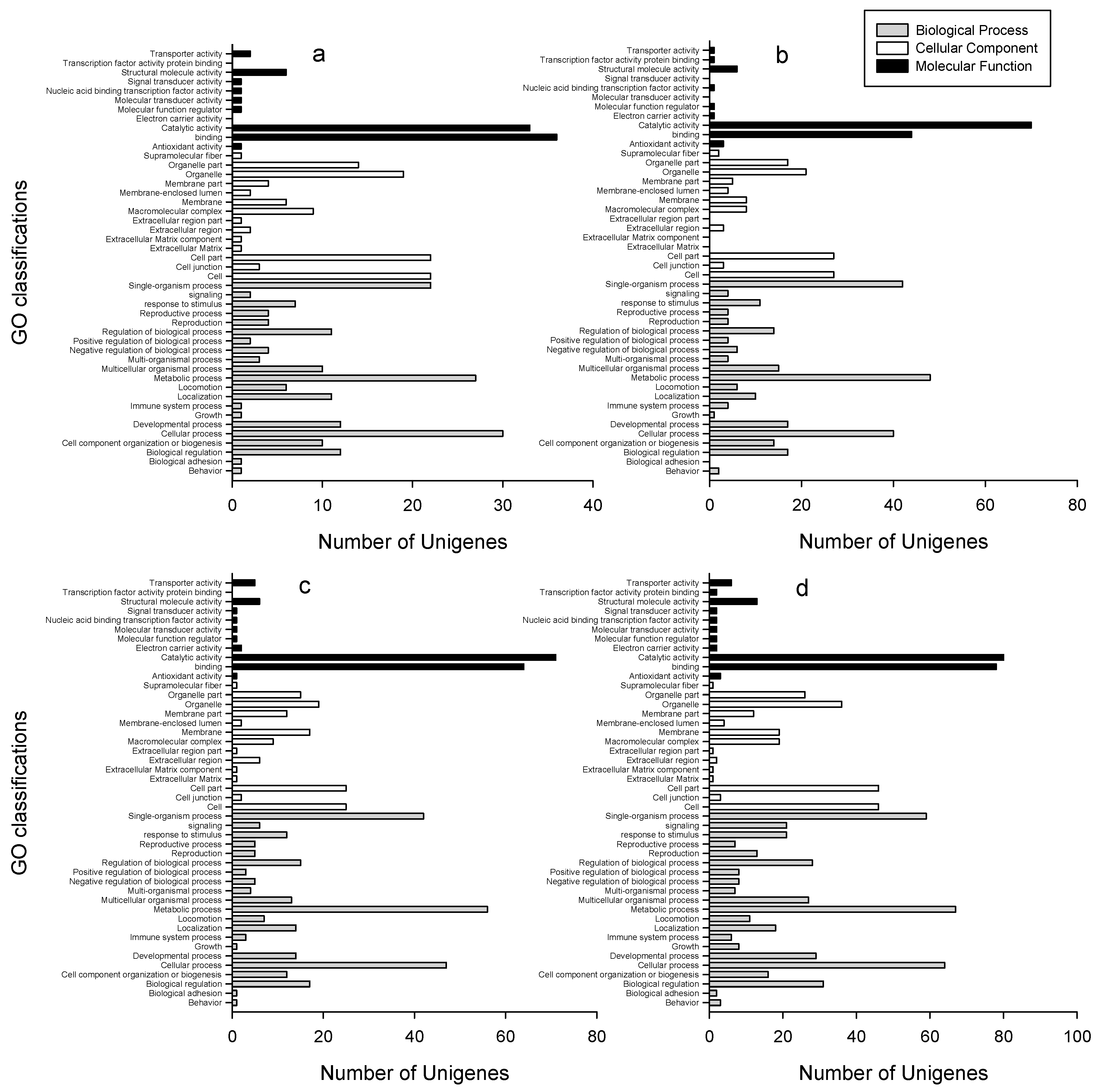
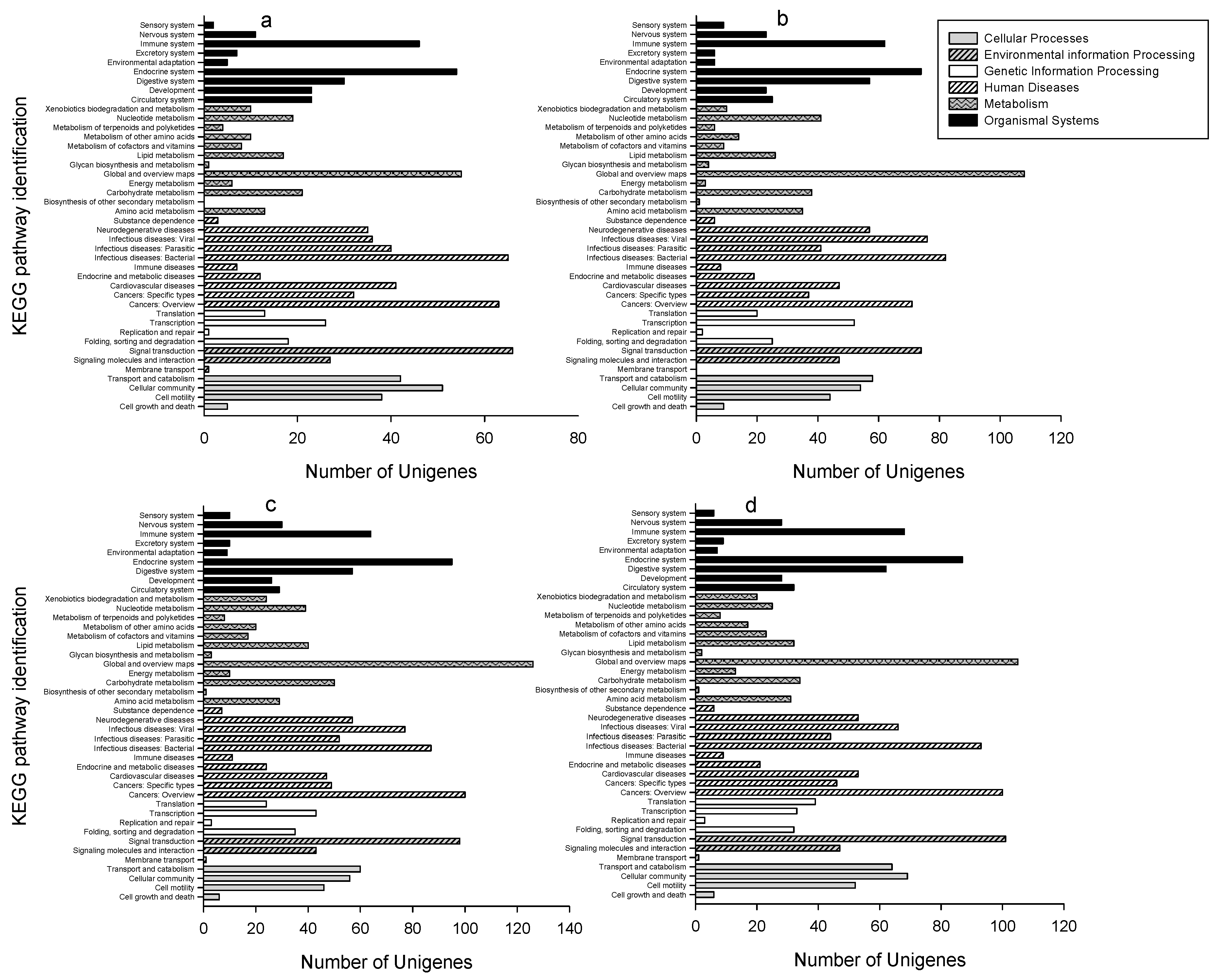
2.3. GO Classifications and KEGG Pathway Identification of DEUs
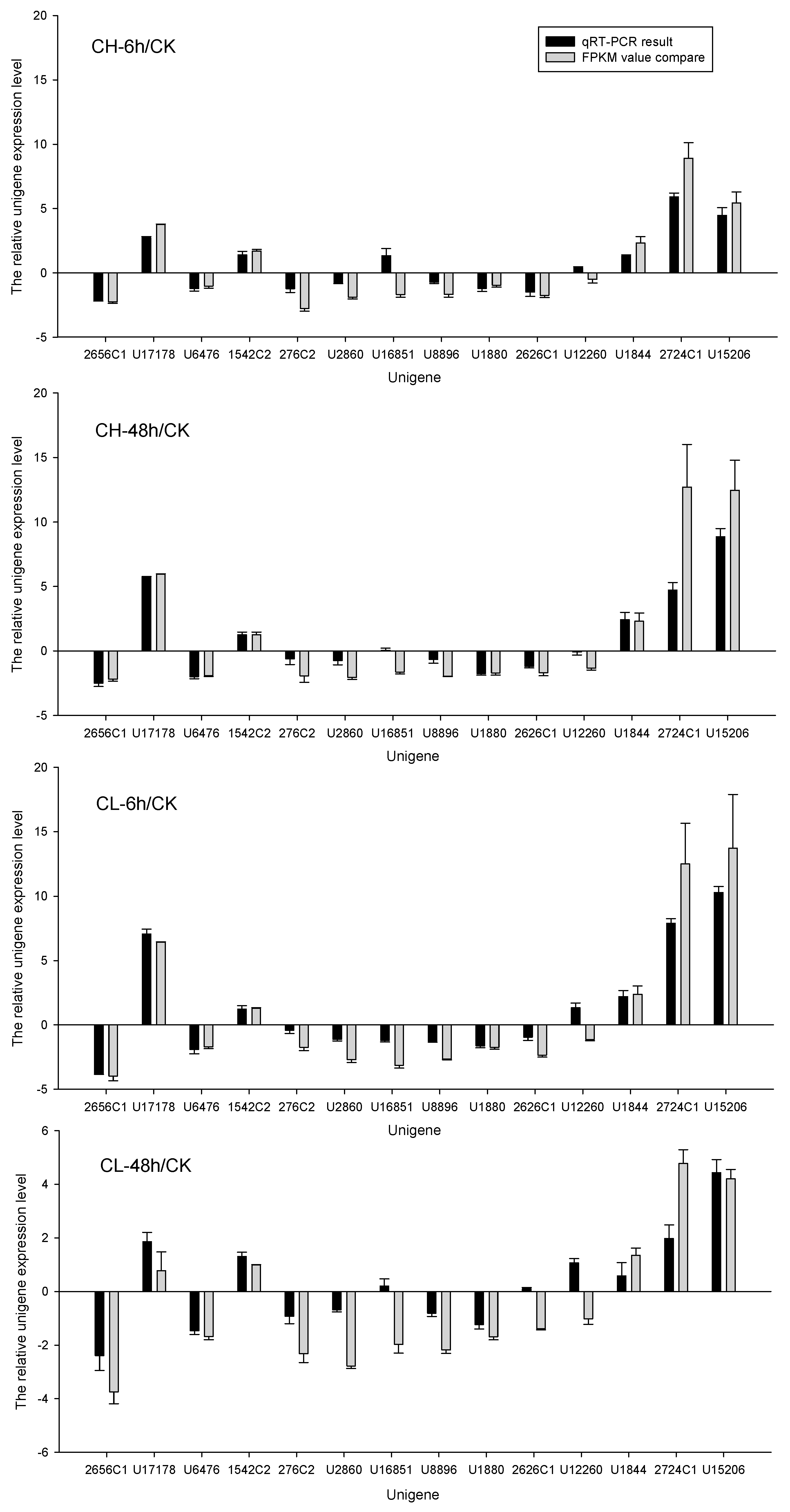
2.4. Insecticide-Metabolism-Related DEUs and Quantitative Real-Time PCR Validation
3. Discussion
4. Materials and Methods
4.1. Insect Rearing and Insecticide Treatment
4.2. RNA Extraction and cDNA Library Preparation
4.3. RNA-Seq Sequencing and DEU Screening
4.4. DEUs, GO Annotation and Pathway Enrichment among Different Treatments
4.5. Sequence Confirmation and Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) Validation
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mei, Z.; Wu, Q.; Zhang, Y.; Hua, L. The biology, ecology and management of Bradysia odoriphaga. Entomol. Knowl. 2003, 40, 396–398. [Google Scholar]
- Zhou, X.; Cao, X.; Shen, Y.; Zhuang, Q.; Zhang, S.; Yu, Y.; Zhang, A. Effects of supplementary nutrition and water on the reproduction and longevity of Bradysia odoriphaga. Chin. J. Appl. Entomol. 2016, 53, 1205–1210. [Google Scholar]
- Dang, Z.; Dong, J.; Gao, Z.; Jia, H.; Zhang, K.; Pan, W. Biology and injury of Bradysia odoriphaga on leek in different types of cultivation. J. Agric. Univ. Hebei 2001, 24, 65–68. [Google Scholar]
- Zhang, Z.; Zhang, Z.; Li, W.; He, M.; Zhu, X.; Lin, W.; Li, X.; Li, W.; Zhang, J.; Bei, Y.; et al. Behavioral responses of female Bradysia odoriphaga Yang et Zhang to volatiles of conspecific larvae, pupae and eggs. Chin. J. Appl. Entomol. 2016, 53, 1198–1204. [Google Scholar]
- Song, J.; Cao, W.; Feng, S.; Du, L. Effectiveness of high virulence Bt resources as a means of controlling Bradysia odoriphaga larvae. Chin. J. Appl. Entomol. 2016, 53, 1217–1224. [Google Scholar]
- Ffrenchconstant, R.H. The molecular genetics of insecticide resistance. Genetics 2013, 194, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Lin, L.; Xie, M.; Zhang, G.; Su, W. De novo sequencing and characterization of the Bradysia odoriphaga (Diptera: Sciaridae) larval transcriptome. Comp. Biochem. Phys. D 2015, 16, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Zhai, Y.; Wang, W.; Chen, H.; Zhou, X.; Zhuang, Q.; Yu, Y.; Li, R. Transcriptome analysis and discovery of genes relevant to development in Bradysia odoriphaga at three developmental stages. PLoS ONE 2016, 11, e0146812. [Google Scholar] [CrossRef] [PubMed]
- Schuster, S.C. Next-generation sequencing transforms today’s biology. Nat. Methods 2008, 5, 16–18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chiodini, R.; Badr, A.; Zhang, G. The impact of next-generation sequencing on genomics. J. Genet. Genom. 2011, 38, 95–109. [Google Scholar] [CrossRef] [PubMed]
