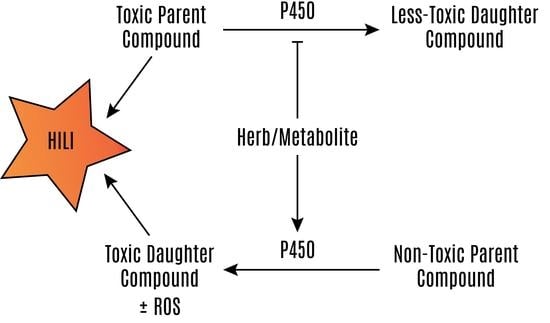
Hepatotoxicity of Herbal Supplements Mediated by Modulation of Cytochrome P450
Abstract
:1. Introduction
2. Literature Search Methodology
3. Herbal Supplements with Potential P450-Associated Hepatotoxicity
3.1. Herbal Supplement Inhibition of P450s
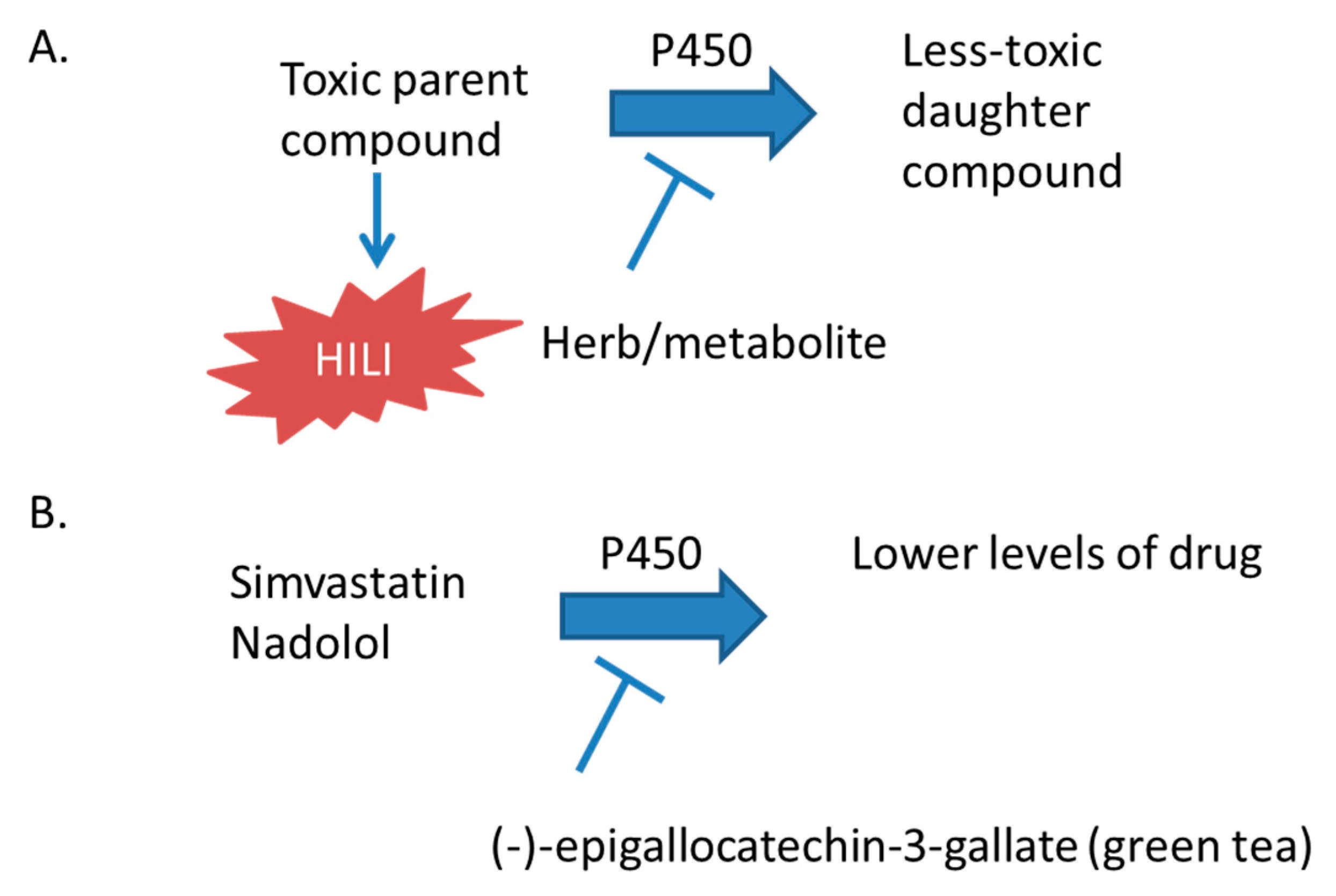
3.1.1. Green Tea
3.1.2. Black Cohosh
3.1.3. Cranberry
3.1.4. Grapefruit
3.1.5. Echinacea
3.1.6. Gardenia
3.2. Herbal Supplement Induction of P450 Enzymes
3.2.1. The Pregnane X Receptor (PXR)
3.2.2. The Constitutive Androstane Receptor (CAR)
3.2.3. St. John’s Wort
3.2.4. Gingko Biloba
3.2.5. Ginseng
3.2.6. Piperine
3.2.7. Garlic
3.3. Herbal Supplement Hepatotoxicity Mediated by the P450 System
3.3.1. Characterization of Liver Injury by Pathophysiology
3.3.2. Characterization of Liver Injury by Pathogenesis
3.3.3. Assessment of Causality
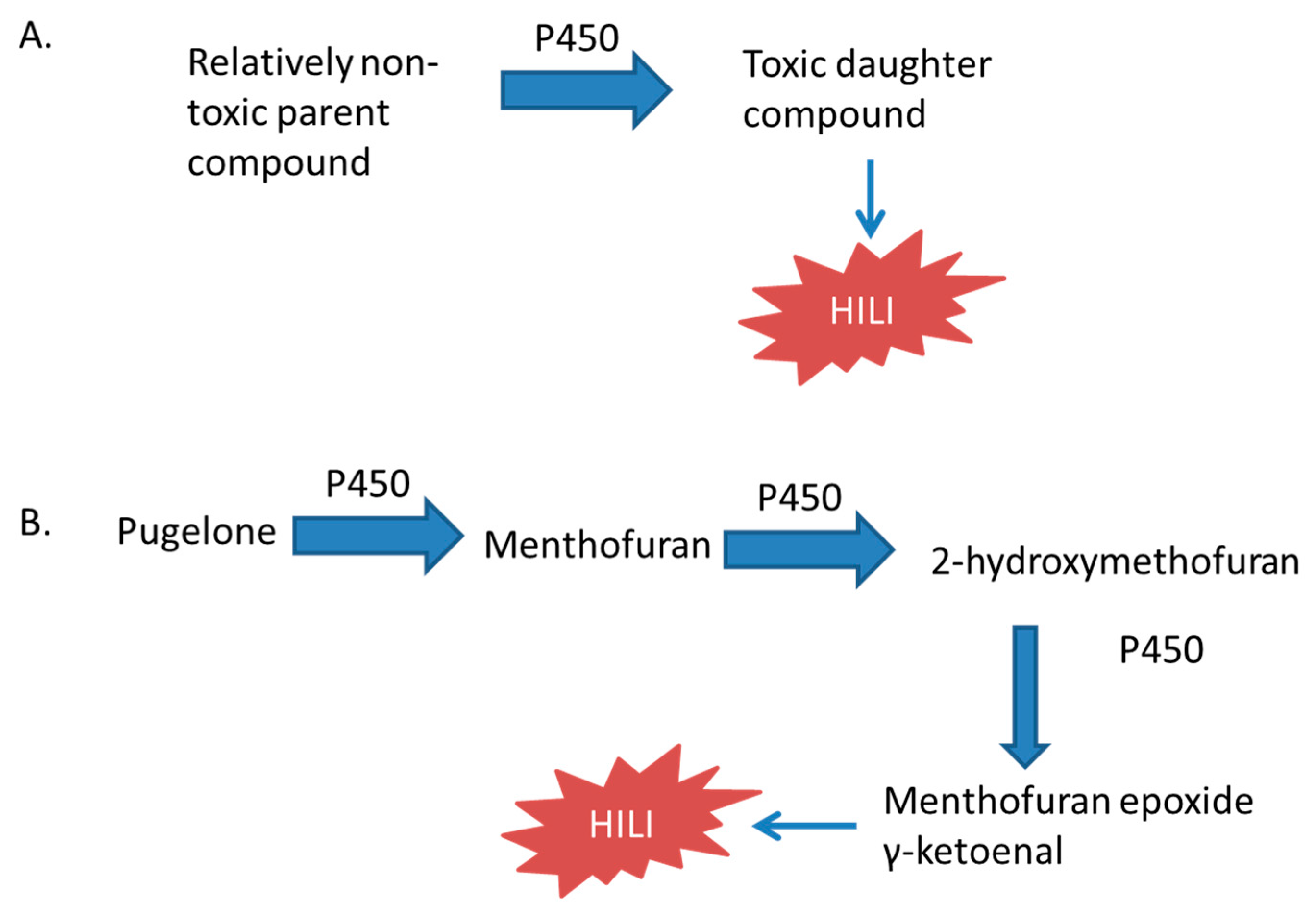
3.3.4. Peppermint Oil, Pennyroyal, and Menthol
3.3.5. Camphor
3.3.6. Germander
3.3.7. Pyrrolizidine Alkaloids
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
References
- Tucker, G.T.; Houston, J.B.; Huang, S.M. Optimizing drug development: Strategies to assess drug metabolism/transporter interaction potential-toward a consensus. Clin. Pharmacol. Ther. 2001, 70, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Matthews, H.B.; Lucier, G.W.; Fisher, K.D. Medicinal herbs in the United States: Research needs. Environ. Health Perspect. 1999, 107, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Bessone, F.; Hernandez, N.; Lucena, M.I.; Andrade, R.J.; Latin Dili Network, L.; Spanish Dili, R. The latin american DILI registry experience: A successful ongoing collaborative strategic initiative. Int. J. Mol. Sci. 2016, 17, 313. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, P.H. Drug-induced liver injury network causality assessment: Criteria and experience in the United States. Int. J. Mol. Sci. 2016, 17, 201. [Google Scholar] [CrossRef] [PubMed]
- Misaka, S.; Miyazaki, N.; Fukushima, T.; Yamada, S.; Kimura, J. Effects of green tea extract and (−)-epigallocatechin-3-gallate on pharmacokinetics of nadolol in rats. Phytomedicine 2013, 20, 1247–1250. [Google Scholar] [CrossRef] [PubMed]
- Kliewer, S.A.; Moore, J.T.; Wade, L.; Staudinger, J.L.; Watson, M.A.; Jones, S.A.; McKee, D.D.; Oliver, B.B.; Willson, T.M.; Zetterstrom, R.H.; et al. An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway. Cell 1998, 92, 73–82. [Google Scholar] [CrossRef]
- Lehmann, J.M.; McKee, D.D.; Watson, M.A.; Willson, T.M.; Moore, J.T.; Kliewer, S.A. The human orphan nuclear receptor PXR is activated by compounds that regulate CYP3A4 gene expression and cause drug interactions. J. Clin. Investig. 1998, 102, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Cherian, M.T.; Chai, S.C.; Chen, T. Small-molecule modulators of the constitutive androstane receptor. Expert Opin. Drug Metab. Toxicol. 2015, 11, 1099–1114. [Google Scholar] [CrossRef] [PubMed]
- Honkakoski, P.; Zelko, I.; Sueyoshi, T.; Negishi, M. The nuclear orphan receptor CAR-retinoid X receptor heterodimer activates the phenobarbital-responsive enhancer module of the CYP2B gene. Mol. Cell. Biol. 1998, 18, 5652–5658. [Google Scholar] [CrossRef] [PubMed]
- Piyachaturawat, P.; Kingkaeohoi, S.; Toskulkao, C. Potentiation of carbon tetrachloride hepatotoxicity by piperine. Drug Chem. Toxicol. 1995, 18, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Gurley, B.J.; Gardner, S.F.; Hubbard, M.A.; Williams, D.K.; Gentry, W.B.; Cui, Y.; Ang, C.Y. Clinical assessment of effects of botanical supplementation on cytochrome P450 phenotypes in the elderly: St John’s wort, garlic oil, panax ginseng and ginkgo biloba. Drugs Aging 2005, 22, 525–539. [Google Scholar] [CrossRef] [PubMed]
- Gurley, B.J.; Gardner, S.F.; Hubbard, M.A.; Williams, D.K.; Gentry, W.B.; Cui, Y.; Ang, C.Y. Cytochrome P450 phenotypic ratios for predicting herb-drug interactions in humans. Clin. Pharmacol. Ther. 2002, 72, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.S.; Zhou, G.; Zhu, B.; Wu, J.; Wang, J.G.; Abd El-Aty, A.M.; Li, T.; Liu, J.; Yang, T.L.; Wang, D.; et al. St John’s wort induces both cytochrome P450 3A4-catalyzed sulfoxidation and 2C19-dependent hydroxylation of omeprazole. Clin. Pharmacol. Ther. 2004, 75, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.S.; Zhu, B.; Abd El-Aty, A.M.; Zhou, G.; Li, Z.; Wu, J.; Chen, G.L.; Liu, J.; Tang, Z.R.; An, W.; et al. The influence of St John’s wort on CYP2C19 activity with respect to genotype. J. Clin. Pharmacol. 2004, 44, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Raji, M.A.; Kuo, Y.F.; Snih, S.A.; Sharaf, B.M.; Loera, J.A. Ethnic differences in herb and vitamin/mineral use in the elderly. Ann. Pharmacother. 2005, 39, 1019–1023. [Google Scholar] [CrossRef] [PubMed]
- Bruno, J.J.; Ellis, J.J. Herbal use among us elderly: 2002 national health interview survey. Ann. Pharmacother. 2005, 39, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Dergal, J.M.; Gold, J.L.; Laxer, D.A.; Lee, M.S.; Binns, M.A.; Lanctot, K.L.; Freedman, M.; Rochon, P.A. Potential interactions between herbal medicines and conventional drug therapies used by older adults attending a memory clinic. Drugs Aging 2002, 19, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Canter, P.H.; Ernst, E. Herbal supplement use by persons aged over 50 years in britain: Frequently used herbs, concomitant use of herbs, nutritional supplements and prescription drugs, rate of informing doctors and potential for negative interactions. Drugs Aging 2004, 21, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Thuluvath, P.J. Complementary and alternative medicine in hepatology: Review of the evidence of efficacy. Clin. Gastroenterol. Hepatol. 2007, 5, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Sanfelix Genoves, J.; Palop Larrea, V.; Rubio Gomis, E.; Martinez-Mir, I. Consumption of medicinal herbs and medicines. Aten. Primaria 2001, 28, 311–314. [Google Scholar] [CrossRef]
- Stjernberg, L.; Berglund, J.; Halling, A. Age and gender effect on the use of herbal medicine products and food supplements among the elderly. Scand. J. Prim. Health Care 2006, 24, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Gordon, W.P.; Huitric, A.C.; Seth, C.L.; McClanahan, R.H.; Nelson, S.D. The metabolism of the abortifacient terpene, (R)-(+)-pulegone, to a proximate toxin, menthofuran. Drug Metab. Dispos. 1987, 15, 589–594. [Google Scholar] [PubMed]
- Khojasteh-Bakht, S.C.; Chen, W.; Koenigs, L.L.; Peter, R.M.; Nelson, S.D. Metabolism of (R)-(+)-pulegone and (R)-(+)-menthofuran by human liver cytochrome P-450s: Evidence for formation of a furan epoxide. Drug Metab. Dispos. 1999, 27, 574–580. [Google Scholar] [PubMed]
- Lassila, T.; Mattila, S.; Turpeinen, M.; Pelkonen, O.; Tolonen, A. Tandem mass spectrometric analysis of S- and N-linked glutathione conjugates of pulegone and menthofuran and identification of P450 enzymes mediating their formation. Rapid Commun. Mass Spectrom. 2016, 30, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Tsintis, P.; La Mache, E. Cioms and ich initiatives in pharmacovigilance and risk management: Overview and implications. Drug Saf. 2004, 27, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Ioannides, C.; Lewis, D.F. Cytochromes P450 in the bioactivation of chemicals. Curr. Top. Med. Chem. 2004, 4, 1767–1788. [Google Scholar] [CrossRef] [PubMed]
- Brewer, C.T.; Chen, T. PXR variants: The impact on drug metabolism and therapeutic responses. Acta Pharm. Sin. B 2016, 6, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Foti, R.S.; Pearson, J.T.; Rock, D.A.; Wahlstrom, J.L.; Wienkers, L.C. In vitro inhibition of multiple cytochrome P450 isoforms by xanthone derivatives from mangosteen extract. Drug Metab. Dispos. 2009, 37, 1848–1855. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Jiang, B.; Nuntanakorn, P.; Kennelly, E.J.; Shord, S.; Lawal, T.O.; Mahady, G.B. Fukinolic acid derivatives and triterpene glycosides from black cohosh inhibit CYP isozymes, but are not cytotoxic to HEP-G2 cells in vitro. Curr. Drug Saf. 2010, 5, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Misaka, S.; Kawabe, K.; Onoue, S.; Werba, J.P.; Giroli, M.; Tamaki, S.; Kan, T.; Kimura, J.; Watanabe, H.; Yamada, S. Effects of green tea catechins on cytochrome P450 2B6, 2C8, 2C19, 2D6 and 3A activities in human liver and intestinal microsomes. Drug Metab. Pharmacokinet. 2013, 28, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Misaka, S.; Kawabe, K.; Onoue, S.; Werba, J.P.; Giroli, M.; Watanabe, H.; Yamada, S. Green tea extract affects the cytochrome P450 3A activity and pharmacokinetics of simvastatin in rats. Drug Metab. Pharmacokinet. 2013, 28, 514–518. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Akimoto, I.; Motegi, K.; Yoshimura, T.; Wada, K.; Nishizono, N.; Oda, K. Synthetic models related to methoxalen and menthofuran-cytochrome P450 (CYP) 2A6 interactions. Benzofuran and coumarin derivatives as potent and selective inhibitors of CYP2A6. Chem. Pharm. Bull. 2013, 61, 997–1001. [Google Scholar] [CrossRef] [PubMed]
