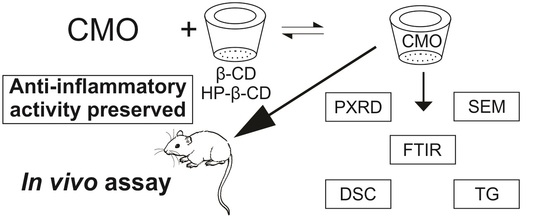
Inclusion Complexes of Copaiba (Copaifera multijuga Hayne) Oleoresin and Cyclodextrins: Physicochemical Characterization and Anti-Inflammatory Activity
Abstract
:1. Introduction
2. Results and Discussion
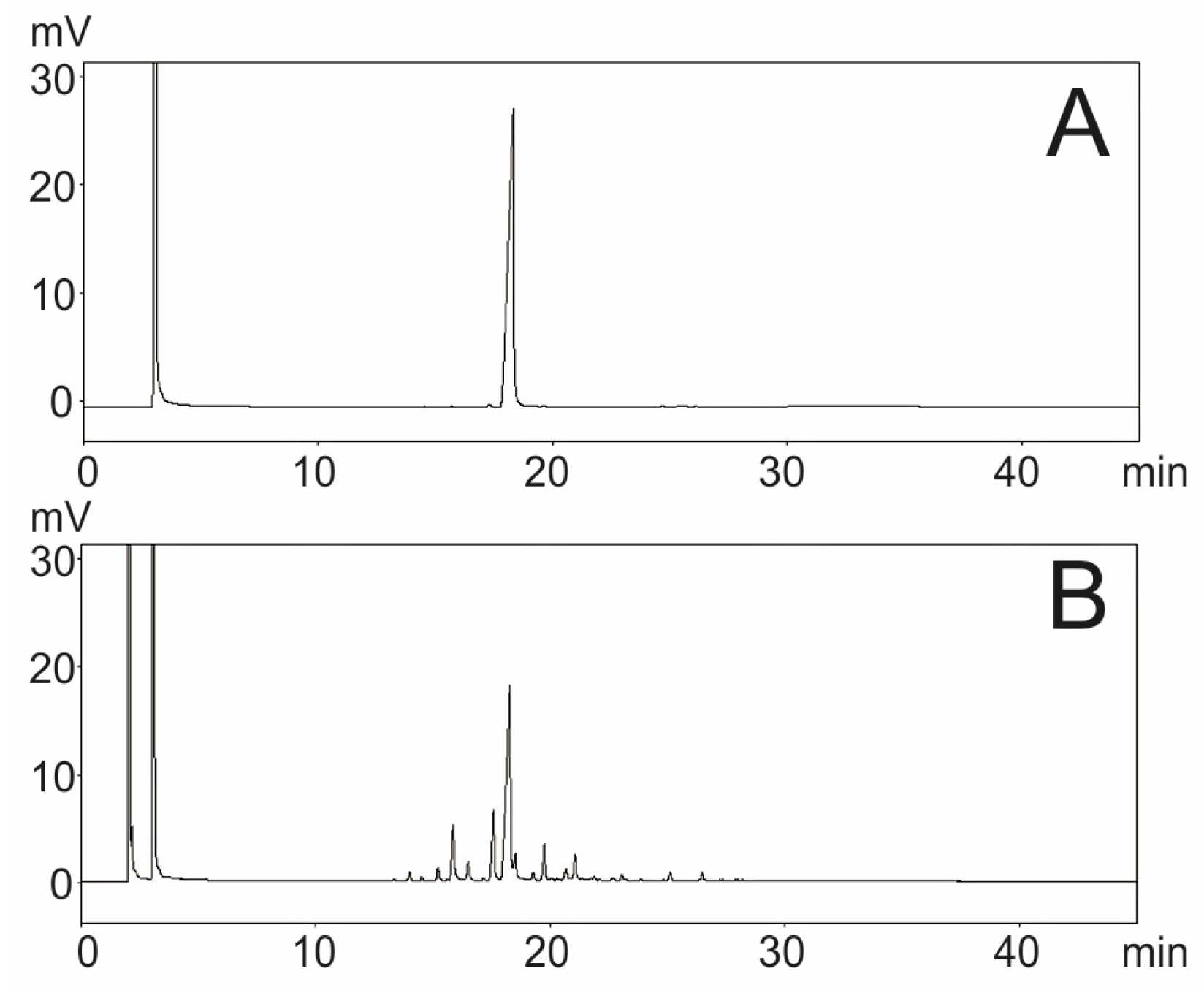
2.1. Quantification of β-Caryophyllene in CMO by Gas Chromatography with Flame Ionization Detector
2.2. Physicochemical Characterization
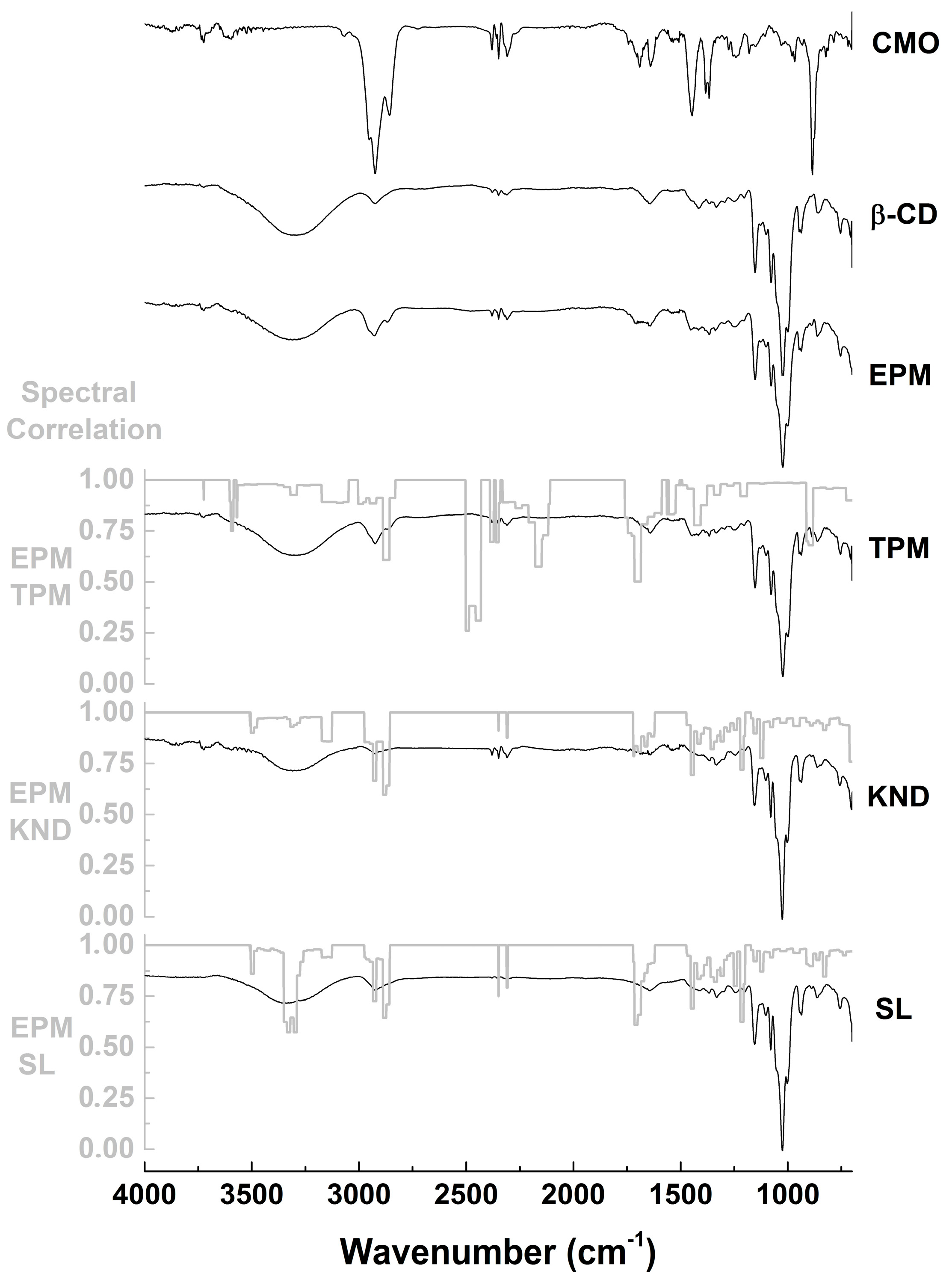
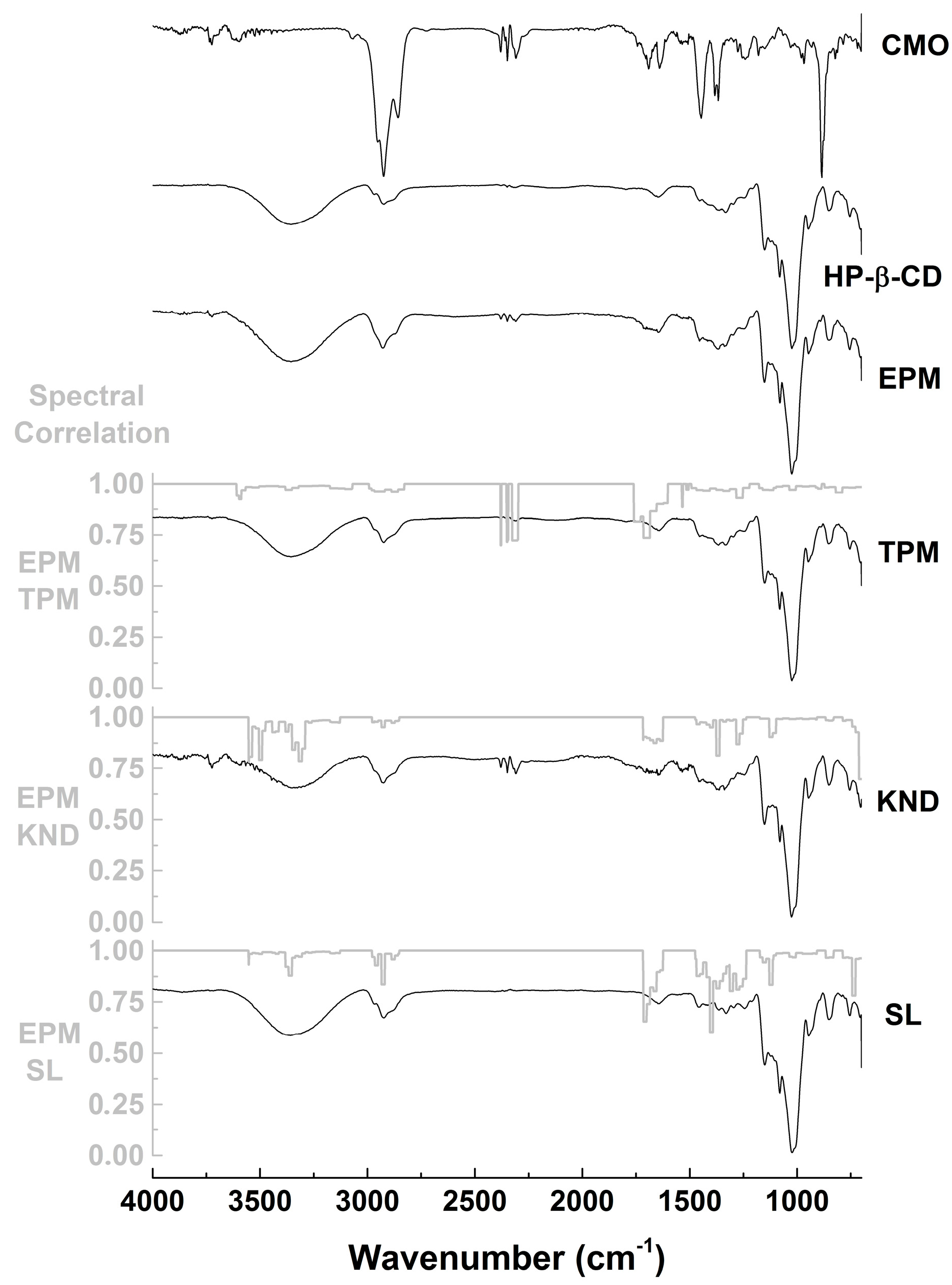
2.2.1. Fourier-Transform Infrared (FTIR) Spectroscopy
2.2.2. Scanning Electronic Microscopy (SEM)
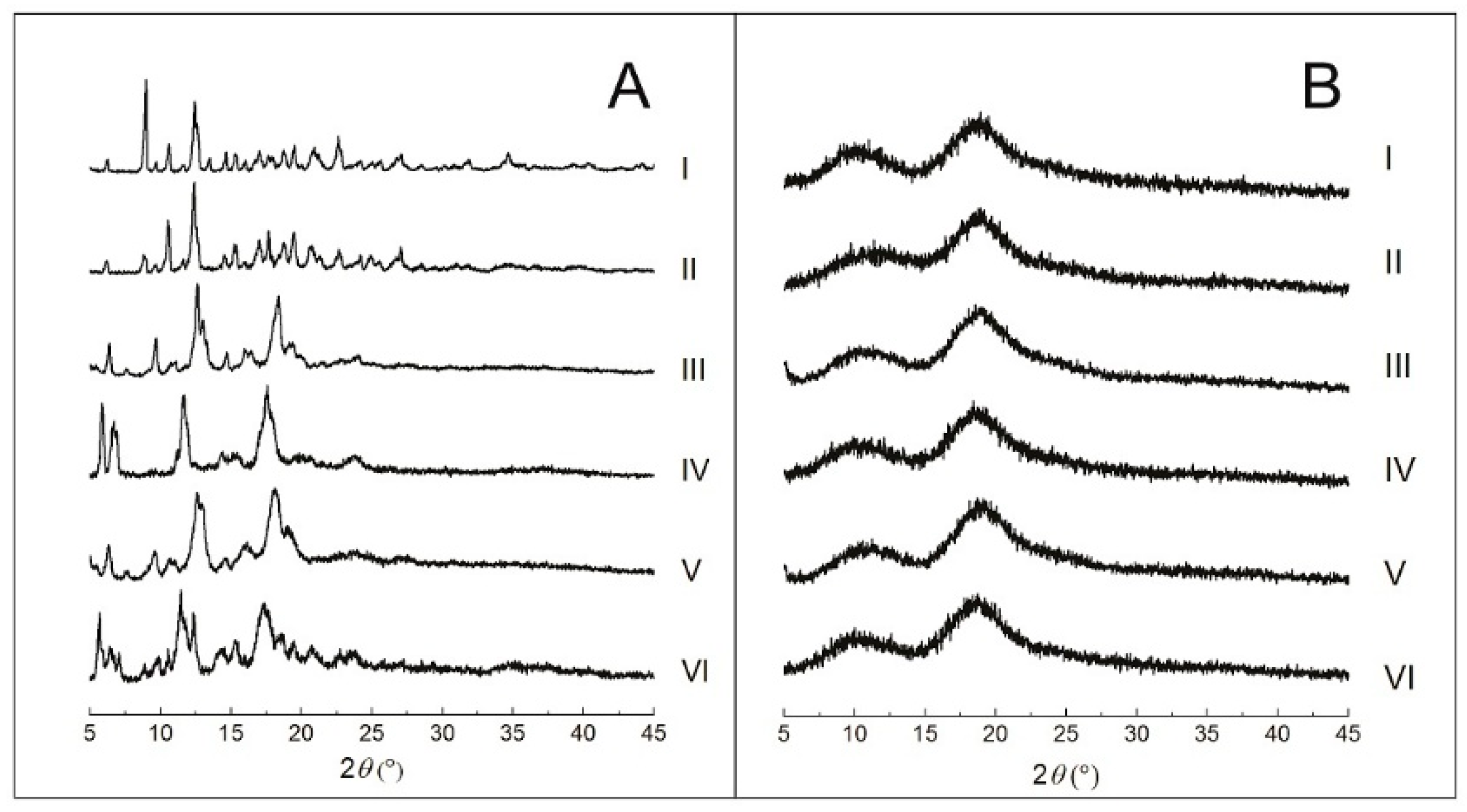
2.2.3. Powder X-Ray Diffraction (PXRD)
2.2.4. Thermogravimetric Analysis (TGA)
2.2.5. Differential Scanning Calorimetry (DSC)
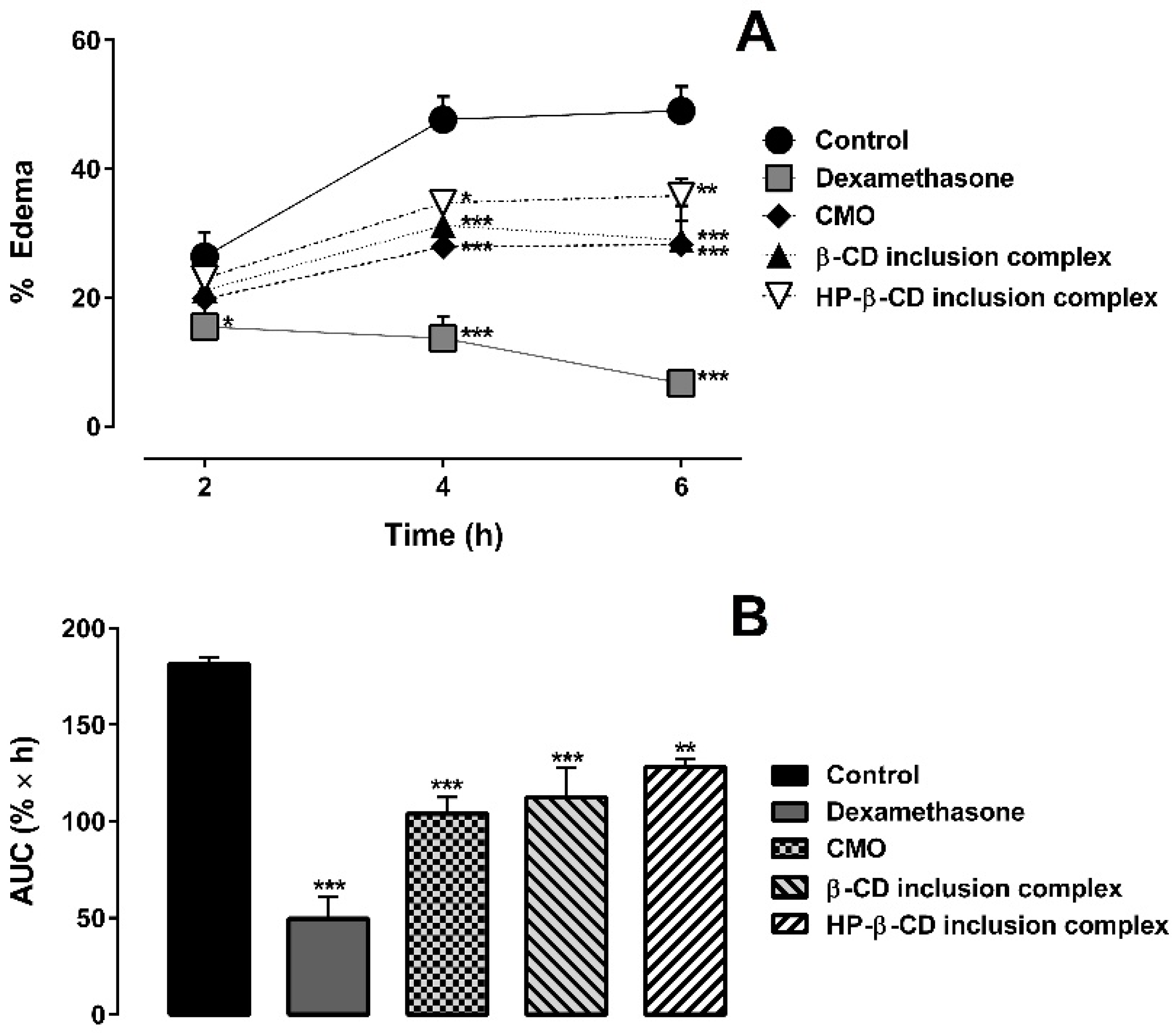
2.3. Carrageenan-Induced Hind Paw Edema Test
3. Materials and Methods
3.1. Materials
3.2. Quantification of β-Caryophyllene on CMO by Gas Chromatography with Flame Ionization Detector (GC-FID)
3.3. Preparation of Inclusion Complexes
3.3.1. Physical Mixture (PM)
3.3.2. Kneading (KND)
3.3.3. Slurry (SL)
3.4. Physicochemical Characterization
3.4.1. Fourier-Transform Infrared (FTIR) Spectroscopy
3.4.2. Scanning Electron Microscopy (SEM)
3.4.3. Powder X-ray Diffraction (PXRD)
3.4.4. Thermogravimetric Analysis (TGA)
3.4.5. Differential Scanning Calorimetry (DSC)
3.5. Evaluation of Anti-Inflammatory Activity
3.5.1. Animals
3.5.2. Induction of Paw Edema
3.5.3. Nitrite Determination
3.5.4. Myeloperoxidase (MPO) Activity
3.5.5. Statistical Analysis
4. Conclusions
5. Patents
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| β-CD | β-Cyclodextrin |
| CDs | Cyclodextrins |
| CMO | Copaifera multijuga oleoresin |
| DSC | Differential scanning calorimetry |
| FTIR | Fourier-transform infrared |
| HP-β-CD | Hydroxypropyl-β-cyclodextrin |
| HP-γ-CD | Hydroxypropyl-γ-cyclodextrin |
| KND | Kneading |
| PM | Physical mixture |
| PXRD | Powder X-ray diffraction |
| RM-β-CD | Randomly methylated-β-cyclodextrin |
| SBE-β-CD | Sulfobutylether-β-cyclodextrin |
| SEM | Scanning electronic microscopy |
| SL | Slurry |