- Van Nimwegen, K.; Soest, R.; Veltman, J.; Nelen, M.; Wilt, G.; Vissers, L.; Grutters, J. Is the $1000 Genome as Near as We Think? A Cost Analysis of Next-Generation Sequencing. Clin. Chem. 2016, 62, 1458–1464. [Google Scholar] [CrossRef] [PubMed]
- Mamidala, P.; Wijeratne, A.; Wijeratne, S.; Kornacker, K.; Sudhamalla, B.; Rivera-Vega, L.; Hoelmer, A.; Meulia, T.; Jones, S.; Mittapalli, O. RNA-Seq and molecular docking reveal multi-level pesticide resistance in the bed bug. BMC Genom. 2012, 13, 6. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Zhu, X.; Xie, W.; Wu, Q.; Wang, S.; Guo, Z.; Xu, B.; Li, X.; Zhou, X.; Zhang, Y. Midgut transcriptome response to a Cry toxin in the diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae). Gene 2014, 533, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Bajda, S.; Dermauw, W.; Greenhalgh, R.; Nauen, R.; Tirry, L.; Clark, R.; Van Leeuwen, T. Transcriptome profiling of a spirodiclofen susceptible and resistant strain of the European red mite Panonychus ulmi using strand-specific RNA-seq. BMC Genom. 2015, 16, 974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Wu, D.; Zhang, Y.; Zhou, H.; Lai, T.; Ding, W. RNA-Seq Analysis Reveals Candidate Targets for Curcumin against Tetranychus cinnabarinus. Biomed. Res. Int. 2016, 2016, 2796260. [Google Scholar] [PubMed]
- Cui, F.; Li, M.; Chang, H.; Mao, Y.; Zhang, H.; Lu, L.; Yan, S.; Lang, M.; Liu, L.; Qiao, C. Carboxylesterase-mediated insecticide resistance: Quantitative increase induces broader metabolic resistance than qualitative change. Pestic. Biochem. Physiol. 2015, 121, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Al-Ayedh, H.; Rizwan-ul-Haq, M.; Hussain, A.; Aljabr, A.M. Insecticidal potency of RNAi-based catalase knockdown in Rhynchophorus ferrugineus (Oliver) (Coleoptera: Curculionidae). Pest Manag. Sci. 2016, 72, 2118–2127. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Zhang, X.; Zhang, Y. P450—Mediated Insecticide Detoxification and Its Implication in Insecticide Efficacy. In Recent Advances in Entomological Research; Liu, T., Kang, L., Eds.; Springer: Berlin, Germany, 2011; pp. 229–245. [Google Scholar]
- Enayati, A.; Ranson, H.; Hemingway, J. Insect glutathione transferases and insecticide resistance. Insect Mol. Biol. 2005, 14, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Hollingworth, R.; Ahammadsahib, K. Inhibitors of respiratory complex I: Mechanisms, pesticidal actions and toxicology. Rev. Pestic. Toxicol. 1995, 3, 277–302. [Google Scholar]
