Treatment Approaches to Moderate to Severe Psoriasis
Abstract
:1. Factors Influencing Psoriasis Severity
2. Treatments Goals
3. Indications for Systemic Therapy
4. The Long-Term Management of Psoriasis
5. Non-Pharmacological Treatment Approaches
5.1. Smoking Cessation Interventions
5.2. Weight Reduction Interventions
5.3. Interventions on Psychiatric Comorbidities
6. Treatment Approaches in Special Patient Populations
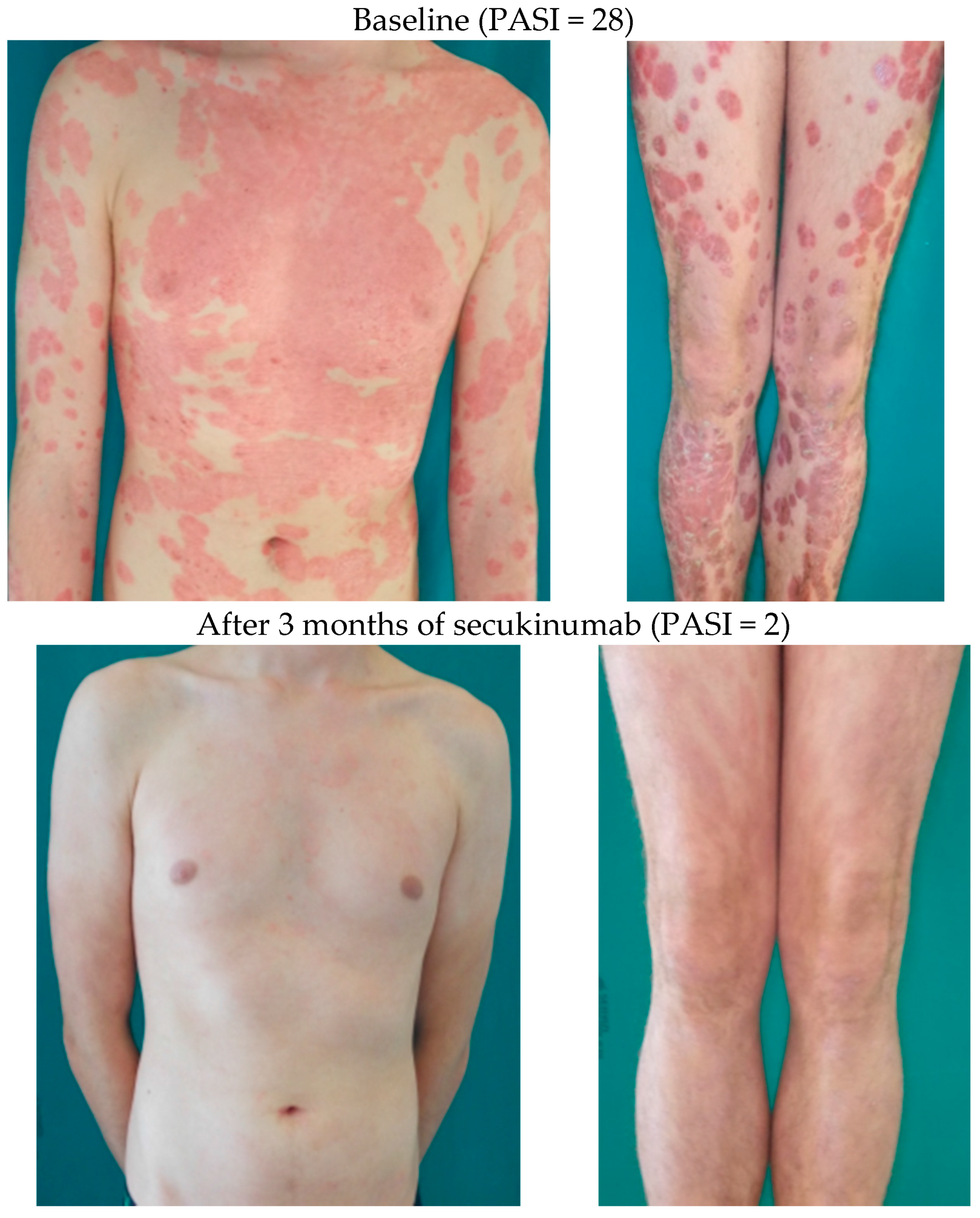
6.1. Systemic Therapy of Psoriasis in Children
6.2. Systemic Therapy of Psoriasis in the Elderly
6.3. Pregnancy
6.4. Patients with a History of Neoplasm
6.5. Patients Undergoing Surgical Procedures
7. Conclusions
Conflicts of Interest
Abbreviations
| PsA | psoriatic arthritis |
| UVB | ultraviolet B |
| nb | narrow band |
| PUVA | psoralen and ultraviolet A |
| IL | interleukin |
| IFN | interferon |
| TNF-α | tumor necrosis factor-α |
References
- Robinson, A.; Kardos, M.; Kimball, A.B. Physician Global Assessment (PGA) and Psoriasis Area and Severity Index (PASI): Why do both? A systematic analysis of randomized controlled trials of biologic agents for moderate to severe plaque psoriasis. J. Am. Acad. Dermatol. 2012, 66, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Finlay, A.Y. Patient-reported outcome measures in psoriasis: Assessing the assessments. Br. J. Dermatol. 2015, 172, 1178–1179. [Google Scholar] [CrossRef] [PubMed]
- Nast, A.; Gisondi, P.; Ormerod, A.D.; Saiag, P.; Smith, C.; Spuls, P.I.; Arenberger, P.; Bachelez, H.; Barker, J.; Dauden, E.; et al. European S3-Guidelines on the systemic treatment of psoriasis vulgaris—Update 2015--Short version—EDF in cooperation with EADV and IPC. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 2277–2294. [Google Scholar] [CrossRef] [PubMed]
- Finlay, A.Y. Current severe psoriasis and the rule of tens. Br. J. Dermatol. 2005, 152, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Mrowietz, U.; Kragballe, K.; Reich, K.; Spuls, P.; Griffiths, C.E.; Nast, A.; Franke, J.; Antoniou, C.; Arenberger, P.; Balieva, F.; et al. Definition of treatment goals for moderate to severe psoriasis: A European consensus. Arch. Dermatol. Res. 2011, 303, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guideline on Clinical Investigation of Medicinal Products Indicated for the Treatment of Psoriasis 2004. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003329.pdf (accessed on 30 September 2017).
- Gisondi, P.; Altomare, G.; Ayala, F.; Bardazzi, F.; Bianchi, L.; Chiricozzi, A.; Costanzo, A.; Conti, A.; Dapavo, P.; De Simone, C.; et al. Italian guidelines on the systemic treatments of moderate-to-severe plaque psoriasis. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 774–790. [Google Scholar] [CrossRef] [PubMed]