| TGA | Thermogravimetric analysis |
| TPM | Theoretical physical mixture |
| γ-CD | γ-Cyclodextrin |
References
- Basile, A.C.; Sertié, J.A.A.; Freitas, P.C.D.; Zanini, A.C. Anti-inflammatory activity of oleoresin from brazilian Copaifera. J. Ethnopharmacol. 1988, 22, 101–109. [Google Scholar] [CrossRef]
- Gomes, N.M.; Rezende, C.M.; Fontes, S.P.; Matheus, M.E.; Pinto, A.C.; Fernandes, P.D. Characterization of the antinociceptive and anti-inflammatory activities of fractions obtained from Copaifera multijuga Hayne. J. Ethnopharmacol. 2010, 128, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Veiga Junior, V.F.; Pinto, A.C. O gênero Copaifera L. Quim. Nova 2002, 25, 273–286. [Google Scholar] [CrossRef]
- Veiga Junior, V.F.; Zunino, L.; Patitucci, M.L.; Pinto, A.C.; Calixto, J.B. The inhibition of paw oedema formation caused by the oil of Copaifera multijuga Hayne and its fractions. J. Pharm. Pharmacol. 2006, 58, 1405–1410. [Google Scholar] [CrossRef] [PubMed]
- Veiga Junior, V.F.; Rosas, E.C.; Carvalho, M.V.; Henriques, M.G.M.O.; Pinto, A.C. Chemical composition and anti-inflammatory activity of copaiba oils from Copaifera cearensis Huber ex Ducke, Copaifera reticulata Ducke and Copaifera multijuga Hayne—A comparative study. J. Ethnopharmacol. 2007, 112, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, E.S.; Passos, G.F.; Medeiros, R.; Cunha, F.M.; Ferreira, J.; Campos, M.M.; Pianowski, L.F.; Calixto, J.B. Anti-inflammatory effects of compounds alpha-humulene and (−)-trans-caryophyllene isolated from the essential oil of Cordia verbenacea. Eur. J. Pharmacol. 2007, 569, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Sabulal, B.; Dan, M.; John, A.; Kurup, R.; Pradeep, N.S.; Valsamma, R.K.; George, V. Caryophyllene-rich rhizome oil of Zingiber nimmonii from South India: Chemical characterization and antimicrobial activity. Phytochemistry 2006, 67, 2469–2473. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.O.; Ueda-Nakamura, T.; Dias Filho, B.P.; Veiga Junior, V.F.; Pinto, A.C.; Nakamura, C.V. Antimicrobial activity of Brazilian copaiba oils obtained from different species of the Copaifera genus. Mem. Inst. Oswaldo Cruz 2008, 103, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, K.R.F.; Veiga Junior, V.F.; Rocha, W.C.; Bandeira, M.F.C.L. In vitro assessment of antibacterial activity of a dental cement constituted of a Copaifera multijuga Hayne oil-resin. Rev. Bras. Farmacogn. 2008, 18, 733–738. [Google Scholar] [CrossRef]
- Di Sotto, A.; Mazzanti, G.; Carbone, F.; Hrelia, P.; Maffei, F. Inhibition by β-caryophyllene of ethyl methanesulfonate-induced clastogenicity in cultured human lymphocytes. Mutat. Res. 2010, 699, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Bahi, A.; Mansouri, S.A.; Memari, E.A.; Ameri, M.A.; Nurulain, S.M.; Ojha, S. β-Caryophyllene, a CB2 receptor agonist produces multiple behavioral changes relevant to anxiety and depression in mice. Physiol. Behav. 2014, 135, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Galdino, P.M.; Nascimento, M.V.M.; Florentino, I.F.; Lino, R.C.; Fajemiroye, J.O.; Chaibub, B.A.; Paula, J.R.; Lima, T.C.M.; Costa, E.A. The anxiolytic-like effect of an essential oil derived from Spiranthera odoratissima A. St. Hil. leaves and its major component, β-caryophyllene, in male mice. Prog. Neuropsychopharmacol. 2012, 38, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Klauke, A.L.; Racz, I.; Pradier, B.; Markert, A.; Zimmer, A.M.; Gertsch, J.; Zimmer, A. The cannabinoid CB2 receptor-selective phytocannabinoid beta-caryophyllene exerts analgesic effects in mouse models of inflammatory and neuropathic pain. Eur. Neuropsychopharmacol. 2014, 24, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Izumi, E.; Ueda-Nakamura, T.; Veiga Junior, V.F.; Pinto, A.C.; Nakamura, C.V. Terpenes from Copaifera Demonstrated in vitro Antiparasitic and Synergic Activity. J. Med. Chem. 2012, 55, 2994–3001. [Google Scholar] [CrossRef] [PubMed]
- Marques, H.M.C. A review on cyclodextrin encapsulation of essential oils and volatiles. Flavour Fragr. J. 2010, 25, 313–326. [Google Scholar] [CrossRef]
- Brewster, M.E.; Loftsson, T. Cyclodextrins as pharmaceutical solubilizers. Adv. Drug Deliv. 2007, 59, 645–666. [Google Scholar] [CrossRef] [PubMed]