- Borovsky, D.; Rabindran, S.; Dawson, W.; Powell, C.; Iannotti, D.; Morris, T.; Shabanowitz, J.; Hunt, D.; DeBondt, H.; DeLoof, A. Expression of Aedes trypsin-modulating oostatic factor on the virion of TMV: A potential larvicide. Proc. Natl. Acad. Sci. USA 2006, 103, 18963–18968. [Google Scholar] [CrossRef] [PubMed]
- Lomate, P.; Sangole, K.; Sunkar, R.; Hivrale, V. Superoxide dismutase activities in the midgut of Helicoverpa armigera larvae: Identification and biochemical properties of a manganese superoxide dismutase. Open Access Insect Physiol. 2015, 5, 13–20. [Google Scholar]
- Menozzi, P.; An Shi, M.; Lougarre, A.; Tang, Z.; Fournier, D. Mutations of acetylcholinesterase which confer insecticide resistance in Drosophila melanogaster populations. BMC Evol. Biol. 2004, 4, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ffrench-constant, R.; Rocheleau, T.; Steichen, J.; Chalmers, A. A point mutation in a Drosophila GABA receptor confers insecticide resistance. Nature 1993, 363, 449–451. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Williamson, M.; Lansdell, S.; Denholm, I.; Han, Z.; Millar, N. A nicotinic acetylcholine receptor mutation conferring target-site resistance to imidacloprid in Nilaparvata lugens (brown planthopper). Proc. Natl. Acad. Sci. USA 2005, 102, 8420–8425. [Google Scholar] [CrossRef] [PubMed]
- Bloomquist, J. Ion channels as targets for insecticides. Ann. Rev. Entomol. 1996, 41, 163–190. [Google Scholar] [CrossRef] [PubMed]
- Zlotkin, E. The insect voltage-gated sodium channel as target of insecticides. Ann. Rev. Entomol. 1999, 44, 429–455. [Google Scholar] [CrossRef] [PubMed]
- Perry, T.; Batterham, P.; Daborn, P. The biology of insecticidal activity and resistance. Insect Biochem. Mol. 2011, 41, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Eddleston, M.; Buckley, N.; Eyer, P.; Dawson, A. Management of acute organophosphorus pesticide poisoning. Lancet 2008, 371, 597–607. [Google Scholar] [CrossRef]
- Ma, J.; Wang, R.; Li, X.; Gao, B.; Chen, S. Transcriptome and gene expression analysis of Cylas formicarius (Coleoptera: Brentidae) during different development stages. J. Insect Sci. 2016, 16, 63. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Lin, L.; Xie, M.; Zhang, G.; Su, W. De novo sequencing, assembly and characterization of antennal transcriptome of Anomala corpulenta Motschulsky (Coleoptera: Rutelidae). PLoS ONE 2014, 9, e114238. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Huang, Y.; Zhang, Y.; Wang, X.; Yang, H.; Yu, O.; Dai, W.; Fang, C. Transcriptome profiling of fruit development and maturation in Chinese white pear (Pyrus bretschneideri Rehd). BMC Genom. 2013, 14, 823. [Google Scholar] [CrossRef] [PubMed]
