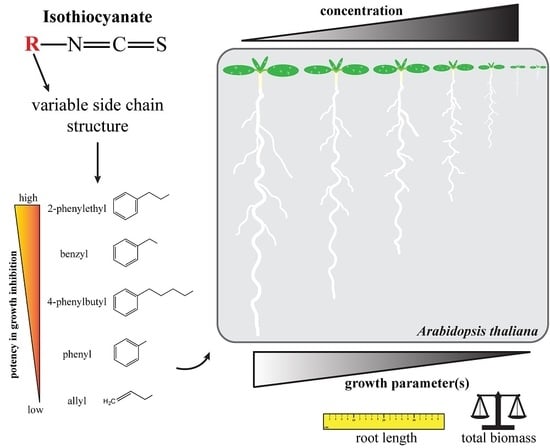
Glucosinolate-Derived Isothiocyanates Inhibit Arabidopsis Growth and the Potency Depends on Their Side Chain Structure
Abstract
:1. Introduction
2. Results
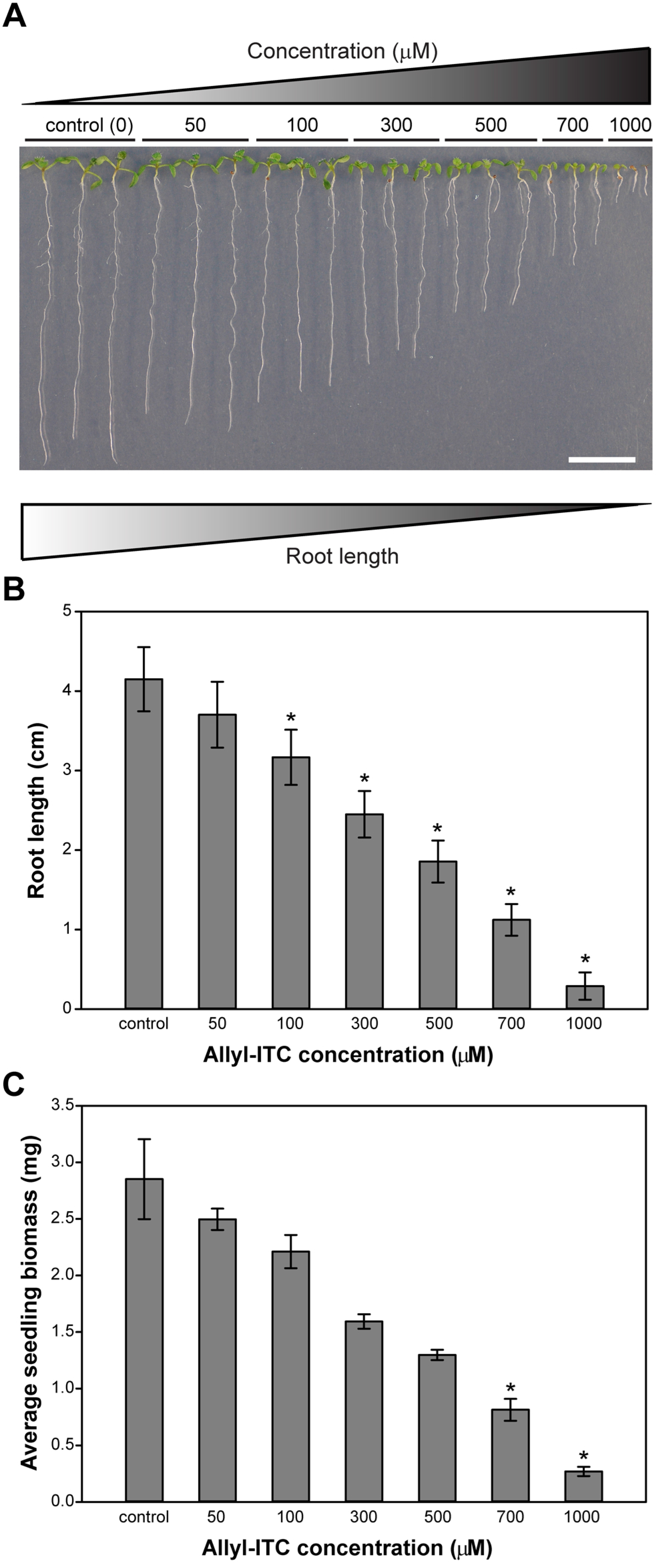
2.1. Allylisothiocyanate (Allyl-ITC) Inhibited Root Growth of Arabidopsis thaliana In Vitro
2.2. Allyl-ITC Reduced the Total Biomass of Arabidopsis thaliana In Vitro
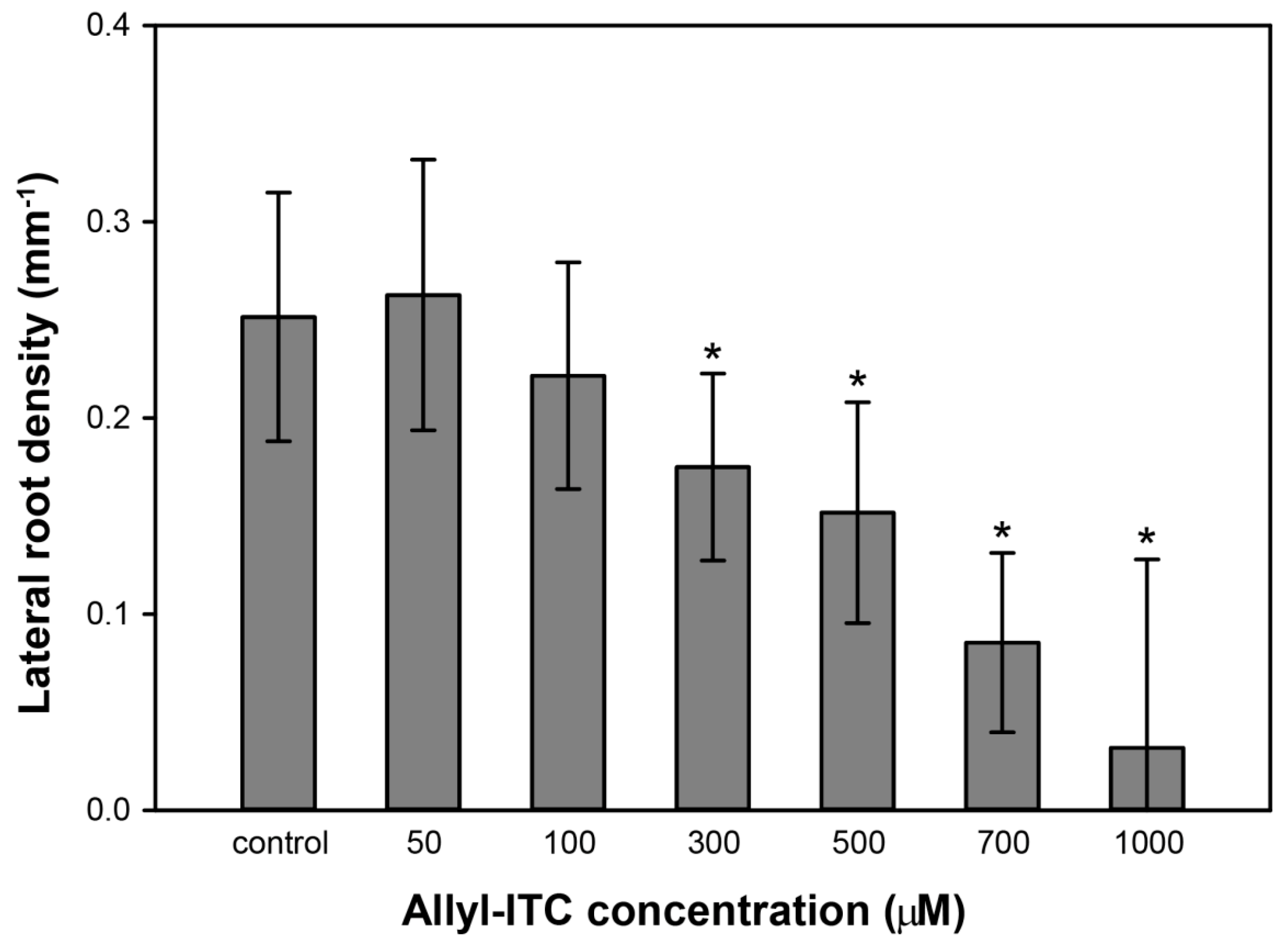
2.3. Allyl-ITC Induced Other Root Morphogenic Changes
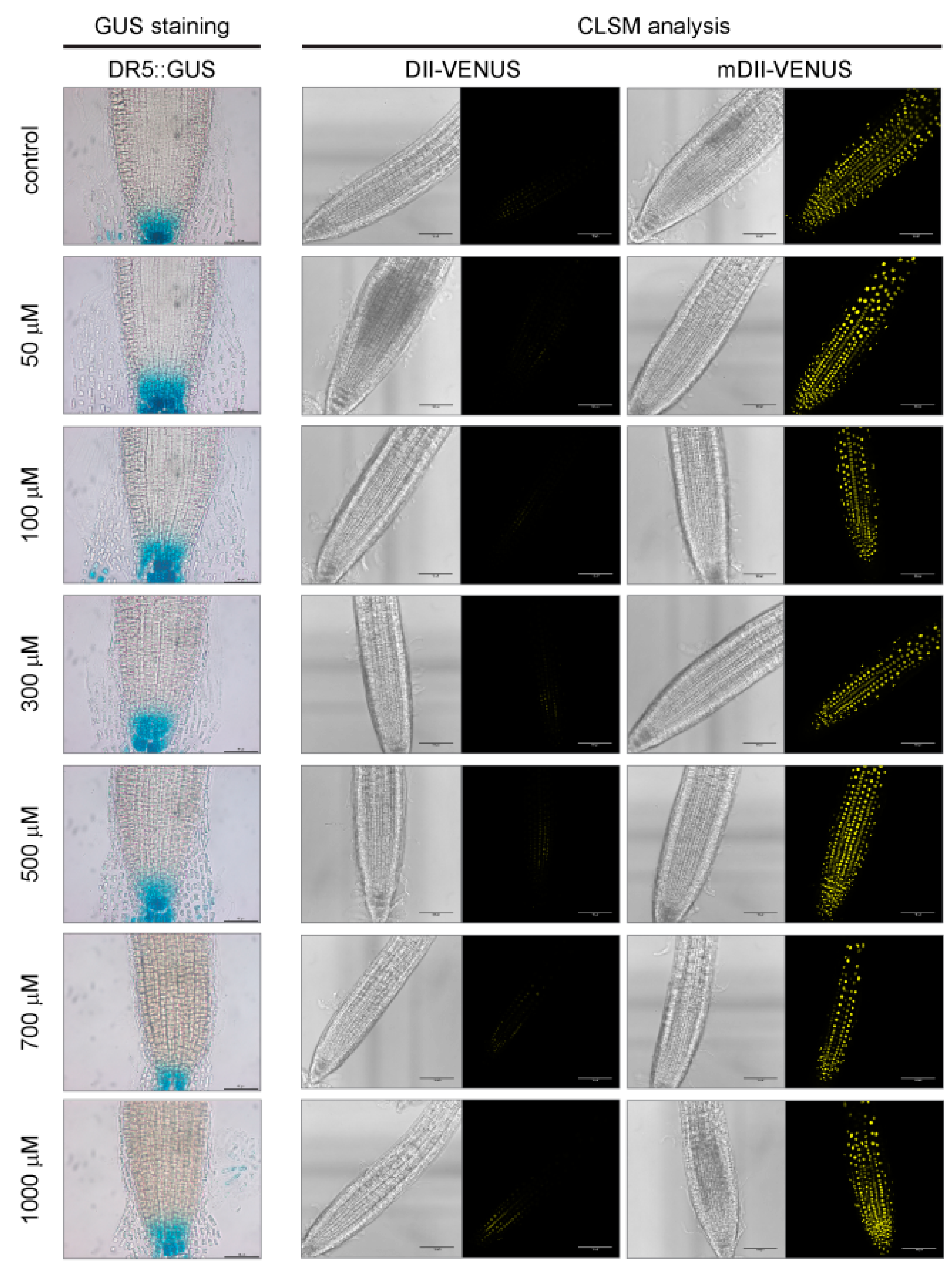
2.4. A Role for Auxin or Ethylene in Root Growth Inhibition Triggered by Allyl-ITC Could Not Be Established
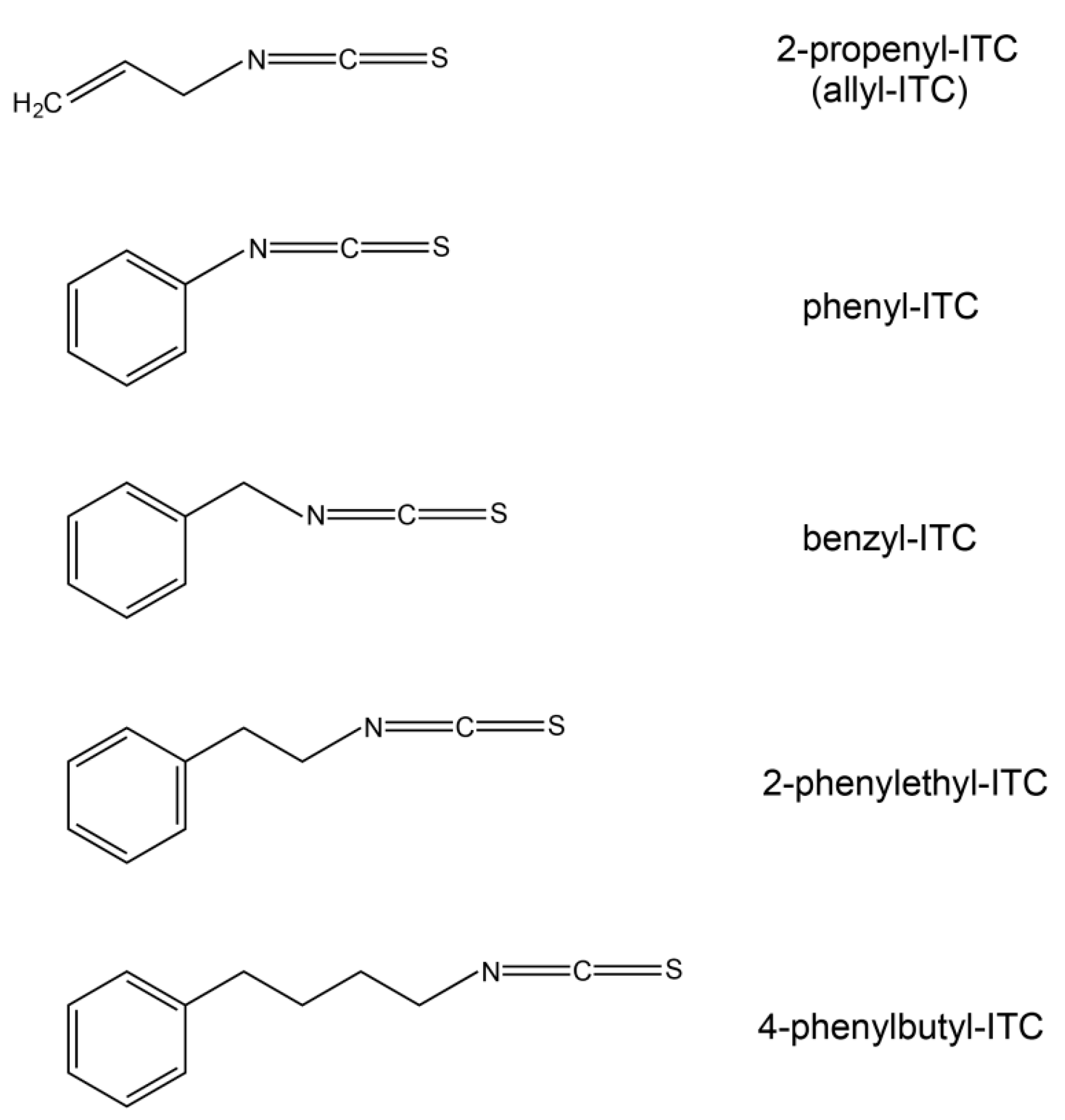
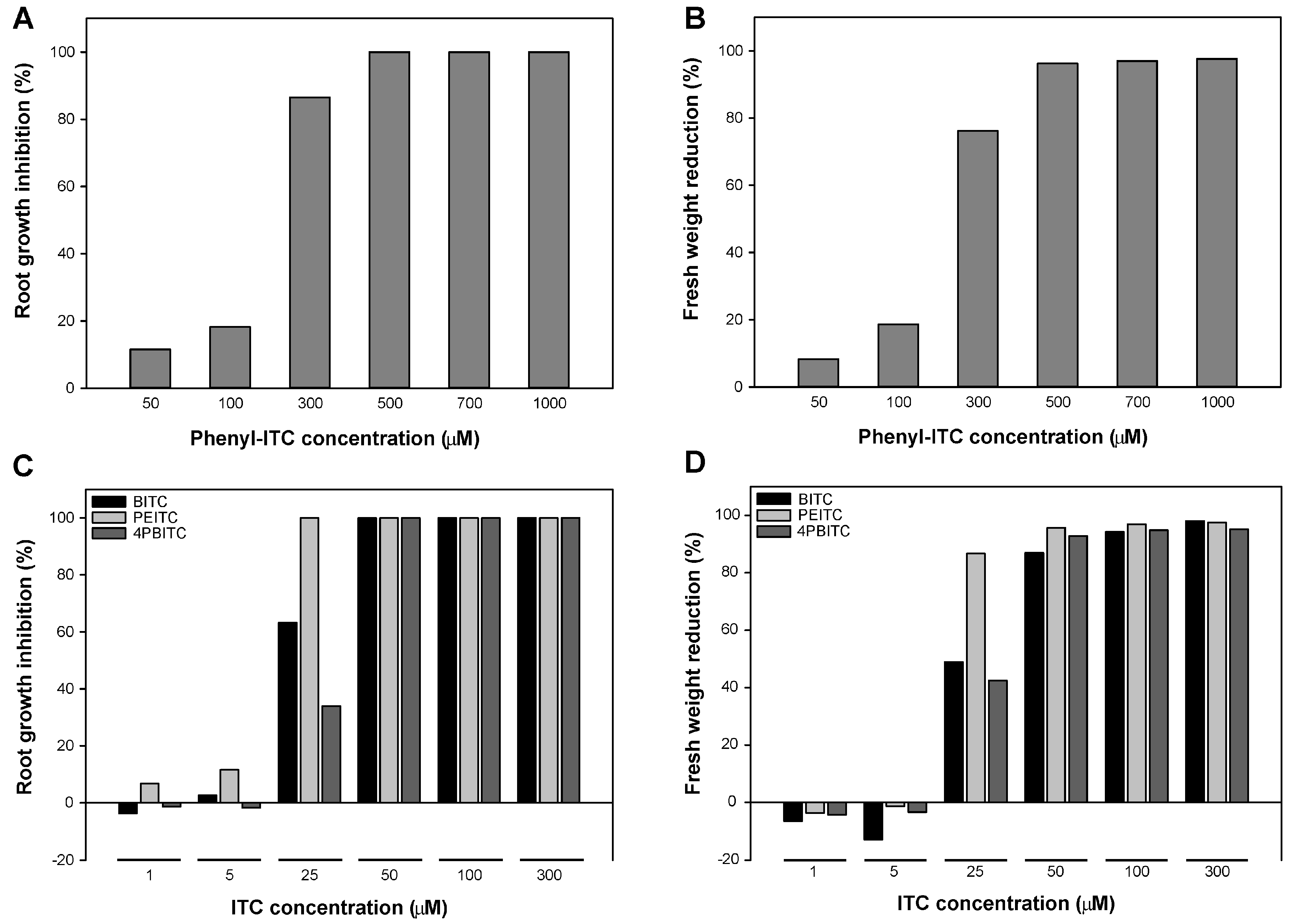
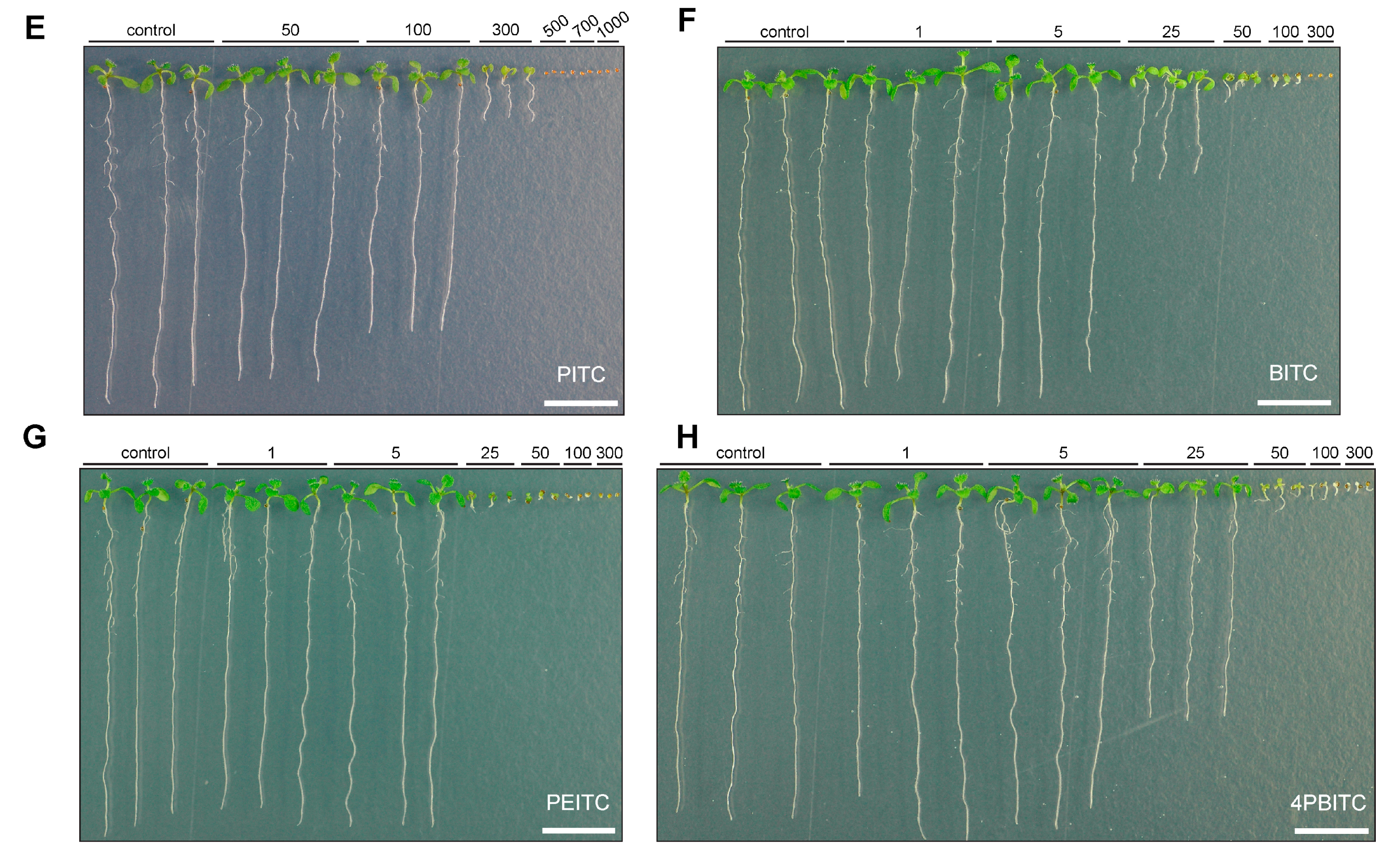
2.5. Effects on Arabidopsis Growth Depended on ITC Side Chain Structure
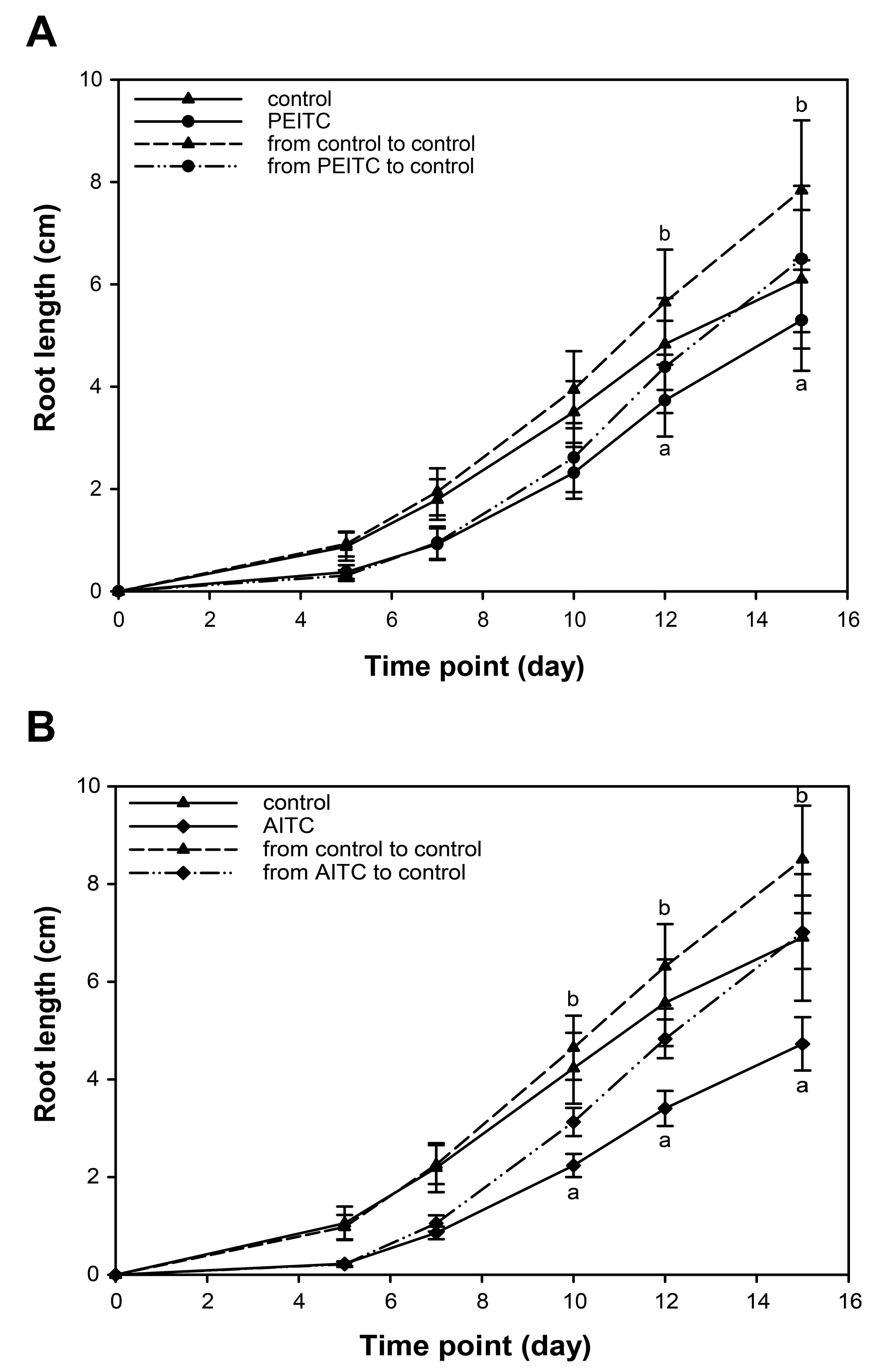
2.6. Root Growth Recovered When Seedlings Were Removed from Exposure to ITCs
3. Discussion
4. Materials and Methods
4.1. Isothiocyanates
4.2. Plant Material and Growth Conditions
4.3. Treatment with Isothiocyanates and Assessment of Growth Parameters
4.4. Recovery of Root Growth from Isothiocyanate Exposure
4.5. Histochemical Analysis of GUS Activity
4.6. Confocal Laser Scanning Microscopy (CLSM) Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bones, A.M.; Rossiter, J.T. The myrosinase-glucosinolate system, its organisation and biochemistry. Physiol. Plant. 1996, 97, 194–208. [Google Scholar] [CrossRef]
- Kissen, R.; Rossiter, J.T.; Bones, A.M. The “mustard oil bomb”: Not so easy to assemble?! Localization, expression and distribution of the components of the myrosinase enzyme system. Phytochem. Rev. 2009, 8, 69–86. [Google Scholar] [CrossRef]
- Agerbirk, N.; Olsen, C.E. Glucosinolate structures in evolution. Phytochemistry 2012, 77, 16–45. [Google Scholar] [CrossRef] [PubMed]
- Bones, A.M.; Rossiter, J.T. The enzymic and chemically induced decomposition of glucosinolates. Phytochemistry 2006, 67, 1053–1067. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.Y.; Kissen, R.; Bones, A.M. Characterization of recombinant nitrile-specifier proteins (NSPs) of Arabidopsis thaliana: Dependency on Fe(II) ions and the effect of glucosinolate substrate and reaction conditions. Phytochemistry 2012, 84, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Zabala, M.D.; Grant, M.; Bones, A.M.; Bennett, R.; Lim, Y.S.; Kissen, R.; Rossiter, J.T. Characterisation of recombinant epithiospecifier protein and its over-expression in Arabidopsis thaliana. Phytochemistry 2005, 66, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-Kostova, A.T.; Kostov, R.V. Glucosinolates and isothiocyanates in health and disease. Trends Mol. Med. 2012, 18, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Traka, M.; Mithen, R. Glucosinolates, isothiocyanates and human health. Phytochem. Rev. 2009, 8, 269–282. [Google Scholar] [CrossRef]