- Kramlinger, V.M.; von Weymarn, L.B.; Murphy, S.E. Inhibition and inactivation of cytochrome P450 2A6 and cytochrome P450 2A13 by menthofuran, beta-nicotyrine and menthol. Chem. Biol. Interact. 2012, 197, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.J.; Wang, H.W.; Liu, T.Y.; Chen, Y.C.; Ueng, T.H. Modulation of cytochrome P-450-dependent monooxygenases, glutathione and glutathione s-transferase in rat liver by geniposide from gardenia jasminoides. Food Chem. Toxicol. 1997, 35, 957–965. [Google Scholar] [CrossRef]
- Gao, L.N.; Zhang, Y.; Cui, Y.L.; Yan, K. Evaluation of genipin on human cytochrome P450 isoenzymes and P-glycoprotein in vitro. Fitoterapia 2014, 98, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Sato, Y.; Kumagai, T.; Yoshinari, K.; Nagata, K. Effect of health foods on cytochrome P450-mediated drug metabolism. J. Pharm. Health Care Sci. 2017, 3, 14. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Zhang, F.; Zheng, J. Retrorsine, but not monocrotaline, is a mechanism-based inactivator of P450 3A4. Chem. Biol. Interact. 2010, 183, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Messer, A.; Raquet, N.; Lohr, C.; Schrenk, D. Major furocoumarins in grapefruit juice ii: Phototoxicity, photogenotoxicity, and inhibitory potency vs. Cytochrome P450 3A4 activity. Food Chem. Toxicol. 2012, 50, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Uchida, S.; Miyakawa, S.; Inui, N.; Takeuchi, K.; Watanabe, H.; Namiki, N. Comparison of inhibitory duration of grapefruit juice on organic anion-transporting polypeptide and cytochrome P450 3A4. Biol. Pharm. Bull. 2013, 36, 1936–1941. [Google Scholar] [CrossRef] [PubMed]
- Albassam, A.A.; Mohamed, M.E.; Frye, R.F. Inhibitory effect of six herbal extracts on CYP2C8 enzyme activity in human liver microsomes. Xenobiotica 2015, 45, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Gorski, J.C.; Huang, S.M.; Pinto, A.; Hamman, M.A.; Hilligoss, J.K.; Zaheer, N.A.; Desai, M.; Miller, M.; Hall, S.D. The effect of echinacea (echinacea purpurea root) on cytochrome P450 activity in vivo. Clin. Pharmacol. Ther. 2004, 75, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Gurley, B.J.; Gardner, S.F.; Hubbard, M.A.; Williams, D.K.; Gentry, W.B.; Carrier, J.; Khan, I.A.; Edwards, D.J.; Shah, A. In vivo assessment of botanical supplementation on human cytochrome P450 phenotypes: Citrus aurantium, echinacea purpurea, milk thistle, and saw palmetto. Clin. Pharmacol. Ther. 2004, 76, 428–440. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Sy-Cordero, A.; Graf, T.N.; Brantley, S.J.; Paine, M.F.; Oberlies, N.H. Isolation and identification of intestinal CYP3A inhibitors from cranberry (Vaccinium macrocarpon) using human intestinal microsomes. Planta Med. 2011, 77, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Langhammer, A.J.; Nilsen, O.G. In vitro inhibition of human CYP1A2, CYP2D6, and CYP3A4 by six herbs commonly used in pregnancy. Phytother. Res. 2014, 28, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Mooiman, K.D.; Maas-Bakker, R.F.; Moret, E.E.; Beijnen, J.H.; Schellens, J.H.; Meijerman, I. Milk thistle’s active components silybin and isosilybin: Novel inhibitors of PXR-mediated CYP3A4 induction. Drug Metab. Dispos. 2013, 41, 1494–1504. [Google Scholar] [CrossRef] [PubMed]
- Sunaga, K.; Ohkawa, K.; Nakamura, K.; Ohkubo, A.; Harada, S.; Tsuda, T. Mechanism-based inhibition of recombinant human cytochrome P450 3A4 by tomato juice extract. Biol. Pharm. Bull. 2012, 35, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, A.; Yoshino, M.; Nakamura, H.; Ishii, I.; Watanabe, T.; Kiuchi, M.; Ishikawa, T.; Ohmori, S.; Kitada, M. Identification of inhibitory component in cinnamon—O-methoxycinnamaldehyde inhibits CYP1A2 and CYP2E1. Drug Metab. Pharmacokinet. 2002, 17, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Ito, H.; Hatano, T. Effects of mace and nutmeg on human cytochrome P450 3A4 and 2C9 activity. Biol. Pharm. Bull. 2010, 33, 1977–1982. [Google Scholar] [CrossRef] [PubMed]
- Usia, T.; Watabe, T.; Kadota, S.; Tezuka, Y. Cytochrome P450 2D6 (CYP2D6) inhibitory constituents of catharanthus roseus. Biol. Pharm. Bull. 2005, 28, 1021–1024. [Google Scholar] [CrossRef] [PubMed]
- Mills, E.; Foster, B.C.; van Heeswijk, R.; Phillips, E.; Wilson, K.; Leonard, B.; Kosuge, K.; Kanfer, I. Impact of african herbal medicines on antiretroviral metabolism. AIDS 2005, 19, 95–97. [Google Scholar] [CrossRef] [PubMed]
- Monera, T.G.; Wolfe, A.R.; Maponga, C.C.; Benet, L.Z.; Guglielmo, J. Moringa oleifera leaf extracts inhibit 6beta-hydroxylation of testosterone by CYP3A4. J. Infect. Dev. Ctries. 2008, 2, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Agbonon, A.; Eklu-Gadegbeku, K.; Aklikokou, K.; Gbeassor, M.; Akpagana, K.; Tam, T.W.; Arnason, J.T.; Foster, B.C. In vitro inhibitory effect of west african medicinal and food plants on human cytochrome P450 3A subfamily. J. Ethnopharmacol. 2010, 128, 390–394. [Google Scholar] [CrossRef] [PubMed]
- Deferme, S.; Kamuhabwa, A.; Nshimo, C.; de Witte, P.; Augustijns, P. Screening of tanzanian plant extracts for their potential inhibitory effect on P-glycoprotein mediated efflux. Phytother. Res. 2003, 17, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Van den Bout-van den Beukel, C.J.; Hamza, O.J.; Moshi, M.J.; Matee, M.I.; Mikx, F.; Burger, D.M.; Koopmans, P.P.; Verweij, P.E.; Schoonen, W.G.; van der Ven, A.J. Evaluation of cytotoxic, genotoxic and CYP450 enzymatic competition effects of tanzanian plant extracts traditionally used for treatment of fungal infections. Basic Clin. Pharmacol. Toxicol. 2008, 102, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Dresser, G.K.; Wacher, V.; Wong, S.; Wong, H.T.; Bailey, D.G. Evaluation of peppermint oil and ascorbyl palmitate as inhibitors of cytochrome P450 3A4 activity in vitro and in vivo. Clin. Pharmacol. Ther. 2002, 72, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Sueoka, N.; Suganuma, M.; Sueoka, E.; Okabe, S.; Matsuyama, S.; Imai, K.; Nakachi, K.; Fujiki, H. A new function of green tea: Prevention of lifestyle-related diseases. Ann. N. Y. Acad. Sci. 2001, 928, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Dona, M.; Dell’Aica, I.; Calabrese, F.; Benelli, R.; Morini, M.; Albini, A.; Garbisa, S. Neutrophil restraint by green tea: Inhibition of inflammation, associated angiogenesis, and pulmonary fibrosis. J. Immunol. 2003, 170, 4335–4341. [Google Scholar] [CrossRef] [PubMed]
- Raederstorff, D.G.; Schlachter, M.F.; Elste, V.; Weber, P. Effect of EGCG on lipid absorption and plasma lipid levels in rats. J. Nutr. Biochem. 2003, 14, 326–332. [Google Scholar] [CrossRef]
- Sartippour, M.R.; Shao, Z.M.; Heber, D.; Beatty, P.; Zhang, L.; Liu, C.; Ellis, L.; Liu, W.; Go, V.L.; Brooks, M.N. Green tea inhibits vascular endothelial growth factor (VEGF) induction in human breast cancer cells. J. Nutr. 2002, 132, 2307–2311. [Google Scholar] [PubMed]
- Haqqi, T.M.; Anthony, D.D.; Gupta, S.; Ahmad, N.; Lee, M.S.; Kumar, G.K.; Mukhtar, H. Prevention of collagen-induced arthritis in mice by a polyphenolic fraction from green tea. Proc. Natl. Acad. Sci. USA 1999, 96, 4524–4529. [Google Scholar] [CrossRef] [PubMed]
- Teschke, R.; Wolff, A.; Frenzel, C.; Schulze, J.; Eickhoff, A. Herbal hepatotoxicity: A tabular compilation of reported cases. Liver Int. 2012, 32, 1543–1556. [Google Scholar] [CrossRef] [PubMed]
- Mazzanti, G.; Di Sotto, A.; Vitalone, A. Hepatotoxicity of green tea: An update. Arch. Toxicol. 2015, 89, 1175–1191. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.T.; Hsu, Y.R.; Lii, C.K.; Lin, A.H.; Chang, K.H.; Yang, H.T. Effect of commercially available green and black tea beverages on drug-metabolizing enzymes and oxidative stress in wistar rats. Food Chem. Toxicol. 2014, 70, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, Y.; Wan, X.; Yang, C.S.; Zhang, J. Green tea polyphenol (−)-epigallocatechin-3-gallate triggered hepatotoxicity in mice: Responses of major antioxidant enzymes and the NRF2 rescue pathway. Toxicol. Appl. Pharmacol. 2015, 283, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Emoto, Y.; Yoshizawa, K.; Kinoshita, Y.; Yuki, M.; Yuri, T.; Yoshikawa, Y.; Sayama, K.; Tsubura, A. Green tea extract-induced acute hepatotoxicity in rats. J. Toxicol. Pathol. 2014, 27, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Teschke, R.; Zhang, L.; Melzer, L.; Schulze, J.; Eickhoff, A. Green tea extract and the risk of drug-induced liver injury. Expert Opin. Drug Metab. Toxicol. 2014, 10, 1663–1676. [Google Scholar] [CrossRef] [PubMed]