- Daudén, E.; Puig, L.; Ferrándiz, C.; Sánchez-Carazo, J.L.; Hernanz-Hermosa, J.M. Consensus document on the evaluation and treatment of moderate-to-severe psoriasis: Psoriasis Group of the Spanish Academy of Dermatology and Venereology. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Piaserico, S.; Cazzaniga, S.; Chimenti, S.; Giannetti, A.; Maccarone, M.; Picardo, M.; Peserico, A.; Naldi, L. Efficacy of switching between tumor necrosis factor-alfa inhibitors in psoriasis: Results from the Italian Psocare registry. J. Am. Acad. Dermatol. 2014, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saeki, H.; Imafuku, S.; Abe, M.; Shintani, Y.; Onozuka, D.; Hagihara, A.; Katoh, N.; Murota, H.; Takeuchi, S.; Sugaya, M.; et al. Poor adherence to medication as assessed by the Morisky Medication Adherence Scale-8 and low satisfaction with treatment in 237 psoriasis patients. J. Dermatol. 2015, 42, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Papp, K.; Gottlieb, A.B.; Naldi, L.; Pariser, D.; Ho, V.; Goyal, K.; Fakharzadeh, S.; Chevrier, M.; Calabro, S.; Langholff, W.; et al. Safety surveillance for ustekinumab and other psoriasis treatments from the psoriasis longitudinal assessment and registry (PSOLAR). J. Drugs Dermatol. 2015, 14, 706–714. [Google Scholar] [PubMed]
- Gottlieb, A.B.; Kalb, R.E.; Langley, R.G.; Krueger, G.G.; de Jong, E.M.; Guenther, L.; Goyal, K.; Fakharzadeh, S.; Chevrier, M.; Calabro, S.; et al. Safety observations in 12095 patients with psoriasis enrolled in an international registry (PSOLAR): Experience with infliximab and other systemic and biologic therapies. J. Drugs Dermatol. 2014, 13, 1441–1448. [Google Scholar] [PubMed]
- Reich, K.; Wozel, G.; Zheng, H.; van Hoogstraten, H.J.; Flint, L.; Barker, J. Efficacy and safety of infliximab as continuous or intermittent therapy in patients with moderate-to-severe plaque psoriasis: Results of a randomized, long-term extension trial (RESTORE2). Br. J. Dermatol. 2013, 168, 1325–1334. [Google Scholar] [CrossRef] [PubMed]
- Naldi, L.; Addis, A.; Chimenti, S.; Giannetti, A.; Picardo, M.; Tomino, C.; Maccarone, M.; Chatenoud, L.; Bertuccio, P.; Caggese, E.; et al. Impact of body mass index and obesity on clinical response to systemic treatment for psoriasis. Evidence from the Psocare project. Dermatology 2008, 217, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Song, G.G.; Seo, Y.H.; Kim, J.H.; Choi, S.J.; Ji, J.D.; Lee, Y.H. Association between TNF-α (-308 A/G, -238 A/G, -857 C/T) polymorphisms and responsiveness to TNF-α blockers in spondyloarthropathy, psoriasis and Crohn’s disease: A meta-analysis. Pharmacogenomics 2015, 16, 1427–1437. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Pérez, R.; Solano-López, G.; Cabaleiro, T.; Román, M.; Ochoa, D.; Talegón, M.; Baniandrés, O.; López Estebaranz, J.L.; de la Cueva, P.; Daudén, E.; et al. The polymorphism rs763780 in the IL-17F gene is associated with response to biological drugs in patients with psoriasis. Pharmacogenomics 2015, 16, 1723–1731. [Google Scholar] [CrossRef] [PubMed]