- Hopkins, R.J.; van Dam, N.M.; van Loon, J.J.A. Role of glucosinolates in insect-plant relationships and multitrophic interactions. Annu. Rev. Entomol. 2009, 54, 57–83. [Google Scholar] [CrossRef] [PubMed]
- Piasecka, A.; Jedrzejczak-Rey, N.; Bednarek, P. Secondary metabolites in plant innate immunity: Conserved function of divergent chemicals. New Phytol. 2015, 206, 948–964. [Google Scholar] [CrossRef] [PubMed]
- Gimsing, A.L.; Kirkegaard, J.A. Glucosinolates and biofumigation: Fate of glucosinolates and their hydrolysis products in soil. Phytochem. Rev. 2009, 8, 299–310. [Google Scholar] [CrossRef]
- Norsworthy, J.K.; Meehan, J.T. Herbicidal activity of eight isothiocyanates on Texas panicum (Panicum texanum), large crabgrass (Digitaria sanguinalis), and sicklepod (Senna obtusifolia). Weed Sci. 2005, 53, 515–520. [Google Scholar] [CrossRef]
- Vaughn, S.; Boydston, R. Volatile allelochemicals released by crucifer green manures. J. Chem. Ecol. 1997, 23, 2107–2116. [Google Scholar] [CrossRef]
- Andersson, M.X.; Nilsson, A.K.; Johansson, O.N.; Boztas, G.; Adolfsson, L.E.; Pinosa, F.; Petit, C.G.; Aronsson, H.; Mackey, D.; Tor, M.; et al. Involvement of the electrophilic isothiocyanate sulforaphane in Arabidopsis local defense responses. Plant Physiol. 2015, 167, 251–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hara, M.; Yatsuzuka, Y.; Tabata, K.; Kuboi, T. Exogenously applied isothiocyanates enhance glutathione S-transferase expression in Arabidopsis but act as herbicides at higher concentrations. J. Plant Physiol. 2010, 167, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Vega, L.J.; Krosse, S.; de Graaf, R.M.; Garvi, J.; Garvi-Bode, R.D.; van Dam, N.M. Allelopathic effects of glucosinolate breakdown products in Hanza [Boscia senegalensis (Pers.) Lam.] processing waste water. Front. Plant Sci. 2015, 6, 532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Øverby, A.; Stokland, R.A.; Asberg, S.E.; Sporsheim, B.; Bones, A.M. Allyl isothiocyanate depletes glutathione and upregulates expression of glutathione S-transferases in Arabidopsis thaliana. Front. Plant Sci. 2015, 6, 277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hossain, M.S.; Ye, W.; Hossain, M.A.; Okuma, E.; Uraji, M.; Nakamura, Y.; Mori, I.C.; Murata, Y. Glucosinolate degradation products, isothiocyanates, nitriles, and thiocyanates, induce stomatal closure accompanied by peroxidase-mediated reactive oxygen species production in Arabidopsis thaliana. Biosci. Biotechnol. Biochem. 2013, 77, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Khokon, M.A.; Jahan, M.S.; Rahman, T.; Hossain, M.A.; Muroyama, D.; Minami, I.; Munemasa, S.; Mori, I.C.; Nakamura, Y.; Murata, Y. Allyl isothiocyanate (AITC) induces stomatal closure in Arabidopsis. Plant Cell Environ. 2011, 34, 1900–1906. [Google Scholar] [CrossRef] [PubMed]