- Charles, J. The regulation of expression of insect cuticle protein genes. Insect Biochem. Mol. Biol. 2010, 40, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; McCaffery, A.R. Elucidation of detoxication mechanisms involved in resistance to insecticides in the third instar larvae of a field-selected strain of Helicoverpa armigera with the use of synergists. Pestic. Biochem. Physiol. 1991, 41, 41–52. [Google Scholar] [CrossRef]
- Kostaropoulos, I.; Papadopoulos, A.I.; Metaxakis, A.; Boukouvala, E.; Papadopoulou-Mourkidou, E. Glutathione S-transferase in the defence against pyrethroids in insects. Insect Biochem. Mol. Biol. 2001, 31, 313–319. [Google Scholar] [CrossRef]
- Wang, W.; Lv, Y.; Fang, F.; Hong, S.; Guo, Q.; Hu, S.; Zou, F.; Shi, L.; Lei, Z.; Ma, K.; et al. Identification of proteins associated with pyrethroid resistance by iTRAQ-based quantitative proteomic analysis in Culex pipiens pallens. Parasites Vectors 2015, 8, 95. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Wang, W.; Zhang, D.; Lv, Y.; Zhou, D.; Ma, L.; Shen, B.; Sun, Y.; Zhu, C. The cuticle proteins: A putative role for deltamethrin resistance in Culex pipiens pallens. Parasitol. Res. 2015, 114, 4421–4429. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, R.M.; Ahmed, S.; Mantle, D. Comparative effect of fenitrothion treatment on intracellular protease activities in insecticide-resistant and susceptible strains of Musca domestica L. Comp. Biochem. Phys. C 1999, 124, 337–343. [Google Scholar] [CrossRef]
- Wang, H.; Reitz, S.R.; Wang, L.; Wang, S.; Li, X.; Lei, Z. The mRNA expression profiles of five heat shock protein genes from Frankliniella occidentalis at different stages and their responses to temperatures and insecticides. J. Integr. Agric. 2014, 13, 2196–2210. [Google Scholar] [CrossRef]
- Zhu, F.; Sams, S.; Moural, T.; Haynes, K.; Potter, M.; Palli, S. RNA Interference of NADPH-Cytochrome P450 Reductase Results in Reduced Insecticide Resistance in the Bed Bug, Cimex lectularius. PLoS ONE 2012, 7, e31037. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Y.; Wang, L.; Yao, J.; Guo, H.; Fang, J. Knockdown of NADPH-cytochrome P450 reductase results in reduced resistance to buprofezin in the small brown planthopper, Laodelphax striatellus (fallén). Pestic. Biochem. Physiol. 2016, 127, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Schuler, M.; Berenbaum, M. Molecular mechanisms of metabolic resistance to synthetic and natural xenobiotics. Annu. Rev. Entomol. 2007, 52, 231–253. [Google Scholar] [CrossRef] [PubMed]