- Gentry-Maharaj, A.; Karpinskyj, C.; Glazer, C.; Burnell, M.; Ryan, A.; Fraser, L.; Lanceley, A.; Jacobs, I.; Hunter, M.S.; Menon, U. Use and perceived efficacy of complementary and alternative medicines after discontinuation of hormone therapy: A nested United Kingdom collaborative trial of ovarian cancer screening cohort study. Menopause 2015, 22, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Wu, L.; Wang, Q.; Yang, B.; Kuang, H. New 9,19-cycloartenol glycosides isolated from the roots of cimicifuga simplex and their anti-inflammatory effects. Bioorg. Med. Chem. Lett. 2014, 24, 5688–5691. [Google Scholar] [CrossRef] [PubMed]
- Lim, T.Y.; Considine, A.; Quaglia, A.; Shawcross, D.L. Subacute liver failure secondary to black cohosh leading to liver transplantation. BMJ Case Rep. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Enbom, E.T.; Le, M.D.; Oesterich, L.; Rutgers, J.; French, S.W. Mechanism of hepatotoxicity due to black cohosh (Cimicifuga racemosa): Histological, immunohistochemical and electron microscopy analysis of two liver biopsies with clinical correlation. Exp. Mol. Pathol. 2014, 96, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Franco, D.L.; Kale, S.; Lam-Himlin, D.M.; Harrison, M.E. Black cohosh hepatotoxicity with autoimmune hepatitis presentation. Case Rep. Gastroenterol. 2017, 11, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Teschke, R. Black cohosh and suspected hepatotoxicity: Inconsistencies, confounding variables, and prospective use of a diagnostic causality algorithm. A critical review. Menopause 2010, 17, 426–440. [Google Scholar] [CrossRef] [PubMed]
- Yokotani, K.; Chiba, T.; Sato, Y.; Nakanishi, T.; Murata, M.; Umegaki, K. Effect of three herbal extracts on cytochrome P450 and possibility of interaction with drugs. Shokuhin Eiseigaku Zasshi 2013, 54, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Walton, E.W. Topical phytochemicals: Applications for wound healing. Adv. Skin Wound Care 2014, 27, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Schell, J.; Betts, N.M.; Foster, M.; Scofield, R.H.; Basu, A. Cranberries improve postprandial glucose excursions in type 2 diabetes. Food Funct. 2017, 8, 3083–3090. [Google Scholar] [CrossRef] [PubMed]
- Avorn, J.; Monane, M.; Gurwitz, J.H.; Glynn, R.J.; Choodnovskiy, I.; Lipsitz, L.A. Reduction of bacteriuria and pyuria after ingestion of cranberry juice. JAMA 1994, 271, 751–754. [Google Scholar] [CrossRef] [PubMed]
- Moore, G.A. Oranges and lemons: Clues to the taxonomy of citrus from molecular markers. Trends Genet. 2001, 17, 536–540. [Google Scholar] [CrossRef]
- Cingi, C.; Toros, S.Z.; Gurbuz, M.K.; Ince, I.; Cakli, H.; Erdogmus, N.; Karasulu, E.; Kaya, E. Effect of grapefruit juice on bioavailability of montelukast. Laryngoscope 2013, 123, 816–819. [Google Scholar] [CrossRef] [PubMed]
- Chanet, A.; Milenkovic, D.; Deval, C.; Potier, M.; Constans, J.; Mazur, A.; Bennetau-Pelissero, C.; Morand, C.; Berard, A.M. Naringin, the major grapefruit flavonoid, specifically affects atherosclerosis development in diet-induced hypercholesterolemia in mice. J. Nutr. Biochem. 2012, 23, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Cirmi, S.; Maugeri, A.; Ferlazzo, N.; Gangemi, S.; Calapai, G.; Schumacher, U.; Navarra, M. Anticancer potential of citrus juices and their extracts: A systematic review of both preclinical and clinical studies. Front. Pharmacol. 2017, 8, 420. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.J.; Chan, W.K.; Harralson, A.F.; Buffum, J.; Bui, B.C. The effects of grapefruit juice on sertraline metabolism: An in vitro and in vivo study. Clin. Ther. 1999, 21, 1890–1899. [Google Scholar] [CrossRef]
- De la Garza, A.L.; Etxeberria, U.; Haslberger, A.; Aumueller, E.; Martinez, J.A.; Milagro, F.I. Helichrysum and grapefruit extracts boost weight loss in overweight rats reducing inflammation. J. Med. Food 2015, 18, 890–898. [Google Scholar] [CrossRef] [PubMed]
- Holmberg, M.T.; Tornio, A.; Neuvonen, M.; Neuvonen, P.J.; Backman, J.T.; Niemi, M. Grapefruit juice inhibits the metabolic activation of clopidogrel. Clin. Pharmacol. Ther. 2014, 95, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Yotsawimonwat, S.; Rattanadechsakul, J.; Rattanadechsakul, P.; Okonogi, S. Skin improvement and stability of echinacea purpurea dermatological formulations. Int. J. Cosmet. Sci. 2010, 32, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Mousa, H.A. Prevention and treatment of influenza, influenza-like illness, and common cold by herbal, complementary, and natural therapies. J. Evid.-Based Complement. Altern. Med. 2017, 22, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Smejkal, K.; Rjaskova, V. Use of plant extracts as an efficient alternative therapy of respiratory tract infections. Ceska a Slovenska Farmacie 2016, 65, 139–160. [Google Scholar] [PubMed]
- Awortwe, C.; Manda, V.K.; Avonto, C.; Khan, S.I.; Khan, I.A.; Walker, L.A.; Bouic, P.J.; Rosenkranz, B. Echinacea purpurea up-regulates CYP1A2, CYP3A4 and MDR1 gene expression by activation of pregnane X receptor pathway. Xenobiotica 2015, 45, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Tao, W.; Zhang, H.; Xue, W.; Zou, Z.; Wu, H.; Cai, B.; Doron, R.; Chen, G. Instant and persistent antidepressant response of gardenia yellow pigment is associated with acute protein synthesis and delayed upregulation of BDNF expression in the hippocampus. ACS Chem. Neurosci. 2016, 7, 1068–1076. [Google Scholar] [CrossRef] [PubMed]
- Appiah, S.; Revitt, M.; Jones, H.; Vu, M.; Simmonds, M.; Bell, C. Antiinflammatory and hepatoprotective medicinal herbs as potential substitutes for bear bile. Int. Rev. Neurobiol. 2017, 135, 149–180. [Google Scholar] [PubMed]
- Higashino, S.; Sasaki, Y.; Giddings, J.C.; Hyodo, K.; Sakata, S.F.; Matsuda, K.; Horikawa, Y.; Yamamoto, J. Crocetin, a carotenoid from gardenia jasminoides ellis, protects against hypertension and cerebral thrombogenesis in stroke-prone spontaneously hypertensive rats. Phytother. Res. 2014, 28, 1315–1319. [Google Scholar] [CrossRef] [PubMed]
- Yamano, T.; Tsujimoto, Y.; Noda, T.; Shimizu, M.; Ohmori, M.; Morita, S.; Yamada, A. Hepatotoxicity of gardenia yellow color in rats. Toxicol. Lett. 1988, 44, 177–182. [Google Scholar] [PubMed]
- Yang, H.J.; Fu, M.H.; Wu, Z.L.; Liang, R.X.; Huang, L.Q.; Fang, J.; Li, G.; Cao, Y. Experimental studies on hepatotoxicity of rats induced by Fructus gardeniae. Zhongguo Zhong Yao Za Zhi 2006, 31, 1091–1093. [Google Scholar] [PubMed]
- Wei, J.; Zhang, F.; Zhang, Y.; Cao, C.; Li, X.; Li, D.; Liu, X.; Yang, H.; Huang, L. Proteomic investigation of signatures for geniposide-induced hepatotoxicity. J. Proteome Res. 2014, 13, 5724–5733. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Jin, M.; Ande, A.; Sinha, N.; Silverstein, P.S.; Kumar, A. Alcohol consumption effect on antiretroviral therapy and HIV-1 pathogenesis: Role of cytochrome P450 isozymes. Expert Opin. Drug Metab. Toxicol. 2012, 8, 1363–1375. [Google Scholar] [CrossRef] [PubMed]
- Pal, D.; Kwatra, D.; Minocha, M.; Paturi, D.K.; Budda, B.; Mitra, A.K. Efflux transporters- and cytochrome P-450-mediated interactions between drugs of abuse and antiretrovirals. Life Sci. 2011, 88, 959–971. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Chai, X.; Yu, L.; Chen, S.; Zeng, S. Identification of novel pregnane X receptor activators from traditional chinese medicines. J. Ethnopharmacol. 2011, 136, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.G.; Liu, H.S.; Zhang, X.X.; Xiao, Y.; Lu, B.B.; Ma, Z.C.; Liang, Q.D.; Tang, X.L.; Xiao, C.R.; Tan, H.L.; et al. Screening of pregnane X receptor activation from ginsenosides. Yao Xue Xue Bao 2013, 48, 144–148. [Google Scholar] [PubMed]
- Wang, Y.M.; Lin, W.; Chai, S.C.; Wu, J.; Ong, S.S.; Schuetz, E.G.; Chen, T. Piperine activates human pregnane x receptor to induce the expression of cytochrome P450 3A4 and multidrug resistance protein 1. Toxicol. Appl. Pharmacol. 2013, 272, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ferguson, S.S.; Negishi, M.; Goldstein, J.A. Induction of human CYP2C9 by rifampicin, hyperforin, and phenobarbital is mediated by the pregnane x receptor. J. Pharmacol. Exp. Ther. 2004, 308, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Godtel-Armbrust, U.; Metzger, A.; Kroll, U.; Kelber, O.; Wojnowski, L. Variability in PXR-mediated induction of CYP3A4 by commercial preparations and dry extracts of St. John’s wort. Naunyn Schmiedebergs Arch. Pharmacol. 2007, 375, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Yeung, E.Y.; Sueyoshi, T.; Negishi, M.; Chang, T.K. Identification of ginkgo biloba as a novel activator of pregnane x receptor. Drug Metab. Dispos. 2008, 36, 2270–2276. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Stanton, J.D.; Tolson, A.H.; Luo, Y.; Wang, H. Bioactive terpenoids and flavonoids from ginkgo biloba extract induce the expression of hepatic drug-metabolizing enzymes through pregnane X receptor, constitutive androstane receptor, and aryl hydrocarbon receptor-mediated pathways. Pharm. Res. 2009, 26, 872–882. [Google Scholar] [CrossRef] [PubMed]