- Kissen, R.; Overby, A.; Winge, P.; Bones, A.M. Allyl-isothiocyanate treatment induces a complex transcriptional reprogramming including heat stress, oxidative stress and plant defence responses in Arabidopsis thaliana. BMC Genom. 2016, 17, 740. [Google Scholar] [CrossRef] [PubMed]
- Sporsheim, B.; Øverby, A.; Bones, A.M. Allyl isothiocyanate inhibits actin-dependent intracellular transport in Arabidopsis thaliana. Int. J. Mol. Sci. 2015, 16, 29134–29147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Øverby, A.; Baevre, M.S.; Thangstad, O.P.; Bones, A.M. Disintegration of microtubules in Arabidopsis thaliana and bladder cancer cells by isothiocyanates. Front. Plant Sci. 2015, 6, 6. [Google Scholar] [CrossRef] [Green Version]
- Åsberg, S.E.; Bones, A.M.; Øverby, A. Allyl isothiocyanate affects the cell cycle of Arabidopsis thaliana. Front. Plant Sci. 2015, 6, 364. [Google Scholar] [CrossRef] [Green Version]
- Overvoorde, P.; Fukaki, H.; Beeckman, T. Auxin control of root development. Cold Spring Harb. Perspect. Biol. 2010, 2, a001537. [Google Scholar] [CrossRef] [PubMed]
- Petricka, J.J.; Winter, C.M.; Benfey, P.N. Control of Arabidopsis root development. Annu. Rev. Plant Biol. 2012, 63, 563–590. [Google Scholar] [CrossRef] [PubMed]
- Kazan, K. Auxin and the integration of environmental signals into plant root development. Ann. Bot. 2013, 112, 1655–1665. [Google Scholar] [CrossRef] [PubMed]
- Perrot-Rechenmann, C. Cellular responses to auxin: Division versus expansion. Cold Spring Harb. Perspect. Biol. 2010, 2, a001446. [Google Scholar] [CrossRef] [PubMed]
- Takatsuka, H.; Umeda, M. Hormonal control of cell division and elongation along differentiation trajectories in roots. J. Exp. Bot. 2014, 65, 2633–2643. [Google Scholar] [CrossRef] [PubMed]
- Fukaki, H.; Tasaka, M. Hormone interactions during lateral root formation. Plant Mol. Biol. 2009, 69, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Marchant, A.; Bhalerao, R.; Casimiro, I.; Eklof, J.; Casero, P.J.; Bennett, M.; Sandberg, G. AUX1 promotes lateral root formation by facilitating indole-3-acetic acid distribution between sink and source tissues in the Arabidopsis seedling. Plant Cell 2002, 14, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Swarup, K.; Benkova, E.; Swarup, R.; Casimiro, I.; Peret, B.; Yang, Y.; Parry, G.; Nielsen, E.; de Smet, I.; Vanneste, S.; et al. The auxin influx carrier LAX3 promotes lateral root emergence. Nat. Cell Biol. 2008, 10, 946–954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benkova, E.; Michniewicz, M.; Sauer, M.; Teichmann, T.; Seifertova, D.; Jurgens, G.; Friml, J. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 2003, 115, 591–602. [Google Scholar] [CrossRef]