- Feyereisen, R. Insect P450 enzymes. Annu. Rev. Entomol. 1999, 44, 507–533. [Google Scholar] [CrossRef] [PubMed]
- Fang, S. Insect glutathione S-transferase: A review of comparative genomic studies and response to xenobiotics. Bull. Insectol. 2012, 65, 265–271. [Google Scholar]
- Claudianos, C.; Ranson, H.; Johnson, R.; Biswas, S.; Schuler, M.; Berenbaum, M.; Feyereisen, R.; Oakeshott, J. A deficit of detoxification enzymes: Pesticide sensitivity and environmental response in the honeybee. Insect Mol. Biol. 2006, 15, 615–636. [Google Scholar] [CrossRef] [PubMed]
- Friedman, R. Genomic organization of the glutathione S-transferase family in insects. Mol. Phylogenet. Evol. 2011, 61, 924–932. [Google Scholar] [CrossRef] [PubMed]
- Small, G.J.; Hemingway, J. Molecular characterization of the amplified carboxylesterase gene associated with organophosphorus insecticide resistance in the brown planthopper, Nilaparvata lugens. Insect Mol. Biol. 2000, 9, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Hemingway, J.; Karunaratne, S.H. Mosquito carboxylesterases: A review of the molecular biology and biochemistry of a major insecticide resistance mechanism. Med. Vet. Entomol. 1998, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.; Pan, Y.; Bi, R.; Gao, X.; Chen, X.; Peng, T.; Zhang, M.; Zhang, H.; Hu, X.; Shang, Q. Elevated expression of esterase and cytochrome P450 are related with lambda–cyhalothrin resistance and lead to cross resistance in Aphis glycines Matsumura, Pest. Biochem. Physiol. 2015, 118, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.; Lin, Z.; Wang, H.; Liu, S.; Chang, H.; Reeck, G.; Qiao, C.; Raymond, M.; Kang, L. Two single mutations commonly cause qualitative change of nonspecific carboxylesterases in insects. Insect Biochem. Mol. Biol. 2011, 41, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Newcomb, R.D.; Campbell, P.M.; Ollis, D.L.; Cheah, E.; Russell, R.J.; Oakeshott, J.G. A single amino acid substitution converts a carboxylesterase to an organophosphorus hydrolase and confers insecticide resistance on a blowfly. Proc. Natl. Acad. Sci. USA 1997, 94, 7464–7468. [Google Scholar] [CrossRef] [PubMed]
- Devonshire, A.L.; Heidari, R.; Bell, K.L.; Campbell, P.M.; Campbell, B.E.; Odgers, W.A.; Oakeshott, J.G.; Russell, R.J. Kinetic efficiency of mutant carboxylesterases implicated in organophosphate insecticide resistance. Pestic. Biochem. Physiol. 2003, 76, 1–13. [Google Scholar] [CrossRef]