- Yin, O.Q.; Tomlinson, B.; Waye, M.M.; Chow, A.H.; Chow, M.S. Pharmacogenetics and herb-drug interactions: Experience with ginkgo biloba and omeprazole. Pharmacogenetics 2004, 14, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Raucy, J.L. Regulation of CYP3A4 expression in human hepatocytes by pharmaceuticals and natural products. Drug Metab. Dispos. 2003, 31, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Sachdeva, K.; Liu, J.; Ford, M.; Yang, D.; Khan, I.A.; Chichester, C.O.; Yan, B. Desmethoxyyangonin and dihydromethysticin are two major pharmacological kavalactones with marked activity on the induction of CYP3A23. Drug Metab. Dispos. 2004, 32, 1317–1324. [Google Scholar] [CrossRef] [PubMed]
- Kluth, D.; Banning, A.; Paur, I.; Blomhoff, R.; Brigelius-Flohe, R. Modulation of pregnane X receptor- and electrophile responsive element-mediated gene expression by dietary polyphenolic compounds. Free Radic. Biol. Med. 2007, 42, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Noordine, M.L.; Cherbuy, C.; Vaugelade, P.; Pascussi, J.M.; Duee, P.H.; Thomas, M. Different activation patterns of rat xenobiotic metabolism genes by two constituents of garlic. Carcinogenesis 2006, 27, 2090–2095. [Google Scholar] [CrossRef] [PubMed]
- Watkins, R.E.; Maglich, J.M.; Moore, L.B.; Wisely, G.B.; Noble, S.M.; Davis-Searles, P.R.; Lambert, M.H.; Kliewer, S.A.; Redinbo, M.R. 2.1 a crystal structure of human PXR in complex with the St. John’s wort compound hyperforin. Biochemistry 2003, 42, 1430–1438. [Google Scholar] [CrossRef] [PubMed]
- Zollner, G.; Wagner, M.; Trauner, M. Nuclear receptors as drug targets in cholestasis and drug-induced hepatotoxicity. Pharmacol. Ther. 2010, 126, 228–243. [Google Scholar] [CrossRef] [PubMed]
- Chen, T. Nuclear receptor drug discovery. Curr. Opin. Chem. Biol. 2008, 12, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.M.; Ong, S.S.; Chai, S.C.; Chen, T. Role of CAR and PXR in xenobiotic sensing and metabolism. Expert Opin. Drug Metab. Toxicol. 2012, 8, 803–817. [Google Scholar] [CrossRef] [PubMed]
- Aleksunes, L.M.; Klaassen, C.D. Coordinated regulation of hepatic phase i and ii drug-metabolizing genes and transporters using AhR-, CAR-, PXR-, PPARα-, and Nrf2-null mice. Drug Metab. Dispos. 2012, 40, 1366–1379. [Google Scholar] [CrossRef] [PubMed]
- Gardner-Stephen, D.; Heydel, J.M.; Goyal, A.; Lu, Y.; Xie, W.; Lindblom, T.; Mackenzie, P.; Radominska-Pandya, A. Human PXR variants and their differential effects on the regulation of human UDP-glucuronosyltransferase gene expression. Drug Metab. Dispos. 2004, 32, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Oladimeji, P.O.; Lin, W.; Brewer, C.T.; Chen, T. Glucose-dependent regulation of pregnane x receptor is modulated by AMP-activated protein kinase. Sci. Rep. 2017, 7, 46751. [Google Scholar] [CrossRef] [PubMed]
- Lamba, V.; Yasuda, K.; Lamba, J.K.; Assem, M.; Davila, J.; Strom, S.; Schuetz, E.G. PXR (NR1I2): Splice variants in human tissues, including brain, and identification of neurosteroids and nicotine as PXR activators. Toxicol. Appl. Pharmacol. 2004, 199, 251–265. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Singh, S.V.; Singh, S.P.; Mu, Y.; Lee, J.H.; Saini, S.P.; Toma, D.; Ren, S.; Kagan, V.E.; Day, B.W.; et al. Orphan nuclear receptor pregnane X receptor sensitizes oxidative stress responses in transgenic mice and cancerous cells. Mol. Endocrinol. 2006, 20, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Shah, Y.; Cheung, C.; Guo, G.L.; Feigenbaum, L.; Krausz, K.W.; Idle, J.R.; Gonzalez, F.J. The pregnane X receptor gene-humanized mouse: A model for investigating drug-drug interactions mediated by cytochromes P450 3A. Drug Metab. Dispos. 2007, 35, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Baes, M.; Gulick, T.; Choi, H.S.; Martinoli, M.G.; Simha, D.; Moore, D.D. A new orphan member of the nuclear hormone receptor superfamily that interacts with a subset of retinoic acid response elements. Mol. Cell. Biol. 1994, 14, 1544–1552. [Google Scholar] [CrossRef] [PubMed]
- Sugatani, J.; Kojima, H.; Ueda, A.; Kakizaki, S.; Yoshinari, K.; Gong, Q.H.; Owens, I.S.; Negishi, M.; Sueyoshi, T. The phenobarbital response enhancer module in the human bilirubin UDP-glucuronosyltransferase UGT1A1 gene and regulation by the nuclear receptor CAR. Hepatology 2001, 33, 1232–1238. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhang, J.; Chua, S.S.; Qatanani, M.; Han, Y.; Granata, R.; Moore, D.D. Induction of bilirubin clearance by the constitutive androstane receptor (CAR). Proc. Natl. Acad. Sci. USA 2003, 100, 4156–4161. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, T.; Sueyoshi, T.; Zelko, I.; Moore, R.; Washburn, K.; Negishi, M. Phenobarbital-responsive nuclear translocation of the receptor CAR in induction of the CYP2b gene. Mol. Cell. Biol. 1999, 19, 6318–6322. [Google Scholar] [CrossRef] [PubMed]
- Moore, L.B.; Parks, D.J.; Jones, S.A.; Bledsoe, R.K.; Consler, T.G.; Stimmel, J.B.; Goodwin, B.; Liddle, C.; Blanchard, S.G.; Willson, T.M.; et al. Orphan nuclear receptors constitutive androstane receptor and pregnane X receptor share xenobiotic and steroid ligands. J. Biol. Chem. 2000, 275, 15122–15127. [Google Scholar] [CrossRef] [PubMed]
- Maglich, J.M.; Parks, D.J.; Moore, L.B.; Collins, J.L.; Goodwin, B.; Billin, A.N.; Stoltz, C.A.; Kliewer, S.A.; Lambert, M.H.; Willson, T.M.; et al. Identification of a novel human constitutive androstane receptor (CAR) agonist and its use in the identification of CAR target genes. J. Biol. Chem. 2003, 278, 17277–17283. [Google Scholar] [CrossRef] [PubMed]
- Stojanovic, N.M.; Samardzic, L.; Randjelovic, P.J.; Radulovic, N.S. Prevalence of self-medication practice with herbal products among non-psychotic psychiatric patients from southeastern serbia: A cross-sectional study. Saudi Pharm. J. 2017, 25, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Agollo, M.C.; Miszputen, S.J.; Diament, J. Hypericum perforatum-induced hepatotoxicity with possible association with copaiba (Copaifera langsdorffii Desf): Case report. Einstein 2014, 12, 355–357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, J.R. Ginkgo biloba extract for age-related macular degeneration. Cochrane Database Syst. Rev. 2013, 31, CD001775. [Google Scholar]
- Abdel-Zaher, A.O.; Farghaly, H.S.M.; El-Refaiy, A.E.M.; Abd-Eldayem, A.M. Protective effect of the standardized extract of ginkgo biloba (EGB761) against hypertension with hypercholesterolemia-induced renal injury in rats: Insights in the underlying mechanisms. Biomed. Pharmacother. 2017, 95, 944–955. [Google Scholar] [CrossRef] [PubMed]
- Coelho, C.; Tyler, R.; Ji, H.; Rojas-Roncancio, E.; Witt, S.; Tao, P.; Jun, H.J.; Wang, T.C.; Hansen, M.R.; Gantz, B.J. Survey on the effectiveness of dietary supplements to treat tinnitus. Am. J. Audiol. 2016, 25, 184–205. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.F.; Huang, L.B.; Zhong, Y.B.; Zhou, Q.H.; Wang, H.L.; Zheng, G.Q.; Lin, Y. An overview of systematic reviews of ginkgo biloba extracts for mild cognitive impairment and dementia. Front. Aging Neurosci. 2016, 8, 276. [Google Scholar] [CrossRef] [PubMed]
- Maeda, J.; Inoue, K.; Ichimura, R.; Takahashi, M.; Kodama, Y.; Saito, N.; Yoshida, M. Essential role of constitutive androstane receptor in ginkgo biloba extract induced liver hypertrophy and hepatocarcinogenesis. Food Chem. Toxicol. 2015, 83, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Reay, J.L.; Scholey, A.B.; Kennedy, D.O. Panax ginseng (G115) improves aspects of working memory performance and subjective ratings of calmness in healthy young adults. Hum. Psychopharmacol. 2010, 25, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Scholey, A.; Ossoukhova, A.; Owen, L.; Ibarra, A.; Pipingas, A.; He, K.; Roller, M.; Stough, C. Effects of american ginseng (Panax quinquefolius) on neurocognitive function: An acute, randomised, double-blind, placebo-controlled, crossover study. Psychopharmacology 2010, 212, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Gum, S.I.; Jo, S.J.; Ahn, S.H.; Kim, S.G.; Kim, J.T.; Shin, H.M.; Cho, M.K. The potent protective effect of wild ginseng (Panax ginseng C.A. Meyer) against benzo[α]pyrene-induced toxicity through metabolic regulation of CYP1A1 and GSTs. J. Ethnopharmacol. 2007, 112, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Bilgi, N.; Bell, K.; Ananthakrishnan, A.N.; Atallah, E. Imatinib and panax ginseng: A potential interaction resulting in liver toxicity. Ann. Pharmacother. 2010, 44, 926–928. [Google Scholar] [CrossRef] [PubMed]
- Bajad, S.; Bedi, K.L.; Singla, A.K.; Johri, R.K. Antidiarrhoeal activity of piperine in mice. Planta Med. 2001, 67, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Chiu, N.T.; Tomlinson Guns, E.S.; Adomat, H.; Jia, W.; Deb, S. Identification of human cytochrome P450 enzymes involved in the hepatic and intestinal biotransformation of 20(S)-protopanaxadiol. Biopharm. Drug Dispos. 2014, 35, 104–118. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Tyagi, A.K. Historical spice as a future drug: Therapeutic potential of piperlongumine. Curr. Pharm. Des. 2016, 22, 4151–4159. [Google Scholar] [CrossRef] [PubMed]