- Kurkov, S.V.; Loftsson, T. Cyclodextrins. Int. J. Pharm. 2013, 453, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Brewster, M.E. Pharmaceutical applications of cyclodextrins: Basic science and product development. J. Pharm. Pharmacol. 2010, 62, 1607–1621. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Brewster, M.E. Cyclodextrins as Functional Excipients: Methods to Enhance Complexation Efficiency. J. Pharm. Sci. 2012, 101, 3019–3032. [Google Scholar] [CrossRef] [PubMed]
- Szente, L.; Szejtli, J. Cyclodextrins as food ingredientes. Trends Food Sci. Technol. 2004, 15, 137–142. [Google Scholar] [CrossRef]
- Galvão, J.G.; Silva, V.F.; Ferreira, S.G.; França, F.R.M.; Santos, D.A.; Freitas, L.S.; Alves, P.B.; Araújo, A.A.S.; Cavalcanti, S.C.H.; Nunes, R.S. β-cyclodextrin inclusion complexes containing Citrus sinensis (L.) Osbeck essential oil: An alternative to control Aedes aegypti larvae. Thermochim. Acta 2015, 608, 14–19. [Google Scholar] [CrossRef]
- Santos, P.L.; Araújo, A.A.S.; Quintans, J.S.S.; Oliveira, M.G.B.; Brito, R.G.; Serafini, M.R.; Menezes, P.P.; Santos, M.R.V.; Alves, P.B.; Lucca Júnior, W.; et al. Preparation, Characterization, and Pharmacological Activity of Cymbopogon winterianus Jowitt ex Bor (Poaceae) Leaf Essential Oil of β-Cyclodextrin Inclusion Complexes. Evid. Based Complement. Altern. Med. 2015, 2015, 502454. [Google Scholar] [CrossRef] [PubMed]
- Siqueira-Lima, P.S.; Araújo, A.A.S.; Lucchese, A.M.; Quintans, J.S.S.; Menezes, P.P.; Alves, P.B.; Lucca Júnior, W.; Santos, M.R.V.; Bonjardim, L.R.; Quintans-Júnior, L.J. β-Cyclodextrin Complex Containing Lippia grata Leaf Essential Oil Reduces Orofacial Nociception in Mice—Evidence of Possible Involvement of Descending Inhibitory Pain Modulation Pathway. Basic Clin. Pharmacol. Toxicol. 2013, 114, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, S.S.; Araújo, A.A.S.; Brito, R.G.; Serafini, M.R.; Menezes, P.P.; DeSantana, J.M.; Lucca Júnior, W.; Alves, P.B.; Blank, A.F.; Oliveira, R.C.M.; et al. Cyclodextrin-Complexed Ocimum basilicum Leaves Essential Oil Increases Fos Protein Expression in the Central Nervous System and Produce an Antihyperalgesic Effect in Animal Models for Fibromyalgia. Int. J. Mol. Sci. 2015, 16, 547–563. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, R.M.; Webster, F.X. Spectrometric Identification of Organic Compounds, 6th ed.; Wiley: New York, NY, USA, 1998. [Google Scholar]