- Zhang, C.; Ma, Z.; Zhang, X.; Wu, H. Transcriptomic alterations in Sitophilus zeamais in response to allyl isothiocyanate fumigation. Pestic. Biochem. Physiol. 2017, 137, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Mei, Z.; Wu, Q.; Zhang, Y.; Hua, L. Life tables of the laboratory population of Bradysia odoriphaga Yang et Zhang (Diptera: Mycetophilidae) at different temperatures. Acta Entomol. Sin. 2004, 47, 219–222. [Google Scholar]
- Langmead, B.; Trapnell, C.; Pop, M.; Salzberg, S. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009, 10, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Conesa, A.; Götz, S.; García-Gómez, J.; Terol, J.; Talón, M.; Robles, M. Blast2GO: A universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 2005, 21, 3674–3676. [Google Scholar] [CrossRef] [PubMed]
- Ashburner, M.; Ball, C.; Blake, J.; Botstein, D.; Butler, H.; Cherry, J.; Davis, A.; Dolinski, K.; Dwight, S.; Eppig, J.; et al. Gene Ontology: Tool for the unification of biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Araki, M.; Goto, S.; Hattori, M.; Hirakawa, M.; Itoh, M.; Katayama, T.; Kawashima, S.; Okuda, S.; Tokimatsu, T.; et al. KEGG for linking genomes to life and the environment. Nucleic Acids Res. 2008, 36, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Xu, J.; Palli, R.; Ferguson, J.; Palli, S. Ingested RNA interference for managing the populations of the Colorado potato beetle, Leptinotarsa decemlineata. Pest Manag. Sci. 2011, 67, 175–182. [Google Scholar] [CrossRef] [PubMed]
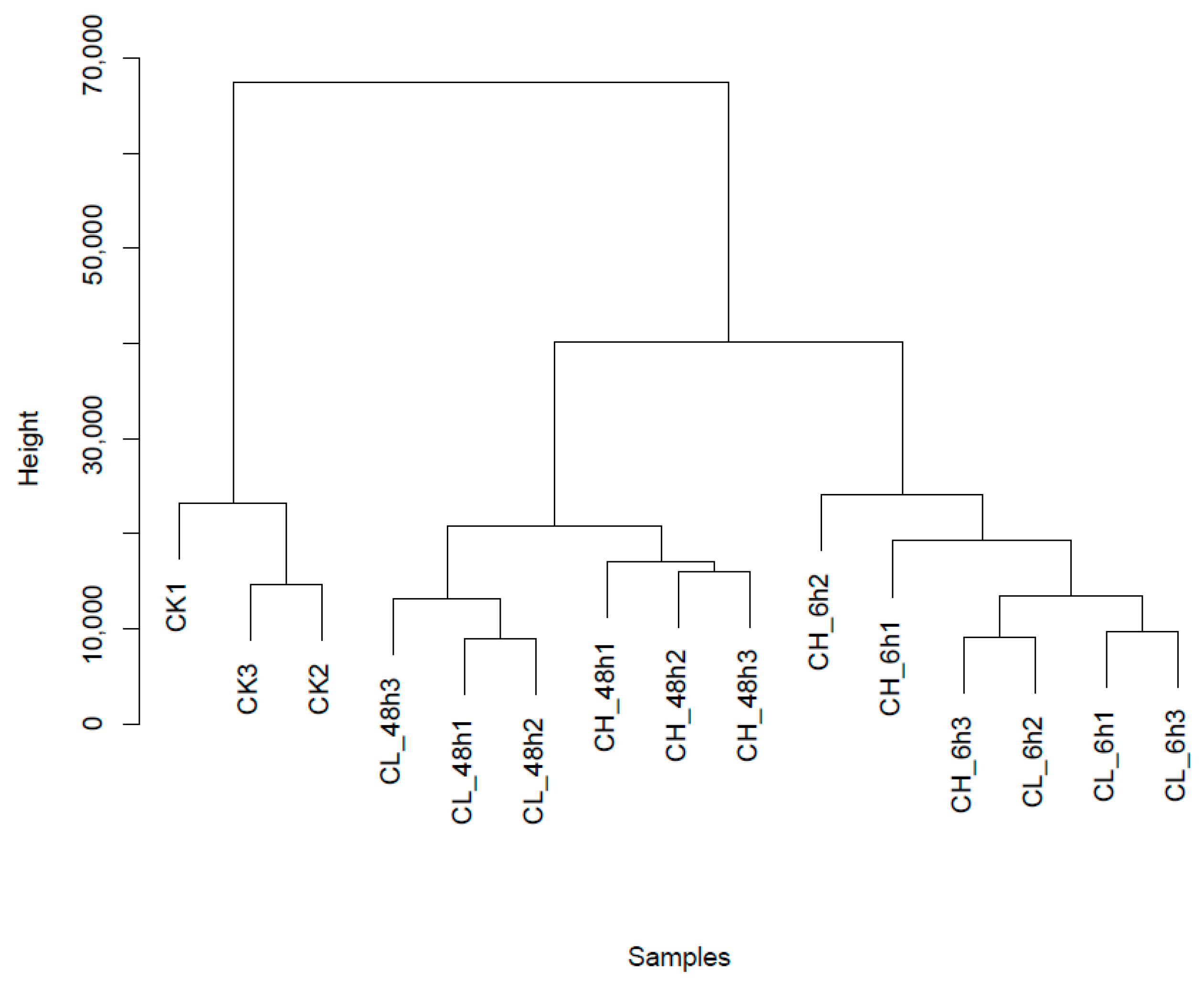
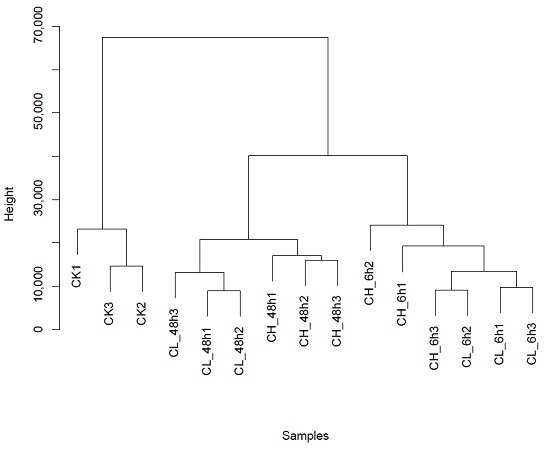
| Sample | Clean Reads | Clean Reads (%) | Clean Nucleotides (bp) | >Q20 (%) | GC (%) | Total Mapped Reads (%) |
|---|---|---|---|---|---|---|
| CK1 | 24,129,440 | 99.96 | 1.21 × 109 | 98.18 | 40.70 | 88.36 |
| CK2 | 24,122,549 | 99.94 | 1.21 × 109 | 98.28 | 39.85 | 83.93 |
| CK3 | 24,117,751 | 99.91 | 1.21 × 109 | 98.34 | 38.76 | 83.85 |
| CH_6h1 | 23,946,892 | 99.95 | 1.20 × 109 | 95.52 | 40.35 | 80.97 |
| CH_6h2 | 23,953,030 | 99.98 | 1.20 × 109 | 98.43 | 41.06 | 86.17 |
| CH_6h3 | 23,940,066 | 99.92 | 1.20 × 109 | 98.21 | 40.62 | 83.63 |
| CH_48h1 | 23,949,969 | 99.96 | 1.20 × 109 | 98.37 | 40.20 | 83.07 |
| CH_48h2 | 23,741,893 | 99.10 | 1.19 × 109 | 98.18 | 40.79 | 84.57 |
| CH_48h3 | 23,944,154 | 99.94 | 1.20 × 109 | 98.35 | 41.53 | 84.96 |
| CL_6h1 | 23,945,882 | 99.95 | 1.20 × 109 | 98.13 | 40.16 | 80.98 |