- Talamonti, M.; Galluzzo, M.; van den Reek, J.M.; de Jong, E.M.; Lambert, J.L.W.; Malagoli, P.; Bianchi, L.; Costanzo, A. Role of the HLA-C*06 allele in clinical response to ustekinumab: Evidence from real life in a large cohort of European patients. Br. J. Dermatol. 2017, 177, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.D.; Feldman, S.R. Comorbidities in patients with psoriasis: The role of the dermatologist. J. Am. Acad. Dermatol. 2017, 77, 191–192. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Savanelli, M.C.; di Somma, C.; Napolitano, M.; Megna, M.; Colao, A.; Savastano, S. Vitamin D and its role in psoriasis: An overview of the dermatologist and nutritionist. Rev. Endocr. Metab. Disord. 2017, 18, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Kinahan, C.E.; Mazloom, S.; Fernandez, A.P. Impact of smoking on response to systemic treatment in patients with psoriasis: A retrospective case-control study. Br. J. Dermatol. 2015, 172, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Jensen, P.; Christensen, R.; Zachariae, C.; Geiker, N.R.; Schaadt, B.K.; Stender, S.; Hansen, P.R.; Astrup, A.; Skov, L. Long-term effects of weight reduction on the severity of psoriasis in a cohort derived from a randomized trial: A prospective observational follow-up study. Am. J. Clin. Nutr. 2016, 104, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Balato, N.; di Somma, C.; Macchia, P.E.; Napolitano, M.; Savanelli, M.C.; Esposito, K.; Colao, A.; Savastano, S. Nutrition and psoriasis: Is there any association between the severity of the disease and adherence to the Mediterranean diet? J. Transl. Med. 2015, 13, 18. [Google Scholar] [CrossRef] [PubMed]
- Gisondi, P. High prevalence of alcohol use disorders in patients with inflammatory skin diseases applies to both psoriasis and eczema. Br. J. Dermatol. 2017, 177, 606–607. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, J.; Wozel, G.; Garzarolli, M.; Viehweg, A.; Bauer, M.; Leopold, K. Effectiveness of interdisciplinary vs. dermatological care of moderate-to-severe psoriasis: A pragmatic randomised controlled trial. Acta Derm.-Venereol. 2014, 94, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Torres, R.M.; Paradela, S.; Fonseca, E. Psoriasis in patients older than 65 years. A comparative study with younger adult psoriatic patients. J. Nutr. Health Aging 2012, 16, 586–591. [Google Scholar] [CrossRef] [PubMed]
- Murase, J.E.; Heller, M.M.; Butler, D.C. Safety of dermatologic medications in pregnancy and lactation: Part I. Pregnancy. J. Am. Acad. Dermatol. 2014, 70, 401. [Google Scholar] [CrossRef] [PubMed]
- Menter, A.; Korman, N.J.; Elmets, C.A.; Feldman, S.R.; Gelfand, J.M.; Gordon, K.B.; Gottlieb, A.; Koo, J.Y.; Lebwohl, M.; Lim, H.W.; et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 5. Guidelines of care for the treatment of psoriasis with phototherapy and photochemotherapy. J. Am. Acad. Dermatol. 2010, 62, 114–135. [Google Scholar] [CrossRef] [PubMed]