- Abarca, R.L.; Rodríguez, F.J.; Guarda, A.; Galotto, M.J.; Bruna, J.E. Characterization of beta-cyclodextrin inclusion complexes containing an essential oil component. Food Chem. 2016, 196, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Passos, J.J.; Sousa, F.B.; Mundim, I.M.; Bonfim, R.R.; Melo, R.; Viana, A.F.; Stolz, E.D.; Borsoi, M.; Rates, S.M.K.; Sinisterra, R.D. In vivo evaluation of the highly soluble oral β-cyclodextrin—Sertraline supramolecular complexes. Int. J. Pharm. 2012, 436, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Medarević, D.; Kachrimanis, K.; Djurić, Z.; Ibrić, S. Influence of hydrophilic polymers on the complexation of carbamazepine with hydroxypropyl-β-cyclodextrin. Eur. J. Pharm. Sci. 2015, 78, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Mura, P. Analytical techniques for characterization of cyclodextrin complexes in the solid state: A review. J. Pharm. Biomed. 2015, 113, 226–238. [Google Scholar] [CrossRef] [PubMed]
- Rezende, B.A.; Cortes, S.F.; Sousa, F.B.; Lula, I.S.; Schmitt, M.; Sinisterra, R.D.; Lemos, V.S. Complexation with β-cyclodextrin confers oral activity on the flavonoid dioclein. Int. J. Pharm. 2009, 367, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Bulani, V.D.; Kothavade, P.S.; Kundaikar, H.S.; Gawali, N.B.; Chowdhury, A.A.; Degani, M.S.; Juvekar, A.R. Inclusion complex of ellagic acid with β-cyclodextrin: Characterization and in vitro anti-inflammatory evaluation. J. Mol. Struct. 2016, 1105, 308–315. [Google Scholar] [CrossRef]
- Melo, P.N.; Barbosa, E.G.; Garnero, C.; Caland, L.B.; Fernandes-Pedrosa, M.F.; Longhi, M.R.; Silva-Júnior, A.A. Interaction pathways of specific co-solvents with hydroxypropyl-β-cyclodextrin inclusion complexes with benznidazole in liquid and solid phase. J. Mol. Liq. 2016, 223, 350–359. [Google Scholar] [CrossRef]