| CL_6h2 | 23,949,511 | 99.96 | 1.20 × 109 | 98.22 | 40.29 | 83.80 |
| CL_6h3 | 23,948,469 | 99.96 | 1.20 × 109 | 98.01 | 40.31 | 81.25 |
| CL_48h1 | 23,919,748 | 99.84 | 1.20 × 109 | 98.30 | 40.62 | 83.34 |
| CL_48h2 | 23,949,005 | 99.96 | 1.20 × 109 | 97.89 | 40.33 | 81.82 |
| CL_48h3 | 23,945,861 | 99.95 | 1.20 × 109 | 98.43 | 40.67 | 82.78 |
| Unigene ID | Annotation | Expression Compared to Control | Fold Change | KEGG Annotation |
|---|---|---|---|---|
| CL2310.Contig3 | malate synthase A | Up | 14.06 | K01638 |
| CL546.Contig2 | glycine-rich cell wall structural protein | Up | 14.06 | K10385 |
| Unigene21344 | spliceosome-associated protein | Up | 13.96 | K13806 |
| CL2019.Contig2 | keratin, type II cytoskeletal | Up | 13.37 | K11422 |
| Unigene14906 | gastrointestinal growth factor | Up | 12.78 | - |
| CL2724.Contig1 | cuticle protein | Up | 12.70 | K03006 |
| Unigene15206 | cuticle protein | Up | 12.46 | K03006 |
| CL1023.Contig2 | Lysosomal protein | Up | 12.13 | - |
| Unigene11830 | glycine-rich cell wall structural protein | Up | 11.97 | K13098 |
| CL2019.Contig1 | keratin, type II cytoskeletal | Up | 11.92 | K07605 |
| Unigene22580 | acyl-CoA desaturase | Down | −6.93 | K00507 |
| Unigene21624 | endocuticle structural glycoprotein | Down | −8.62 | - |
| Unigene343 | cell target antigen | Down | −9.15 | - |
| CL534.Contig2 | activating signal cointegrator | Down | −9.17 | K13114 |
| CL1382.Contig3 | adhesion G-protein coupled receptor | Down | −9.44 | - |
| Unigene1839 | Full=Flexible cuticle protein | Down | −9.92 | - |
| CL2888.Contig2 | aldo−keto reductase protein | Down | −10.00 | K00011 |
| CL572.Contig1 | BTB/POZ domain-containing protein | Down | −10.32 | K10457 |
| CL347.Contig2 | Succinyl-CoA ligase (ADP/GDP-forming) subunit α, mitochondrial | Down | −10.63 | - |
| Unigene6098 | Zonadhesin | Down | −14.62 | K07604 |
| Unigene ID | Annotation | Expression Compared to Control | Fold Change | KEGG Annotation |
|---|---|---|---|---|
| CL2903.Contig2 | Titin | Up | 11.58 | K14721 |
| Unigene14493 | zinc finger protein | Up | 10.16 | K09228 |
| CL884.Contig2 | Mediator of RNA polymerase | Up | 10.12 | K15169 |
| CL2635.Contig1 | serine/threonine-protein kinase | Up | 9.78 | K08791 |
| Unigene3337 | amino acid adenylation domain-containing protein | Up | 9.72 | K00142 |
| Unigene5847 | tyrosine-protein phosphatase | Up | 9.52 | K18040 |
| CL234.Contig1 | AT-rich interactive domain-containing protein | Up | 9.46 | K07874 |
| CL565.Contig3 | transcription factor | Up | 9.38 | K09269 |