- Blilou, I.; Xu, J.; Wildwater, M.; Willemsen, V.; Paponov, I.; Friml, J.; Heidstra, R.; Aida, M.; Palme, K.; Scheres, B. The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature 2005, 433, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Swarup, R.; Perry, P.; Hagenbeek, D.; van der Straeten, D.; Beemster, G.T.; Sandberg, G.; Bhalerao, R.; Ljung, K.; Bennett, M.J. Ethylene upregulates auxin biosynthesis in Arabidopsis seedlings to enhance inhibition of root cell elongation. Plant Cell 2007, 19, 2186–2196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruzicka, K.; Ljung, K.; Vanneste, S.; Podhorska, R.; Beeckman, T.; Friml, J.; Benkova, E. Ethylene regulates root growth through effects on auxin biosynthesis and transport-dependent auxin distribution. Plant Cell 2007, 19, 2197–2212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortega-Martinez, O.; Pernas, M.; Carol, R.J.; Dolan, L. Ethylene modulates stem cell division in the Arabidopsis thaliana root. Science 2007, 317, 507–510. [Google Scholar] [CrossRef] [PubMed]
- Muday, G.K.; Rahman, A.; Binder, B.M. Auxin and ethylene: Collaborators or competitors? Trends Plant Sci. 2012, 17, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.R.; Negi, S.; Sukumar, P.; Muday, G.K. Ethylene inhibits lateral root development, increases IAA transport and expression of PIN3 and PIN7 auxin efflux carriers. Development 2011, 138, 3485–3495. [Google Scholar] [CrossRef] [PubMed]
- Negi, S.; Ivanchenko, M.G.; Muday, G.K. Ethylene regulates lateral root formation and auxin transport in Arabidopsis thaliana. Plant J. 2008, 55, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Wittstock, U.; Burow, M. Glucosinolate breakdown in Arabidopsis: Mechanism, regulation and biological significance. Arabidopsis Book 2010, 8, e0134. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.D.; Tokuhisa, J.G.; Reichelt, M.; Gershenzon, J. Variation of glucosinolate accumulation among different organs and developmental stages of Arabidopsis thaliana. Phytochemistry 2003, 62, 471–481. [Google Scholar] [CrossRef]
- Clossais-Besnard, N.; Larher, F. Physiological role of glucosinolates in Brassica napus. Concentration and distribution pattern of glucosinolates among plant organs during a complete life cycle. J. Sci. Food Agric. 1991, 56, 25–38. [Google Scholar] [CrossRef]
- James, D.C.; Rossiter, J.T. Development and characteristics of myrosinase in Brassica napus during early seedling growth. Physiol. Plant. 1991, 82, 163–170. [Google Scholar] [CrossRef]
- Stepanova, A.N.; Yun, J.; Likhacheva, A.V.; Alonso, J.M. Multilevel interactions between ethylene and auxin in Arabidopsis roots. Plant Cell 2007, 19, 2169–2185. [Google Scholar] [CrossRef] [PubMed]
- Ulmasov, T.; Murfett, J.; Hagen, G.; Guilfoyle, T.J. Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell 1997, 9, 1963–1971. [Google Scholar] [CrossRef] [PubMed]
- Brunoud, G.; Wells, D.M.; Oliva, M.; Larrieu, A.; Mirabet, V.; Burrow, A.H.; Beeckman, T.; Kepinski, S.; Traas, J.; Bennett, M.J.; et al. A novel sensor to map auxin response and distribution at high spatio-temporal resolution. Nature 2012, 482, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Kissen, R.; Pope, T.W.; Grant, M.; Pickett, J.A.; Rossiter, J.T.; Powell, G. Modifying the alkylglucosinolate profile in Arabidopsis thaliana alters the tritrophic interaction with the herbivore Brevicoryne brassicae and parasitoid Diaeretiella rapae. J. Chem. Ecol. 2009, 35, 958–969. [Google Scholar] [CrossRef] [PubMed]