- Del Campo, M.; Kosaki, K.; Bennett, F.C.; Jones, K.L. Developmental delay in fetal aminopterin/methotrexate syndrome. Teratology 1999, 60, 10–12. [Google Scholar] [CrossRef]
- Branche, J.; Cortot, A.; Bourreille, A.; Coffin, B.; de Vos, M.; de Saussure, P.; Seksik, P.; Marteau, P.; Lemann, M.; Colombel, J.F. Cyclosporine treatment of steroid-refractory ulcerative colitis during pregnancy. Inflamm. Bowel Dis. 2009, 15, 1044–1048. [Google Scholar] [CrossRef] [PubMed]
- Yiu, Z.Z.; Griffiths, C.E.; Warren, R.B. Safety of biological therapies for psoriasis: Effects on reproductive potential and outcomes in male and female patients. Br. J. Dermatol. 2014, 171, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Nast, A.; Spuls, P.I.; van der Kraaij, G.; Gisondi, P.; Paul, C.; Ormerod, A.D.; Saiag, P.; Smith, C.H.; Dauden, E.; de Jong, E.M.; et al. European S3-Guideline on the systemic treatment of psoriasis vulgaris—Update Apremilast and Secukinumab—EDF in cooperation with EADV and IPC. J. Eur. Acad. Dermatol. Venereol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Fabiano, A.; de Simone, C.; Gisondi, P.; Piaserico, S.; Lasagni, C.; Pellacani, G.; Conti, A. Management of patients with psoriasis treated with biological drugs needing a surgical treatment. Drug Dev. Res. 2014, 75, S24–S26. [Google Scholar] [CrossRef] [PubMed]
- Bakkour, W.; Purssell, H.; Chinoy, H.; Griffiths, C.E.; Warren, R.B. The risk of post-operative complications in psoriasis and psoriatic arthritis patients on biologic therapy undergoing surgical procedures. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Hannen, R.; Udeh-Momoh, C.; Upton, J.; Wright, M.; Michael, A.; Gulati, A.; Rajpopat, S.; Clayton, N.; Halsall, D.; Burrin, J.; Flower, R.; et al. Dysfunctional Skin-Derived Glucocorticoid Synthesis Is a Pathogenic Mechanism of Psoriasis. J. Investig. Dermatol. 2017, 137, 1630–1637. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gisondi, P.; Del Giglio, M.; Girolomoni, G. Treatment Approaches to Moderate to Severe Psoriasis. Int. J. Mol. Sci. 2017, 18, 2427. https://doi.org/10.3390/ijms18112427
Gisondi P, Del Giglio M, Girolomoni G. Treatment Approaches to Moderate to Severe Psoriasis. International Journal of Molecular Sciences. 2017; 18(11):2427. https://doi.org/10.3390/ijms18112427
Chicago/Turabian StyleGisondi, Paolo, Micol Del Giglio, and Giampiero Girolomoni. 2017. "Treatment Approaches to Moderate to Severe Psoriasis" International Journal of Molecular Sciences 18, no. 11: 2427. https://doi.org/10.3390/ijms18112427
APA StyleGisondi, P., Del Giglio, M., & Girolomoni, G. (2017). Treatment Approaches to Moderate to Severe Psoriasis. International Journal of Molecular Sciences, 18(11), 2427. https://doi.org/10.3390/ijms18112427