- Ziment, I. History of the treatment of chronic bronchitis. Respiration 1991, 58, 37–42. [Google Scholar] [CrossRef] [PubMed]
- De Souza Grinevicius, V.M.; Kviecinski, M.R.; Santos Mota, N.S.; Ourique, F.; Porfirio Will Castro, L.S.; Andreguetti, R.R.; Gomes Correia, J.F.; Filho, D.W.; Pich, C.T.; Pedrosa, R.C. Piper nigrum ethanolic extract rich in piperamides causes ROS overproduction, oxidative damage in DNA leading to cell cycle arrest and apoptosis in cancer cells. J. Ethnopharmacol. 2016, 189, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.H.; Bhandary, B.; Lee, E.M.; Park, J.K.; Jeong, K.S.; Kim, I.K.; Kim, H.R.; Chae, H.J. The roles of ER stress and P450 2E1 in CCL(4)-induced steatosis. Int. J. Biochem. Cell Biol. 2011, 43, 1469–1482. [Google Scholar] [CrossRef] [PubMed]
- Stjernberg, L.; Berglund, J. Garlic as an insect repellent. JAMA 2000, 284, 831. [Google Scholar] [CrossRef] [PubMed]
- Fisher, C.D.; Augustine, L.M.; Maher, J.M.; Nelson, D.M.; Slitt, A.L.; Klaassen, C.D.; Lehman-McKeeman, L.D.; Cherrington, N.J. Induction of drug-metabolizing enzymes by garlic and allyl sulfide compounds via activation of constitutive androstane receptor and nuclear factor E2-related factor 2. Drug Metab. Dispos. 2007, 35, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- Sueyoshi, T.; Green, W.D.; Vinal, K.; Woodrum, T.S.; Moore, R.; Negishi, M. Garlic extract diallyl sulfide (DAS) activates nuclear receptor CAR to induce the sult1e1 gene in mouse liver. PLoS ONE 2011, 6, e21229. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.V.; Pal, R.; Vaiphei, K.; Singh, K. Garlic hepatotoxicity: Safe dose of garlic. Trop. Gastroenterol. 2006, 27, 26–30. [Google Scholar] [PubMed]
- Oboh, G. Tropical green leafy vegetables prevent garlic-induced hepatotoxicity in the rat. J. Med. Food 2006, 9, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Letsyo, E.; Jerz, G.; Winterhalter, P.; Lindigkeit, R.; Beuerle, T. Incidence of pyrrolizidine alkaloids in herbal medicines from german retail markets: Risk assessments and implications to consumers. Phytother. Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.P.; Zhang, M.P.; Wang, K.Y.; Sun, C.Y.; Wang, Y. Research achievements on ginsenosides biosynthesis from panax ginseng. Zhongguo Zhong Yao Za Zhi 2016, 41, 4292–4302. [Google Scholar] [PubMed]
- Stickel, F.; Poschl, G.; Seitz, H.K.; Waldherr, R.; Hahn, E.G.; Schuppan, D. Acute hepatitis induced by greater celandine (Chelidonium majus). Scand. J. Gastroenterol. 2003, 38, 565–568. [Google Scholar] [CrossRef] [PubMed]
- Hardeman, E.; Van Overbeke, L.; Ilegems, S.; Ferrante, M. Acute hepatitis induced by greater celandine (Chelidonium majus). Acta Gastroenterol. Belg. 2008, 71, 281–282. [Google Scholar] [PubMed]
- Teschke, R.; Glass, X.; Schulze, J. Herbal hepatotoxicity by greater celandine (Chelidonium majus): Causality assessment of 22 spontaneous reports. Regul. Toxicol. Pharmacol. 2011, 61, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Teschke, R.; Frenzel, C.; Glass, X.; Schulze, J.; Eickhoff, A. Greater celandine hepatotoxicity: A clinical review. Ann. Hepatol. 2012, 11, 838–848. [Google Scholar] [PubMed]
- Valentao, P.; Carvalho, M.; Carvalho, F.; Fernandes, E.; das Neves, R.P.; Pereira, M.L.; Andrade, P.B.; Seabra, R.M.; Bastos, M.L. Hypericum androsaemum infusion increases tert-butyl hydroperoxide-induced mice hepatotoxicity in vivo. J. Ethnopharmacol. 2004, 94, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Dag, M.; Ozturk, Z.; Aydnl, M.; Koruk, I.; Kadayfc, A. Postpartum hepatotoxicity due to herbal medicine teucrium polium. Ann. Saudi Med. 2014, 34, 541–543. [Google Scholar] [CrossRef] [PubMed]
- Sezer, R.G.; Bozaykut, A. Pediatric hepatotoxicity associated with polygermander (Teucrium polium). Clin. Toxicol. 2012, 50, 153. [Google Scholar] [CrossRef] [PubMed]
- Goksu, E.; Kilic, T.; Yilmaz, D. Hepatitis: A herbal remedy germander. Clin. Toxicol. 2012, 50, 158. [Google Scholar] [CrossRef] [PubMed]
- Lekehal, M.; Pessayre, D.; Lereau, J.M.; Moulis, C.; Fouraste, I.; Fau, D. Hepatotoxicity of the herbal medicine germander: Metabolic activation of its furano diterpenoids by cytochrome P450 3A depletes cytoskeleton-associated protein thiols and forms plasma membrane blebs in rat hepatocytes. Hepatology 1996, 24, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Kouzi, S.A.; McMurtry, R.J.; Nelson, S.D. Hepatotoxicity of germander (Teucrium chamaedrys L.) and one of its constituent neoclerodane diterpenes teucrin a in the mouse. Chem. Res. Toxicol. 1994, 7, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Teschke, R. Kava hepatotoxicity—A clinical review. Ann. Hepatol. 2010, 9, 251–265. [Google Scholar] [PubMed]
- Gordon, P.; Khojasteh, S.C. A decades-long investigation of acute metabolism-based hepatotoxicity by herbal constituents: A case study of pennyroyal oil. Drug Metab. Rev. 2015, 47, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Anderson, I.B.; Mullen, W.H.; Meeker, J.E.; Khojasteh Bakht, S.C.; Oishi, S.; Nelson, S.D.; Blanc, P.D. Pennyroyal toxicity: Measurement of toxic metabolite levels in two cases and review of the literature. Ann. Intern. Med. 1996, 124, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Gordon, W.P.; Forte, A.J.; McMurtry, R.J.; Gal, J.; Nelson, S.D. Hepatotoxicity and pulmonary toxicity of pennyroyal oil and its constituent terpenes in the mouse. Toxicol. Appl. Pharmacol. 1982, 65, 413–424. [Google Scholar] [CrossRef]
- Bakerink, J.A.; Gospe, S.M., Jr.; Dimand, R.J.; Eldridge, M.W. Multiple organ failure after ingestion of pennyroyal oil from herbal tea in two infants. Pediatrics 1996, 98, 944–947. [Google Scholar] [PubMed]
- Sztajnkrycer, M.D.; Otten, E.J.; Bond, G.R.; Lindsell, C.J.; Goetz, R.J. Mitigation of pennyroyal oil hepatotoxicity in the mouse. Acad. Emerg. Med. 2003, 10, 1024–1028. [Google Scholar] [CrossRef] [PubMed]
- Thorup, I.; Wurtzen, G.; Carstensen, J.; Olsen, P. Short term toxicity study in rats dosed with pulegone and menthol. Toxicol. Lett. 1983, 19, 207–210. [Google Scholar] [CrossRef]
- Madsen, C.; Wurtzen, G.; Carstensen, J. Short-term toxicity study in rats dosed with menthone. Toxicol. Lett. 1986, 32, 147–152. [Google Scholar] [CrossRef]
- Khojasteh, S.C.; Hartley, D.P.; Ford, K.A.; Uppal, H.; Oishi, S.; Nelson, S.D. Characterization of rat liver proteins adducted by reactive metabolites of menthofuran. Chem. Res. Toxicol. 2012, 25, 2301–2309. [Google Scholar] [CrossRef] [PubMed]
- Thorup, I.; Wurtzen, G.; Carstensen, J.; Olsen, P. Short term toxicity study in rats dosed with peppermint oil. Toxicol. Lett. 1983, 19, 211–215. [Google Scholar] [CrossRef]
- Yao, J.; Li, C.G.; Gong, L.K.; Feng, C.C.; Li, C.Z.; Gao, M.; Luan, Y.; Qi, X.M.; Ren, J. Hepatic cytochrome P450s play a major role in monocrotaline-induced renal toxicity in mice. Acta Pharmacol. Sin. 2014, 35, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Ruan, J.; Fu, P.P.; Lin, G. Cytotoxicity of pyrrolizidine alkaloid in human hepatic parenchymal and sinusoidal endothelial cells: Firm evidence for the reactive metabolites mediated pyrrolizidine alkaloid-induced hepatotoxicity. Chem. Biol. Interact. 2016, 243, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Corey, R.; Werner, K.T.; Singer, A.; Moss, A.; Smith, M.; Noelting, J.; Rakela, J. Acute liver failure associated with garcinia cambogia use. Ann. Hepatol. 2016, 15, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, H.; Ahn, J.H.; Suk, H.J. Liver injury induced by herbal extracts containing mistletoe and kudzu. J. Altern. Complement. Med. 2015, 21, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.M.; Chai, S.C.; Brewer, C.T.; Chen, T. Pregnane X receptor and drug-induced liver injury. Expert Opin. Drug Metab. Toxicol. 2014, 10, 1521–1532. [Google Scholar] [CrossRef] [PubMed]
- Woolbright, B.L.; Jaeschke, H. Novel insight into mechanisms of cholestatic liver injury. World J. Gastroenterol. 2012, 18, 4985–4993. [Google Scholar] [CrossRef] [PubMed]
- Schyschka, L.; Sanchez, J.J.; Wang, Z.; Burkhardt, B.; Muller-Vieira, U.; Zeilinger, K.; Bachmann, A.; Nadalin, S.; Damm, G.; Nussler, A.K. Hepatic 3D cultures but not 2D cultures preserve specific transporter activity for acetaminophen-induced hepatotoxicity. Arch. Toxicol. 2013, 87, 1581–1593. [Google Scholar] [CrossRef] [PubMed]
- Navarro, V.J.; Senior, J.R. Drug-related hepatotoxicity. N. Engl. J. Med. 2006, 354, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Suk, K.T.; Kim, D.J.; Kim, C.H.; Park, S.H.; Yoon, J.H.; Kim, Y.S.; Baik, G.H.; Kim, J.B.; Kweon, Y.O.; Kim, B.I.; et al. A prospective nationwide study of drug-induced liver injury in korea. Am. J. Gastroenterol. 2012, 107, 1380–1387. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Fontana, R.J.; Bonkovsky, H.L.; Watkins, P.B.; Davern, T.; Serrano, J.; Yang, H.; Rochon, J. Drug Induced Liver Injury Network. Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the united states. Gastroenterology 2008, 135, 1924–1934. [Google Scholar] [CrossRef] [PubMed]