- Lambrix, V.; Reichelt, M.; Mitchell-Olds, T.; Kliebenstein, D.J.; Gershenzon, J. The Arabidopsis epithiospecifier protein promotes the hydrolysis of glucosinolates to nitriles and influences Trichoplusia ni herbivory. Plant Cell 2001, 13, 2793–2807. [Google Scholar] [CrossRef] [PubMed]
- Kliebenstein, D.J.; Kroymann, J.; Brown, P.; Figuth, A.; Pedersen, D.; Gershenzon, J.; Mitchell-Olds, T. Genetic control of natural variation in Arabidopsis glucosinolate accumulation. Plant Physiol. 2001, 126, 811–825. [Google Scholar] [CrossRef] [PubMed]
- Reichelt, M.; Brown, P.D.; Schneider, B.; Oldham, N.J.; Stauber, E.; Tokuhisa, J.; Kliebenstein, D.J.; Mitchell-Olds, T.; Gershenzon, J. Benzoic acid glucosinolate esters and other glucosinolates from Arabidopsis thaliana. Phytochemistry 2002, 59, 663–671. [Google Scholar] [CrossRef]
- Fahey, J.W.; Zalcmann, A.T.; Talalay, P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 2001, 56, 5–51. [Google Scholar] [CrossRef]
- Petersen, B.L.; Chen, S.; Hansen, C.H.; Olsen, C.E.; Halkier, B.A. Composition and content of glucosinolates in developing Arabidopsis thaliana. Planta 2002, 214, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Sarsby, J.; Towers, M.W.; Stain, C.; Cramer, R.; Koroleva, O.A. Mass spectrometry imaging of glucosinolates in Arabidopsis flowers and siliques. Phytochemistry 2012, 77, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Shroff, R.; Vergara, F.; Muck, A.; Svatos, A.; Gershenzon, J. Nonuniform distribution of glucosinolates in Arabidopsis thaliana leaves has important consequences for plant defense. Proc. Natl. Acad. Sci. USA 2008, 105, 6196–6201. [Google Scholar] [CrossRef] [PubMed]
- Koroleva, O.A.; Davies, A.; Deeken, R.; Thorpe, M.R.; Tomos, A.D.; Hedrich, R. Identification of a new glucosinolate-rich cell type in Arabidopsis flower stalk. Plant Physiol. 2000, 124, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Koroleva, O.A.; Gibson, T.M.; Cramer, R.; Stain, C. Glucosinolate-accumulating S-cells in Arabidopsis leaves and flower stalks undergo programmed cell death at early stages of differentiation. Plant J. 2010, 64, 456–469. [Google Scholar] [CrossRef] [PubMed]
- Hanschen, F.S.; Schreiner, M. Isothiocyanates, nitriles, and epithionitriles from glucosinolates are affected by genotype and developmental stage in Brassica oleracea varieties. Front. Plant Sci. 2017, 8, 1095. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.F.; Chen, S.X. Regulation of plant glucosinolate metabolism. Planta 2007, 226, 1343–1352. [Google Scholar] [CrossRef] [PubMed]