| CL2809.Contig2 | transcription initiation factor | Up | 9.38 | K14650 |
| Unigene2261 | histone acetyltransferase | Up | 9.25 | K11306 |
| CL2708.Contig1 | aldose reductase | Down | −7.81 | K00011 |
| Unigene26463 | dienelactone hydrolase family protein | Down | −8.96 | K01061 |
| Unigene21654 | heat shock protein 67 | Down | −9.12 | K01011 |
| Unigene20053 | keratin, type I cytoskeletal | Down | −9.49 | K07604 |
| Unigene456 | serine protease inhibitor | Down | −9.65 | - |
| Unigene15229 | RNA recognition motif domain containing protein | Down | −9.92 | K13195 |
| Unigene13983 | larval serum protein | Down | −10.46 | K00505 |
| Unigene21431 | transmembrane protein | Down | −10.54 | - |
| CL119.Contig1 | cofilin/actin-depolymerizing factor | Down | −13.77 | K05765 |
| CL2528.Contig3 | cytochrome P450 | Down | −14.62 | K14999 |
| Gene Name | CH6h VS. CK | CH48h VS. CK | CL6h VS. CK | CL48h VS. CK | ||||
|---|---|---|---|---|---|---|---|---|
| Up | Down | Up | Down | Up | Down | Up | Down | |
| Cytochrome P450 | 30 | 23 | 35 | 18 | 35 | 35 | 21 | 40 |
| Glutathione S-transferase | 1 | 31 | 1 | 29 | 1 | 37 | 0 | 35 |
| Carboxylesterase | 1 | 2 | 7 | 14 | 4 | 16 | 2 | 13 |
| Acetylcholinesterase | 5 | 2 | 5 | 6 | 7 | 5 | 4 | 6 |
| NADH dehydrogenase | 1 | 11 | 2 | 7 | 2 | 13 | 2 | 11 |
| Catalase | 3 | 1 | 4 | 1 | 2 | 1 | 3 | 1 |
| Trypsin | 10 | 14 | 20 | 34 | 18 | 18 | 8 | 38 |
| Superoxide dismutase | 3 | 2 | 1 | 1 | 1 | 4 | 2 | 0 |
| GABA receptor | 0 | 3 | 0 | 3 | 0 | 3 | 0 | 3 |
| Nicotinic acetylcholine receptor | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 3 |
| Sodium channel | 8 | 0 | 8 | 0 | 7 | 1 | 7 | 1 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Lin, L.; Ali, F.; Xie, M.; Zhang, G.; Su, W. Using Next-Generation Sequencing to Detect Differential Expression Genes in Bradysia odoriphaga after Exposure to Insecticides. Int. J. Mol. Sci. 2017, 18, 2445. https://doi.org/10.3390/ijms18112445
Chen H, Lin L, Ali F, Xie M, Zhang G, Su W. Using Next-Generation Sequencing to Detect Differential Expression Genes in Bradysia odoriphaga after Exposure to Insecticides. International Journal of Molecular Sciences. 2017; 18(11):2445. https://doi.org/10.3390/ijms18112445
Chicago/Turabian StyleChen, Haoliang, Lulu Lin, Farman Ali, Minghui Xie, Guangling Zhang, and Weihua Su. 2017. "Using Next-Generation Sequencing to Detect Differential Expression Genes in Bradysia odoriphaga after Exposure to Insecticides" International Journal of Molecular Sciences 18, no. 11: 2445. https://doi.org/10.3390/ijms18112445