- Meier, Y.; Pauli-Magnus, C.; Zanger, U.M.; Klein, K.; Schaeffeler, E.; Nussler, A.K.; Nussler, N.; Eichelbaum, M.; Meier, P.J.; Stieger, B. Interindividual variability of canalicular ATP-binding-cassette (ABC)-transporter expression in human liver. Hepatology 2006, 44, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Irie, M.; Iwata, K.; Nakane, H.; Yoshikane, M.; Koyama, Y.; Uehara, Y.; Takeyama, Y.; Kitamura, Y.; Sohda, T.; et al. Altered expression of alkaline phosphatase (ALP) in the liver of primary biliary cirrhosis (PBC) patients. Hepatol. Res. 2006, 35, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Fisher, K.; Vuppalanchi, R.; Saxena, R. Drug-induced liver injury. Arch. Pathol. Lab. Med. 2015, 139, 876–887. [Google Scholar] [CrossRef] [PubMed]
- Teschke, R.; Larrey, D.; Melchart, D.; Danan, G. Traditional chinese medicine (TCM) and herbal hepatotoxicity: Rucam and the role of novel diagnostic biomarkers such as microRNAs. Medicines 2016, 3, 18. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.S.; Chung, B.C.; Kwon, O.S.; Jung, B.H. Discovery of common urinary biomarkers for hepatotoxicity induced by carbon tetrachloride, acetaminophen and methotrexate by mass spectrometry-based metabolomics. J. Appl. Toxicol. 2012, 32, 505–520. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; McGill, M.R.; Dorko, K.; Kumer, S.C.; Schmitt, T.M.; Forster, J.; Jaeschke, H. Mechanisms of acetaminophen-induced cell death in primary human hepatocytes. Toxicol. Appl. Pharmacol. 2014, 279, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Lu, J.; Cheng, J.; Wang, L.; Matsubara, T.; Csanaky, I.L.; Klaassen, C.D.; Gonzalez, F.J.; Ma, X. Human PXR modulates hepatotoxicity associated with rifampicin and isoniazid co-therapy. Nat. Med. 2013, 19, 418–420. [Google Scholar] [CrossRef] [PubMed]
- Ganey, P.E.; Luyendyk, J.P.; Maddox, J.F.; Roth, R.A. Adverse hepatic drug reactions: Inflammatory episodes as consequence and contributor. Chem. Biol. Interact. 2004, 150, 35–51. [Google Scholar] [CrossRef] [PubMed]
- Ganey, P.E.; Luyendyk, J.P.; Newport, S.W.; Eagle, T.M.; Maddox, J.F.; Mackman, N.; Roth, R.A. Role of the coagulation system in acetaminophen-induced hepatotoxicity in mice. Hepatology 2007, 46, 1177–1186. [Google Scholar] [CrossRef] [PubMed]
- Tujios, S.; Fontana, R.J. Mechanisms of drug-induced liver injury: From bedside to bench. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Den Braver, M.W.; den Braver-Sewradj, S.P.; Vermeulen, N.P.; Commandeur, J.N. Characterization of cytochrome P450 isoforms involved in sequential two-step bioactivation of diclofenac to reactive P-benzoquinone imines. Toxicol. Lett. 2016, 253, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Kishida, T.; Onozato, T.; Kanazawa, T.; Tanaka, S.; Kuroda, J. Increase in covalent binding of 5-hydroxydiclofenac to hepatic tissues in rats co-treated with lipopolysaccharide and diclofenac: Involvement in the onset of diclofenac-induced idiosyncratic hepatotoxicity. J. Toxicol. Sci. 2012, 37, 1143–1156. [Google Scholar] [CrossRef] [PubMed]
- Lundgren, H.; Martinsson, K.; Cederbrant, K.; Jirholt, J.; Mucs, D.; Madeyski-Bengtson, K.; Havarinasab, S.; Hultman, P. HLA-DR7 and HLA-DQ2: Transgenic mouse strains tested as a model system for ximelagatran hepatotoxicity. PLoS ONE 2017, 12, e0184744. [Google Scholar] [CrossRef] [PubMed]
- Clare, K.E.; Miller, M.H.; Dillon, J.F. Genetic factors influencing drug-induced liver injury: Do they have a role in prevention and diagnosis? Curr. Hepatol. Rep. 2017, 16, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Auerbach, H.E. New-onset acute thrombocytopenia in hospitalized patients: Pathophysiology and diagnostic approach. J. Community Hosp. Intern. Med. Perspect. 2017, 7, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Ghannam, M.; Mansour, S.; Nabulsi, A.; Abdoh, Q. Anticonvulsant hypersensitivity syndrome after phenytoin administration in an adolescent patient: A case report and review of literature. Clin. Mol. Allergy 2017, 15, 14. [Google Scholar] [CrossRef] [PubMed]
- Benichou, C. Standardization of definitions and criteria of causality assessment of adverse drug reactions. Drug-induced liver disorders: Report of an international consensus meeting. Int. J. Clin. Pharmacol. Ther. Toxicol. 1990, 28, 317–322. [Google Scholar]
- Danan, G.; Teschke, R. Rucam in drug and herb induced liver injury: The update. Int. J. Mol. Sci. 2015, 17, 14. [Google Scholar] [CrossRef] [PubMed]
- Benichou, C.; Danan, G.; Flahault, A. Causality assessment of adverse reactions to drugs—II. An original model for validation of drug causality assessment methods: Case reports with positive rechallenge. J. Clin. Epidemiol. 1993, 46, 1331–1336. [Google Scholar] [CrossRef]
- Danan, G.; Benichou, C. Causality assessment of adverse reactions to drugs—I. A novel method based on the conclusions of international consensus meetings: Application to drug-induced liver injuries. J. Clin. Epidemiol. 1993, 46, 1323–1330. [Google Scholar] [CrossRef]
- Yamazaki, F.; Sone, R. Desensitization of menthol-activated cold receptors in lower extremities during local cooling in young women with a cold constitution. J. Physiol. Sci. 2017, 67, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Ciganda, C.; Laborde, A. Herbal infusions used for induced abortion. J. Toxicol. Clin. Toxicol. 2003, 41, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, T.; Nomura, H.; Nakanishi, K.; Fujita, S. Effects of drug metabolism modifiers on pulegone-induced hepatotoxicity in mice. Res. Commun. Chem. Pathol. Pharmacol. 1987, 58, 75–83. [Google Scholar] [PubMed]
- Miyazawa, M.; Marumoto, S.; Takahashi, T.; Nakahashi, H.; Haigou, R.; Nakanishi, K. Metabolism of (+)- and (−)-menthols by CYP2A6 in human liver microsomes. J. Oleo Sci. 2011, 60, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, M.; Ikarashi, N.; Tsukui, M.; Kurokawa, A.; Naito, R.; Suzuki, M.; Yokobori, K.; Ochiai, T.; Ishii, M.; Kusunoki, Y.; et al. Menthol reduces the anticoagulant effect of warfarin by inducing cytochrome P450 2C expression. Eur. J. Pharm. Sci. 2014, 56, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Paul, I.M. Therapeutic options for acute cough due to upper respiratory infections in children. Lung 2012, 190, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Hoy, N.Y.; Leung, A.K.; Metelitsa, A.I.; Adams, S. New concepts in median nail dystrophy, onychomycosis, and hand, foot, and mouth disease nail pathology. ISRN Dermatol. 2012, 2012, 680163. [Google Scholar] [CrossRef] [PubMed]
- Nakahashi, H.; Miyazawa, M. Biotransformation of (−)-camphor by salmonella typhimurium OY1002/2A6 expressing human CYP2A6 and NADPH-P450 reductase. J. Oleo Sci. 2011, 60, 545–548. [Google Scholar] [CrossRef] [PubMed]
- Jerome, S.V.; Hughes, T.F.; Friesner, R.A. Successful application of the DBLOC method to the hydroxylation of camphor by cytochrome P450. Protein Sci. 2016, 25, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Herrera, S.; Bruguera, M. Hepatotoxicity induced by herbs and medicines used to induce weight loss. Gastroenterol. Hepatol. 2008, 31, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Jaradat, N.A.; Ayesh, O.I.; Anderson, C. Ethnopharmacological survey about medicinal plants utilized by herbalists and traditional practitioner healers for treatments of diarrhea in the west bank/palestine. J. Ethnopharmacol. 2016, 182, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Guesmi, F.; Prasad, S.; Tyagi, A.K.; Landoulsi, A. Antinflammatory and anticancer effects of terpenes from oily fractions of teucruim alopecurus, blocker of IκBα kinase, through downregulation of nf-kappab activation, potentiation of apoptosis and suppression of NF-κB-regulated gene expression. Biomed. Pharmacother. 2017, 95, 1876–1885. [Google Scholar] [CrossRef] [PubMed]
- Bosisio, E.; Giavarini, F.; Dell’Agli, M.; Galli, G.; Galli, C.L. Analysis by high-performance liquid chromatography of teucrin A in beverages flavoured with an extract of Teucrium chamaedrys L. Food Addit. Contam. 2004, 21, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Druckova, A.; Mernaugh, R.L.; Ham, A.J.; Marnett, L.J. Identification of the protein targets of the reactive metabolite of teucrin a in vivo in the rat. Chem. Res. Toxicol. 2007, 20, 1393–1408. [Google Scholar] [CrossRef] [PubMed]
- Fau, D.; Lekehal, M.; Farrell, G.; Moreau, A.; Moulis, C.; Feldmann, G.; Haouzi, D.; Pessayre, D. Diterpenoids from germander, an herbal medicine, induce apoptosis in isolated rat hepatocytes. Gastroenterology 1997, 113, 1334–1346. [Google Scholar] [CrossRef] [PubMed]
- Nencini, C.; Galluzzi, P.; Pippi, F.; Menchiari, A.; Micheli, L. Hepatotoxicity of Teucrium chamaedrys L. Decoction: Role of difference in the harvesting area and preparation method. Indian J. Pharmacol. 2014, 46, 181–184. [Google Scholar] [PubMed]