- Rumberger, A.; Marschner, P. 2-Phenylethylisothiocyanate concentration and microbial community composition in the rhizosphere of canola. Soil Biol. Biochem. 2003, 35, 445–452. [Google Scholar] [CrossRef]
- Schreiner, M.; Krumbein, A.; Knorr, D.; Smetanska, I. Enhanced glucosinolates in root exudates of Brassica rapa ssp. rapa mediated by salicylic acid and methyl jasmonate. J. Agric. Food Chem. 2011, 59, 1400–1405. [Google Scholar] [CrossRef] [PubMed]
- Strehmel, N.; Böttcher, C.; Schmidt, S.; Scheel, D. Profiling of secondary metabolites in root exudates of Arabidopsis thaliana. Phytochemistry 2014, 108, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Auger, B.; Pouvreau, J.B.; Pouponneau, K.; Yoneyama, K.; Montiel, G.; Le Bizec, B.; Delavault, P.; Delourme, R.; Simier, P. Germination stimulants of Phelipanche ramosa in the rhizosphere of Brassica napus are derived from the glucosinolate pathway. Mol. Plant Microbe Interact. 2012, 25, 993–1004. [Google Scholar] [CrossRef] [PubMed]
- Mönchgesang, S.; Strehmel, N.; Schmidt, S.; Westphal, L.; Taruttis, F.; Müller, E.; Herklotz, S.; Neumann, S.; Scheel, D. Natural variation of root exudates in Arabidopsis thaliana-linking metabolomic and genomic data. Sci. Rep. 2016, 6, 29033. [Google Scholar] [CrossRef]
- Xu, D.; Hanschen, F.S.; Witzel, K.; Nintemann, S.J.; Nour-Eldin, H.H.; Schreiner, M.; Halkier, B.A. Rhizosecretion of stele-synthesized glucosinolates and their catabolites requires GTR-mediated import in Arabidopsis. J. Exp. Bot. 2017, 68, 3205–3214. [Google Scholar] [CrossRef] [PubMed]
- Bialy, Z.; Oleszek, W.; Lewis, J.; Fenwick, G.R. Allelopathic potential of glucosinolates (mustard oil glycosides) and their degradation products against wheat. Plant Soil 1990, 129, 227–281. [Google Scholar] [CrossRef]
- Hanschen, F.S.; Lamy, E.; Schreiner, M.; Rohn, S. Reactivity and stability of glucosinolates and their breakdown products in foods. Angew. Chem. Int. Ed. 2014, 53, 11430–11450. [Google Scholar] [CrossRef] [PubMed]
- Jiao, D.; Eklind, K.I.; Choi, C.I.; Desai, D.H.; Amin, S.G.; Chung, F.L. Structure-activity relationships of isothiocyanates as mechanism-based inhibitors of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced lung tumorigenesis in A/J mice. Cancer Res. 1994, 54, 4327–4333. [Google Scholar] [PubMed]
- Luang-In, V.; Rossiter, J.T. Stability studies of isothiocyanates and nitriles in aqueous media. Songklanakarin J. Sci. Technol. 2015, 37, 625–630. [Google Scholar]
- Ohta, Y.; Takatani, K.; Kawakishi, S. Decomposition rate of allyl isothiocyanate in aqueous solution. Biosci. Biotechnol. Biochem. 1995, 59, 102–103. [Google Scholar] [CrossRef]
- Kawakishi, S.; Namiki, M. Decomposition of allyl isothiocyanate in aqueous solution. Agric. Biol. Chem. 1969, 33, 452–459. [Google Scholar] [CrossRef]
- Pecháček, R.; Velíšek, J.; Hrabcová, H. Decomposition products of allyl isothiocyanate in aqueous solutions. J. Agric. Food Chem. 1997, 45, 4584–4588. [Google Scholar] [CrossRef]