- Michalska, K.; Gruba, E.; Bocian, W.; Cielecka-Piontek, J. Enantioselective recognition of radezolid by cyclodextrin modified capillary electrokinetic chromatography and electronic circular dichroism. J. Pharm. Biomed. Anal. 2017, 139, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Periasamy, R.; Kothainayaki, S.; Sivakumar, K. Preparation, physicochemical analysis and molecular modeling investigation of 2,2′-Bipyridine: β-Cyclodextrin inclusion complex in solution and solid state. J. Mol. Struct. 2015, 1100, 59–69. [Google Scholar] [CrossRef]
- Zhu, Z.-Y.; Luo, Y.; Liu, Y.; Wang, X.T.; Liu, F.; Guo, M.Z.; Wang, Z.; Liu, A.J.; Zhang, Y.M. Inclusion of chrysin in β-cyclodextrin and its biological activities. J. Drug Deliv. Sci. Technol. 2016, 31, 176–186. [Google Scholar] [CrossRef]
- Spamer, E.; Müller, D.G.; Wessels, P.L.; Venter, J.P. Characterization of the complexes of furosemide with 2-hydroxypropyl-β-cyclodextrin and sulfobutyl ether-7-β-cyclodextrin. Eur. J. Pharm. Sci. 2002, 16, 247–253. [Google Scholar] [CrossRef]
- Tang, P.; Tang, B.; Wang, Q.; Xu, K.; Xiong, X.; Li, H. Effect of hydroxypropyl-β-cyclodextrin on the bounding of salazosulfapyridine to human serum albumin. Int. J. Biol. Macromol. 2016, 92, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Menezes, P.P.; Serafini, M.R.; Carvalho, Y.M.B.G.; Santana, D.V.S.; Lima, B.S.; Quintans-Júnior, L.J.; Marreto, R.N.; Aquino, T.M.; Sabino, A.R.; Scotti, L.; et al. Kinetic and physical-chemical study of the inclusion complex of β-cyclodextrin containing carvacrol. J. Mol. Struct. 2016, 1125, 323–330. [Google Scholar] [CrossRef]
- Hădărugă, N.G.; Hădărugă, D.I.; Isengard, H.-D. Water content of natural cyclodextrins and their essential oil complexes: A comparative study between Karl Fischer titration and thermal methods. Food Chem. 2012, 132, 1741–1748. [Google Scholar] [CrossRef]
- Araujo, D.R.; Tsuneda, S.S.; Cereda, C.M.S.; Carvalho, F.D.G.F.; Preté, P.S.C.; Fernandes, S.A.; Yokaichiya, F.; Franco, M.K.K.D.; Mazzaro, I.; Fraceto, L.F.; et al. Development and pharmacological evaluation of ropivacaine-2-hydroxypropyl-β-cyclodextrin inclusion complex. Eur. J. Pharm. Sci. 2008, 33, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Müller, S.D.; Florentino, D.; Ortmann, C.F.; Martins, F.A.; Danielski, L.G.; Michels, M.; Constantino, L.S.; Petronilho, F.; Reginatto, F.H. Anti-inflammatory and antioxidant activities of aqueous extract of Cecropia glaziovii leaves. J. Ethnopharmacol. 