- Smith, L.W.; Culvenor, C.C. Plant sources of hepatotoxic pyrrolizidine alkaloids. J. Nat. Prod. 1981, 44, 129–152. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Ohkuma, S.; McDermott, W.V.; Trey, C.; Huxtable, R.J. Hepatic venocclusive disease associated with the consumption of pyrrolizidine-containing dietary supplements. Gastroenterology 1985, 88, 1050–1054. [Google Scholar] [CrossRef]
- Valla, D.; Benhamou, J.P. Drug-induced vascular and sinusoidal lesions of the liver. Baillieres Clin. Gastroenterol. 1988, 2, 481–500. [Google Scholar] [CrossRef]
- Bye, S.N.; Dutton, M.F. The inappropriate use of traditional medicines in south africa. J. Ethnopharmacol. 1991, 34, 253–259. [Google Scholar] [CrossRef]
- He, X.; Xia, Q.; Ma, L.; Fu, P.P. 7-cysteine-pyrrole conjugate: A new potential DNA reactive metabolite of pyrrolizidine alkaloids. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2016, 34, 57–76. [Google Scholar] [CrossRef] [PubMed]
- He, Y.Q.; Yang, L.; Liu, H.X.; Zhang, J.W.; Liu, Y.; Fong, A.; Xiong, A.Z.; Lu, Y.L.; Yang, L.; Wang, C.H.; et al. Glucuronidation, a new metabolic pathway for pyrrolizidine alkaloids. Chem. Res. Toxicol. 2010, 23, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Li, L.; Zhong, D.; Shen, S.; Zheng, J.; Chen, X. 9-glutathionyl-6,7-dihydro-1-hydroxymethyl-5 h-pyrrolizine is the major pyrrolic glutathione conjugate of retronecine-type pyrrolizidine alkaloids in liver microsomes and in rats. Chem. Res. Toxicol. 2016, 29, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Sarges, P.; Steinberg, J.M.; Lewis, J.H. Drug-induced liver injury: Highlights from a review of the 2015 literature. Drug Saf. 2016, 39, 801–821. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Kim, H.W.; Lee, H.Y.; Son, C.G. Systematic review on herb-induced liver injury in korea. Food Chem. Toxicol. 2015, 84, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Suzuki, A.; Borlak, J.; Andrade, R.J.; Lucena, M.I. Drug-induced liver injury: Interactions between drug properties and host factors. J. Hepatol. 2015, 63, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Shahbaz, O.; Mahajan, S.; Lewis, J.H. Highlights of drug—And herb-induced liver injury in the literature from 2016: How best to translate new information into clinical practice? Expert Opin. Drug Metab. Toxicol. 2017, 13, 935–951. [Google Scholar] [CrossRef] [PubMed]

| Herbal Supplement | Preparation/Compound | Effect on P450 | CYP450 Reported |
|---|---|---|---|
| Mangosteen (Garcinia mangostana) | Aqueous extract | Inhibition of activity [28]. | 2C8, 2C9, 2C19 |
| Black cohosh (Actaea racemosa L. [syn. Cimifuga racemosa L.]) | Fukinolic acid and cimicfugic acids A and B | Inhibition of activity of purified enzymes [29]. | 1A2, 2D6, 2C9, 3A4 |
| Green tea * (Camellia sinensis) | (−)-epigallocatechin-3-gallate | Inhibition of activity in HLM (human liver micrsomes) and HIM (human intestinal microsomes) [30]. | 2B6, 2C8, 2C19, 2D6, 3A |
| Extract | Inhibition of activity in rat liver microsomes [31]. | 3a | |
| Menthol | Menthofuran | Inhibited activity in HLM [32]. | 2A6 |
| Menthofuran | Inhibited coumarin 7-hyroxylation in purified enzymes [33]. | 2A6, 2A13 | |
| (−)-menthol * | Inhibited coumarin 7-hydroxylation in purified enzymes [33]. | 2A13, 2A6 | |
| Garcinia jasminoides * | Geniposide, extract | Decreased activity in rat liver microsomes [34]. | 3A4 |
| Genipin | Inhibited activity and decreased mRNA and protein expression in HepG2 [35]. | 2C19, 3A4 | |
| Geniposide | Decreased activity in rat livers [34]. | 3a | |
| Garlic (Allium sativum) * | Garlic oil | Inhibited activity reflected by 6-hydroxychlorzoxazone/chlorzaoxazone serum ratios in humans [11,12]. | 2E1 |
| Not noted | Inhibited activity reflected by decreased phenacetin metabolism in HLM [36]. | 1A2 | |
| Retrorsine | Inhibited activity in purified enzymes [37]. | 3A4 | |
| Grapefruit (Citrus paradisi) * | Dhydrobergamottin, gergamottin | Inhibited activity in HLM [38,39]. | 3A4 |
| Juice | Inhibited activity reflected by decreased midazolam concentrations in humans [39]. | 3A4 | |
| Saw palmetto (Serenoa repens) | Extract | Inhibited activity in HLM [40]. | 2C8 |
| Echinacea purpura * | Root Extract (pill) | Inhibition of activity reflected by decreased midazolam hydroxylation in humans [41,42]. | 3A4 |
| Root Extract (pill) | Inhibition of activity as reflected by decreased caffeine metabolism in humans [41,42]. | 1A2 | |
| Marslinic acid, corosolic acid, ursolic acid | Inhibited the activity in HIM [43]. | 3A4 | |
| Cranberry (vaccinium macrocarpon) | Extract | Inhibited activity of purified enzymes [44]. | 1A2, 2D6 |
| Milk thistle (Silybum marianum) | Silybin, isosilybin | Decreased mRNA and inhibited PXR-mediated CYP-Luciferase activity in LS180 cell line [45]. | 3A4 |
| Inhibited promoter activity via hPXR [45]. | 3A4 | ||
| Tomato (Lycopersicon esculentum) | Juice extract | Inhibited activity of purified enzymes [46]. | 3A4 |
| Capsicum (Capsicum annuum L. var. grossum.) | Dried and re-suspended in DMSO | Inhibited activity of purified enzymes [46]. | 3A4 |
| Potato (Solanum tuberosum L.) | Dried and re-suspended in DMSO | Inhibited activity of purified enzymes [46]. | 1A2, 2D6, 3A4 |
| Eggplant (Solanum melongena L.) | Dried and re-suspended in DMSO | Inhibited activity of purified enzymes [46]. | 1A2, 2D6, 3A4 |
| Sweet pepper (Capsicum annuum) | Dried and re-suspended in DMSO | Inhibited activity of purified enzymes [46]. | 1A2, 2D6, 3A4 |
| Black elderberry (Sambucus nigra) | Extract (pill) | Inhibited activity in microsomes overexpressing CYP450 [44]. | 1A2, 2D6, 3A4 |
| Fennel (Foeniculum vulgare) | Extract (tea) | Inhibited activity in microsomes overexpressing CYP450 [44]. | 1A2, 2D6, 3A4 |
| Horsetail (Equisetaceae family) | Extract (tea) | Inhibited activity in microsomes overexpressing CYP450 [44]. | 1A2, 2D6, 3A4 |
| Raspberry leaf (Rubus idaeus) | Extract (pill) | Inhibited activity in microsomes overexpressing CYP450 [44]. | 1A2, 2D6, 3A4 |
| Cinnamon (Cinnamomum verum) | o-methoxy cinnamaldehyde | Inhibited activity in rat liver microsomes [47]. | 1a2, 2e1 |
| Extract | Inhibited activity in microsomes overexpressing CYP450 [48]. | 2C9, 3A4 | |
| Ginger (Zingiber officinale) | Extract | Inhibited activity in microsomes overexpressing CYP450 [48]. | 2C9, 3A4 |
| Mace (Myristica fragrans) | Extract | Inhibited activity in microsomes overexpressing CYP450 [48]. | 2C9, 3A4 |
| Nutmeg (Myristica genus) | Extract | Inhibited activity in microsomes overexpressing CYP450 [48]. | 2C9, 3A4 |
| Valerian (Valeriana officinalis) | Extract | Inhibited activity in HLM [40]. | 2C8 |
| Madagascan medicinal plant (Catharanthus roseus) | Ajmalicine | Inhibited activity in HLM [49]. | 2D6 |
| Vindolene | Inhibited activity in HLM [49]. | 2D6, 3A4 | |
| Serpentine | Inhibited activity in HLM [49]. | 2D6, 3A4 | |
| Southern African medicinal plant (Sutherlandia frutescens) | Extract | Inhibited activity in transfected microsomes [50]. | 3A4 |
| Southern African medicinal plant (Moringa oleifera) | Extract | Inhibited activity reflected by decreased testosterone hydroxylation in HLM [51]. | 3A4 |
| West African medicinal plants | Extract | Inhibited activity in transfected microsomes [52]. | 3A4, 3A5, 3A7 |
| Aframomum cuspidatum | |||
| Aframomum meliguieta | |||
| Harrisonia abyssinica | |||
| Phyllanthus amarus | |||
| Piper guineense | |||
| Lonchocarpus sericeus | |||
| Lipia multiflora | |||
| West African medicinal plants | Extract | Inhibited activity in transfected microsomes [52]. | 3A4, 3A7 |
| Jutropha curcas | |||
| Persia Americana | |||
| Oxytenanthera abyssinica | |||
| Talinum triangulare | |||
| Tanzanian medicinal plant | |||
| Cyphostemma ildebrandtii | |||
| Tanzanian medicinal plant (Acacia nilotica) | Extract | Inhibited activity in transfected microsomes [53,54]. | 2C9, 2C19, 2D6, 3A4 |
| Tanzanian medicinal plants | Extract | Inhibited activity in transfected microsomes [53,54]. | 2C9, 2C19, 3A4 |
| Acacia robusta | |||
| Agauria salicifolia | |||
| Tanzanian medicinal plants | Extract | Inhibited activity in transfected microsomes [53,54]. | 2C9, 3A4 |
| Elaeodendron buchananii | |||
| Sclerocarya birrea | |||
| Peppermint (Mentha piperita) | Oil | Inhibited activity in HLM [55]. | 3A4 |
| Menthol * | Inhibited activity in HLM [55]. | 3A4 | |
| Menthyl acetate | Inhibited activity in HLM [55]. | 3A4 | |
| Ascorbyl palmitate | Inhibited activity in HLM [55]. | 3A4 | |
| Sesamin (Sesamum indicum) | Not noted | Inhibited activity reflected by decreased phenacetin, diclofenac, omeprazole, dextromethorphan, and midazolam metabolism in HLM [36]. | 1A2, 2C9, 2C19, 2D6, 3A4 |
| Tumeric (Curcuma longa) | Not noted | Inhibited activity reflected by decreased diclofenac, omeprazole, dextromethorphan, and midazolam metabolism in HLM [36]. | 2C9, 2C19, 2D6, 3A4 |