- Tsao, R.; Yu, Q.; Friesen, I.; Potter, J.; Chiba, M. Factors affecting the dissolution and degradation of oriental mustard-derived sinigrin and allyl isothiocyanate in aqueous media. J. Agric. Food Chem. 2000, 48, 1898–1902. [Google Scholar] [CrossRef] [PubMed]
- Hanschen, F.S.; Bauer, A.; Mewis, I.; Keil, C.; Schreiner, M.; Rohn, S.; Kroh, L.W. Thermally induced degradation of aliphatic glucosinolates: Identification of intermediary breakdown products and proposed degradation pathways. J. Agric. Food Chem. 2012, 60, 9890–9899. [Google Scholar] [CrossRef] [PubMed]
- De Nicola, G.R.; Montaut, S.; Rollin, P.; Nyegue, M.; Menut, C.; Iori, R.; Tatibouet, A. Stability of benzylic-type isothiocyanates in hydrodistillation-mimicking conditions. J. Agric. Food Chem. 2013, 61, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Iori, R.; Thornalley, P.J. Purification of major glucosinolates from Brassicaceae seeds and preparation of isothiocyanate and amine metabolites. J. Sci. Food Agric. 2006, 86, 1271–1280. [Google Scholar] [CrossRef]
- Rohloff, J.; Bones, A.M. Volatile profiling of Arabidopsis thaliana - putative olfactory compounds in plant communication. Phytochemistry 2005, 66, 1941–1955. [Google Scholar] [CrossRef] [PubMed]
- Rumble, J. CRC Handbook of Chemistry and Physics, 98th ed.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2017; ISBN 978-1-498-78454-2. [Google Scholar]
- Norsworthy, J.K.; Meehan, J.T. Use of isothiocyanates for suppression of Palmer amaranth (Amaranthus palmeri ), pitted morningglory (Ipomoea lacunosa), and yellow nutsedge (Cyperus esculentus). Weed Sci. 2005, 53, 884–890. [Google Scholar] [CrossRef]
- Wu, H.; Feng, J.T.; Lin, K.C.; Zhang, X. Synthesis and herbicidal activity of substituted pyrazole isothiocyanates. Molecules 2012, 17, 12187–12196. [Google Scholar] [CrossRef] [PubMed]
- Conaway, C.C.; Jiao, D.; Chung, F.-L. Inhibition of rat liver cytochrome P450 isozymes by isothiocyanates and their conjugates: A structure-activity relationship study. Carcinogenesis 1996, 17, 2423–2427. [Google Scholar] [CrossRef] [PubMed]
- Morse, M.A.; Eklind, K.I.; Amin, S.G.; Hecht, S.S.; Chung, F.L. Effects of alkyl chain length on the inhibition of NNK-induced lung neoplasia in A/J mice by arylalkyl isothiocyanates. Carcinogenesis 1989, 10, 1757–1759. [Google Scholar] [CrossRef] [PubMed]
- Morse, M.A.; Eklind, K.I.; Hecht, S.S.; Jordan, K.G.; Choi, C.I.; Desai, D.H.; Amin, S.G.; Chung, F.L. Structure-activity relationships for inhibition of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone lung tumorigenesis by arylalkyl isothiocyanates in A/J mice. Cancer Res. 1991, 51, 1846–1850. [Google Scholar] [PubMed]
- Zhao, Z.; Zhang, W.; Stanley, B.A.; Assmann, S.M. Functional proteomics of Arabidopsis thaliana guard cells uncovers new stomatal signaling pathways. Plant Cell 2008, 20, 3210–3226. [Google Scholar] [CrossRef] [PubMed]