2016, 185, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Hasnat, M.A.; Pervin, M.; Cha, K.M.; Kim, S.K.; Lim, B.O. Anti-inflammatory activity on mice of extract of Ganoderma lucidum grown on rice via modulation of MAPK and NF-κB pathways. Phytochemistry 2015, 114, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Quintans-Júnior, L.J.; Araújo, A.A.S.; Brito, R.G.; Santos, P.L.; Quintans, J.S.S.; Menezes, P.P.; Serafini, M.R.; Silva, G.F.; Carvalho, F.M.S.; Brogden, N.K.; et al. β-caryophyllene, a dietary cannabinoid, complexed with β-cyclodextrin produced anti-hyperalgesic effect involving the inhibition of Fos expression in superficial dorsal horn. Life Sci. 2016, 149, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Lavor, E.P.; Navarro, M.V.M.; Freire, F.D.; Aragão, C.F.S.; Raffin, F.N.; Barbosa, E.G.; Moura, T.F.A.L. Application of thermal analysis to the study of antituberculosis drugs–excipient compatibility. J. Therm. Anal. Calorim. 2014, 115, 2303–2309. [Google Scholar] [CrossRef]
- Posadas, I.; Bucci, M.; Roviezzo, F.; Rossi, A.; Parente, L.; Sautebin, L.; Cirino, G. Carrageenan-induced mouse paw oedema is biphasic, age-weight dependent and displays differential nitric oxide cyclooxygenase-2 expression. Brit. J. Pharmacol. 2004, 142, 331–338. [Google Scholar] [CrossRef]
- Kobayashi, C.; Fontanive, T.O.; Enzweiler, B.G.; Bona, L.R.; Massoni, T.; Apel, M.A.; Henriques, A.T.; Richter, M.F.; Ardenghi, P.; Suyenaga, E.S. Pharmacological evaluation of Copaifera multijuga oil in rats. Pharm. Biol. 2011, 49, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Alves-Filho, J.C.; Freitas, A.; Russo, M.; Cunha, F.Q. Toll-like receptor 4 signaling leads to neutrophil migration impairment in polymicrobial sepsis. Crit. Care Med. 2006, 34, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Bradley, P.P.; Priebat, D.A.; Christensen, R.D.; Rothstein, G. Measurement of Cutaneous Inflammation: Estimation of Neutrophil Content with an Enzyme Marker. J. Investig. Dermatol. 1982, 78, 206–209. [Google Scholar] [CrossRef] [PubMed]