- Del Carmen Martínez-Ballesta, M.; Moreno, D.A.; Carvajal, M. The physiological importance of glucosinolates on plant response to abiotic stress in Brassica. Int. J. Mol. Sci. 2013, 14, 11607–11625. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Ballesta Mdel, C.; Muries, B.; Moreno, D.A.; Dominguez-Perles, R.; Garcia-Viguera, C.; Carvajal, M. Involvement of a glucosinolate (sinigrin) in the regulation of water transport in Brassica oleracea grown under salt stress. Physiol. Plant. 2014, 150, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Ballesta, M.; Moreno-Fernandez, D.A.; Castejon, D.; Ochando, C.; Morandini, P.A.; Carvajal, M. The impact of the absence of aliphatic glucosinolates on water transport under salt stress in Arabidopsis thaliana. Front. Plant Sci. 2015, 6, 524. [Google Scholar] [CrossRef] [PubMed]
- Katz, E.; Nisani, S.; Yadav, B.S.; Woldemariam, M.G.; Shai, B.; Obolski, U.; Ehrlich, M.; Shani, E.; Jander, G.; Chamovitz, D.A. The glucosinolate breakdown product indole-3-carbinol acts as an auxin antagonist in roots of Arabidopsis thaliana. Plant J. 2015, 82, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Scarpella, E.; Francis, P.; Berleth, T. Stage-specific markers define early steps of procambium development in Arabidopsis leaves and correlate termination of vein formation with mesophyll differentiation. Development 2004, 131, 3445–3455. [Google Scholar] [CrossRef] [PubMed]
| Mutant Name | Gene Name (ID) | Description |
|---|---|---|
| eir1-1 (pin2-1) | EIR1/PIN2 (At5g57090) | auxin efflux carrier |
| pin3-4 | PIN3 (At1g70940) | regulator of auxin efflux and transport |
| pin4-3 | PIN4 (At2g01420) | putative auxin efflux carrier |
| tir1-1 | TIR1 (At3g62980) | auxin receptor/F-box protein |
| axr2-1 | AXR2/IAA7 (At3g23050) | member of Aux/IAA protein family |
| axr3-1 | AXR3/IAA17 (At1g04250) | member of Aux/IAA protein family |
| axr4-1 | AXR4 (At1g54990) | involved in polar auxin transport |
| axr6-1 | AXR6 (At4g02570) | CUL1 subunit of SCF complex |
| ein2-1 | EIN2 (At5g03280) | involved in ethylene signal transduction |
| etr1-3 | ETR1/EIN1 (At1g66340) | ethylene binding receptor |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urbancsok, J.; Bones, A.M.; Kissen, R. Glucosinolate-Derived Isothiocyanates Inhibit Arabidopsis Growth and the Potency Depends on Their Side Chain Structure. Int. J. Mol. Sci. 2017, 18, 2372. https://doi.org/10.3390/ijms18112372
Urbancsok J, Bones AM, Kissen R. Glucosinolate-Derived Isothiocyanates Inhibit Arabidopsis Growth and the Potency Depends on Their Side Chain Structure. International Journal of Molecular Sciences. 2017; 18(11):2372. https://doi.org/10.3390/ijms18112372
Chicago/Turabian StyleUrbancsok, János, Atle M. Bones, and Ralph Kissen. 2017. "Glucosinolate-Derived Isothiocyanates Inhibit Arabidopsis Growth and the Potency Depends on Their Side Chain Structure" International Journal of Molecular Sciences 18, no. 11: 2372. https://doi.org/10.3390/ijms18112372