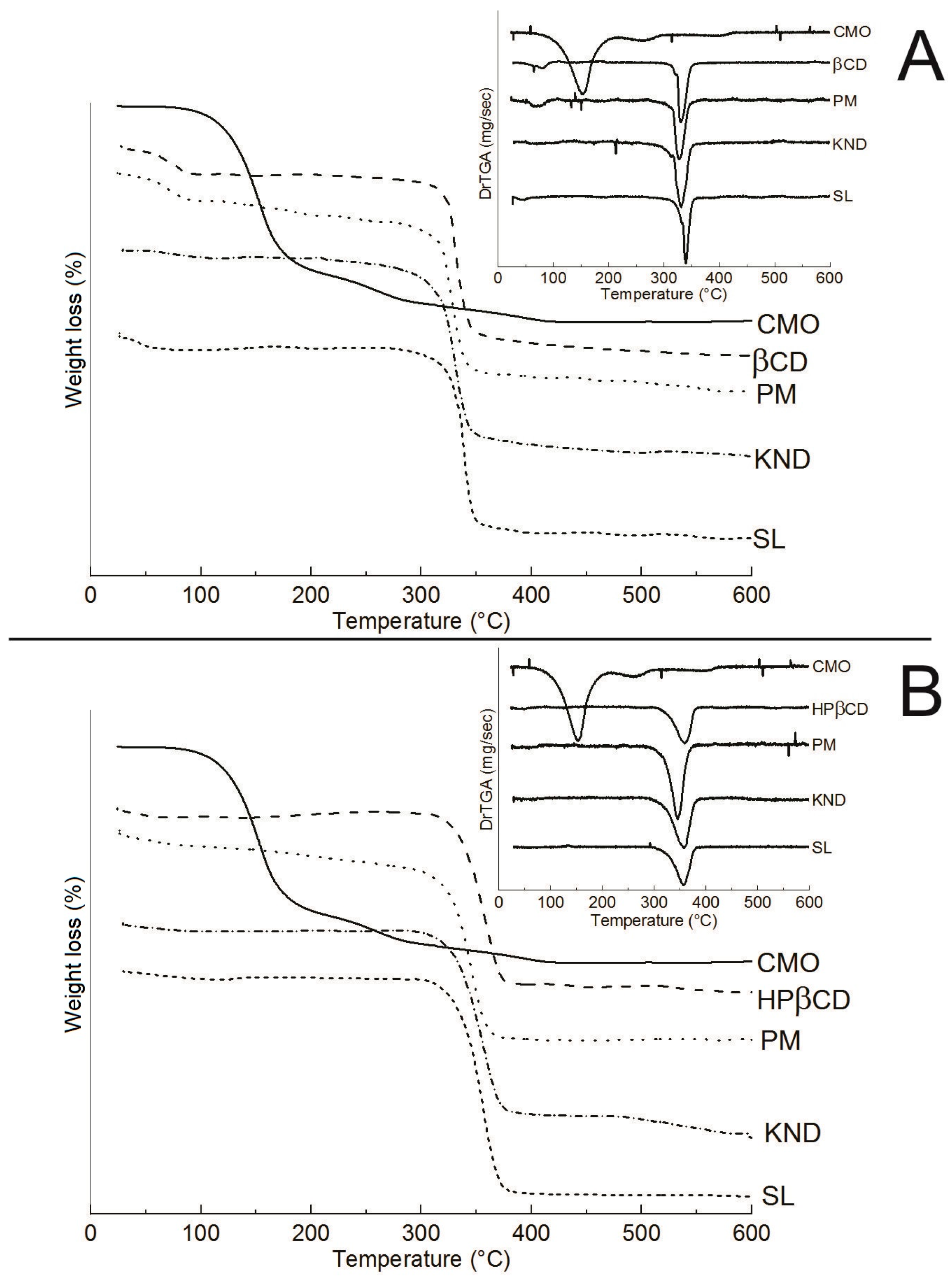


| Sample | Δm1 (%) | Δm2 (%) | Δm3 (%) |
|---|---|---|---|
| 25–200 °C | 200–400 °C | 400–600 °C | |
| CMO | 74.99 | 22.62 | 0.55 |
| β-CD | 13.60 | 79.42 | 6.02 |
| PM (β-CD) | 18.09 | 70.74 | 6.68 |
| KND (β-CD) | 4.06 | 86.40 | 5.34 |
| SL (β-CD) | 6.34 | 75.79 | 1.90 |
| HP-β-CD | 2.93 | 79.79 | 3.88 |
| PM (HP-β-CD) | 11.29 | 80.06 | 0.00 |
| KND (HP-β-CD) | 3.61 | 84.09 | 10.62 |
| SL (HP-β-CD) | 2.63 | 82.96 | 1.10 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinheiro, J.G.d.O.; Tavares, E.D.A.; Silva, S.S.d.; Félix Silva, J.; Carvalho, Y.M.B.G.d.; Ferreira, M.R.A.; Araújo, A.A.d.S.; Barbosa, E.G.; Fernandes Pedrosa, M.D.F.; Soares, L.A.L.; et al. Inclusion Complexes of Copaiba (Copaifera multijuga Hayne) Oleoresin and Cyclodextrins: Physicochemical Characterization and Anti-Inflammatory Activity. Int. J. Mol. Sci. 2017, 18, 2388. https://doi.org/10.3390/ijms18112388
Pinheiro JGdO, Tavares EDA, Silva SSd, Félix Silva J, Carvalho YMBGd, Ferreira MRA, Araújo AAdS, Barbosa EG, Fernandes Pedrosa MDF, Soares LAL, et al. Inclusion Complexes of Copaiba (Copaifera multijuga Hayne) Oleoresin and Cyclodextrins: Physicochemical Characterization and Anti-Inflammatory Activity. International Journal of Molecular Sciences. 2017; 18(11):2388. https://doi.org/10.3390/ijms18112388
Chicago/Turabian StylePinheiro, Jonas Gabriel de Oliveira, Emanuella De Aragão Tavares, Sofia Santos da Silva, Juliana Félix Silva, Yasmim Maria Barbosa Gomes de Carvalho, Magda Rhayanny Assunção Ferreira, Adriano Antunes de Souza Araújo, Euzébio Guimarães Barbosa, Matheus De Freitas Fernandes Pedrosa, Luiz Alberto Lira Soares, and et al. 2017. "Inclusion Complexes of Copaiba (Copaifera multijuga Hayne) Oleoresin and Cyclodextrins: Physicochemical Characterization and Anti-Inflammatory Activity" International Journal of Molecular Sciences 18, no. 11: 2388. https://doi.org/10.3390/ijms18112388