| St. John’s wort (Hypericum perforatum) | Not noted | Inhibited activity reflected by decreased phenacetin, diclofenac, and midazolam metabolism in HLM [36]. | 1A2, 2C9, 3A4 |
| Herbal Supplement | Preparation/Compound | Effect on hPXR |
|---|---|---|
| Gan Gao-Licorice (Radix et Rhizoma Glycyrrhizae) | Extract | Activation of CYP3A4 promoter via hPXR [96]. |
| Huang Qi-Astragalus mebranaceus (Radix Astragali) | Extract | Activation of CYP3A4 promoter via hPXR [96]. |
| Ji Xue Cao-Centella asiatica (Herba Centellae) | Extract | Activation of CYP3A4 promoter via hPXR [96]. |
| Ban Lan Gen-Isatis indigotica (Radix Isatidis) | Extract | Activation of CYP3A4 promoter via hPXR [96]. |
| Jin Yin Hua-Lonicera japonica (Flos Lonicerae Japonicae) | Extract | Activation of CYP3A4 promoter via hPXR [96]. |
| Hong Jing Tian-Rhodiola crenulata (Radix et Rhizoma Rhodiolae Crenulatae) | Extract | Activation of CYP3A4 promoter via hPXR [96]. |
| Da Huang-Rhubarb (Radix et Rhizoma Rhei) | Extract | Activation of CYP3A4 promoter via hPXR [96]. |
| Trans-resveratrol | Activation of CYP3A4 promoter via hPXR [96]. | |
| Fu Ling-Poria cocos (Poria) | Extract | Activation of CYP3A4 promoter via hPXR [96]. |
| Bai Shao-Paeonia lactiflora (Radix Paeoniae Alba) | Extract | Activation of CYP3A4 promoter via hPXR [96]. |
| Sang Qi-Panax notoginseng (Radix et Rhizoma Notoginseng) | Extract * | Activation of CYP3A4 promoter via hPXR [96]. |
| Chuan Xiong-Ligusticum chuanxiong (Rhizoma Chuanxiong) | Extract | Activation of CYP3A4 promoter via hPXR [96]. |
| Dang Gui-Chinese angelica (Radix Angelicae sinensis) | Extract | Activation of CYP3A4 promoter via hPXR [96]. |
| Ligustilide | Activation of CYP3A4 promoter via hPXR [96]. | |
| Sheng Di Huang-Rehmannia root (Radix Rehmanniae) | Extract | Activation of CYP3A4 promoter via hPXR [96]. |
| Yin Yang Huo-Epimedium brevicornum (Herba Epimedii) | Extract | Activation of CYP3A4 promoter via hPXR [96]. |
| Di Gu Pi-Lycium chinense (Cortex Lycii) | Extract | Activation of CYP3A4 promoter via hPXR [96]. |
| Bai Zhu-Atractylodes macrocephala (Rhizoma Atractylodis) | Extract | Activation of CYP3A4 promoter via hPXR [96]. |
| Wu Wei Zi-Schisandra chinensis (Fructus Schisandrae Chinensis) | Extract | Activation of CYP3A4 promoter via hPXR [96]. |
| Schisantherin A | Activation of CYP3A4 promoter via hPXR [96]. | |
| Bai Shao-Paeonia lactiflora (Radix Paeoniae Alba) | Extract | Activation of CYP3A4 promoter via hPXR [96]. |
| Mai Dong-Ophiopogon japonicas (Radix Ophiopogonis) | Extract | Activation of CYP3A4 promoter via hPXR [96]. |
| Hu Zhang-Polygonum multiflorum (Radix Polygoni Multiflori) | Extract | Activation of CYP3A4 promoter via hPXR [96]. |
| Huang Lian-Coptis chinensis (Rhizoma Coptidis) | Extract | Activation of CYP3A4 promoter via hPXR [96]. |
| Berberine hydrochloride | Activation of CYP3A4 promoter via hPXR [96]. | |
| Yin Chen-Artemisia scoparia (Herba Artemisiae Scopariae) | Extract | Activation of CYP3A4 promoter via hPXR [96]. |
| Tian Hua Fen-Trichosanthes kirilowii (Radix Trichosanthis) | Extract | Activation of CYP3A4 promoter via hPXR [96]. |
| Shui Fei Ji-Silybum marianum (Fructus silybi) | Extract | Activation of CYP3A4 promoter via hPXR [96]. |
| Zhi Zi-Gardenia fruit (Fructus gardeniae) | Extract * | Activation of CYP3A4 promoter via hPXR [96]. |
| Ren Shen–Ginseng (Radix et Rhizoma ginseng) * | Ginsenoside F2, protopanaxadiol | Activation of CYP3A4 promoter via hPXR [97]. |
| Panaxatriol, Rg2, pseudoginsenoside F11, Rg1, ginsenodide, Rb3 | Activation of CYP3A4 promoter via hPXR [97]. | |
| Extract | Activation of CYP3A4 promoter via hPXR [96]. | |
| Black pepper (Piper nigrum) * | Piperine | Activation of CYP3A4 promoter via hPXR, increased mRNA and protein in intestinal cell lines and human hepatocytes [98]. |
| St. John’s Wort * (Hypericum perforatum) | Hyperforin | Activation of CYP3A4 promoter via hPXR [99,100]. |
| Hyperforin | Increased CYP2C9 and 3A4 protein and mRNA in human hepatocytes [99,100]. | |
| Extract (pill) | Increased CYP3A4 activity reflected by decreased phenytoin concentrations [12]. | |
| Increased CYP2C19 activity reflected by decreased omeprazole concentrations in humans dependent on CYP2C19 phenotype [13,14]. | ||
| Increased activity reflected by hydroxymidazolam/midazolam serum ratios in humans (CYP3A4) 6-hydroxychlorzoxazone/chlorzaoxazone serum ratios in humans (CYP2E1) [11,12]. | ||
| Gingko biloba * | Extract | Activation of CYP3A4 promoter via hPXR [96,101]. |
| Gingkolide A, Gingkolide B | Activation of CYP3A4 promoter via hPXR [102]. | |
| Increased CYP2B6 and 3A4 mRNA in PHH [102]. | ||
| Leaf extract | Increased activity of CYP2C19 reflected by decreased plasma concentrations of omeprazole and increased 5-hydroxyomeprazole in humans [103]. | |
| Kava Kava * (Piper methysticum) | Extract | Activation of CYP3A4 promoter via hPXR [104]. |
| Desmethoxyangonin, dihydromethysticin | Increased Cyp3a23 mRNA in rat livers [105]. | |
| Echinacea purpura * | Extract | Activation of CYP3A4 promoter via hPXR [87]. |
| Extract | Increased CYP1A2 and 3A4 mRNA in HepG2 [87]. | |
| Extract | Increased CYP1A2 and 3A4 mRNA in HepG2 [87]. | |
| Thyme (Thymus vulgaris) | Extract | Activation of CYP3A4 promoter via hPXR [106]. |
| Clove (Syzygium aromaticum) | Extract | Activation of CYP3A4 promoter via hPXR [106]. |
| Tumeric (Curcuma longa) | Curcumin | Activation of CYP3A4 promoter via hPXR [106]. |
| Red wine (Vitis vinifera) | Resveratrol | Activation of CYP3A4 promoter via hPXR [106]. |
| Southern African medicinal plant (Hypoxis hemerocallidea) | Extract | Activation of CYP3A4 promoter via hPXR [50]. |
| Rooperol | Activation of CYP3A4 promoter via hPXR [50]. | |
| Stimasterol | Activation of CYP3A4 promoter via hPXR [50]. | |
| Southern African medicinal plant (Sutherlandia frutescens) | Extract | Activation of CYP3A4 promoter via hPXR [50]. |
| Tanzanian medicinal plant (Cyphostemma hildebrandtii) | Extract | Activation of CYP3A4 promoter via hPXR [53,54]. |
| Tanzanian medicinal plant (Agauria salicifolia) | Extract | Activation of CYP3A4 promoter via hPXR [53,54]. |
| Tanzanian medicinal plant (Elaeodendron buchananii) | Extract | Activation of CYP3A4 promoter via hPXR [53,54]. |
| Tanzanian medicinal plant (Sclerocarya birrea) | Extract | Activation of CYP3A4 promoter via hPXR [53,54]. |
| Tanzanian medicinal plant (Sterculia Africana) | Extract | Activation of CYP3A4 promoter via hPXR [53,54]. |
| Tanzanian medicinal plant (Turraea holstii) | Extract | Activation of CYP3A4 promoter via hPXR [53,54]. |
| Allspice (Pimenta dioica) | Extract | Increased transcription of CYP3A4 in HepG2 cell line [106]. |
| Grape seed (Vitis vinifera) | Extract | Increased CYP3A4 mRNA in PHH [104]. |
| Garlic (Allium sativum) * | Diallysulfide | Increased Cyp2b1 and 2b2 mRNA in rat livers [107]. |
| Herbal Supplement | Preparation/Compound | Toxicity |
|---|---|---|
| Black cohosh (Actaea racemosa) | Extract | Liver necrosis, autoimmune hepatitis, protein adducts [69,70]. |
| Ginseng (Panax ginseng and P. quinquefolius) | Extract | Possible liver injury in a patient after interaction with imatinib [134]. |
| Greater celandine (Chelidonium majus) | Extract | Reports of hepatocellular injury in humans [148,149,150,151]. |
| Single report of cholestasis in human [148]. | ||
| Black Pepper (Piper nigrum) | Piperine | Increased liver enzymes with CCl4 and hepatic lipid peroxidation in mice [10]. |
| St. John’s Wort (Hypericum perforatum) | Extract | Liver injury associated with copaiba use [125]. |
| Extract | Increased toxicity associated with tert-butyl hydroperoxide [152]. | |
| Green tea (Camellia sinensis) | Epigallocatechin-3-gallate | Hepatotoxic in mice [64]. |
| Extract | Hepatotoxic in rats [65]. | |
| Germander (Lamiaceae family, Teucrim Genus) | Hepatotoxic in humans [61,153,154,155]. | |
| Teucrin A, teuchamaedryn A | Hepatotoxic to isolated rat hepatocytes, CYP3A4 dependent [156]. | |
| Teucrin A | Hepatocellular toxicity in mice [157]. | |
| Gingko biloba | Extract | May be hepatotoxic in mice [130]. |
| Extract | Hepatotoxic in humans [61]. | |
| Camphor (Cinnamomum camphora) | Topical cream | Single report of hepatotoxicity in a human [158]. |
| Kava kava (Piper methysticum) | Extract | Hepatotoxic in humans [61]. |
| Pennyroyal oil (Mentha pulegium and Hedeoma pulegioides) | Oil | Hepatotoxic in mice [159]. |
| Oil | Hepatotoxic in humans [160,161,162]. | |
| (R)-(+)-pulegone | CYP2E1/1A2-dependent hepatotoxicity in mice [163]. | |
| Pulegone, menthol | Hepatotoxic in mice [164]. | |
| Menthone | Hepatotoxic in rats [165,166]. | |
| Pulegone, menthol | Hepatotoxic in rats [164,167]. | |
| Gardenia (Fructus gardenia) | Extract (30% geniposide) | Hepatotoxic in rats [91]. |
| Geniposide | Hepatotoxic in rats [92]. | |
| Extract | Hepatotoxic in rats [92]. | |
| Garlic (Allium sativum) | Homogenate | Hepatotoxic in rats [144,145]. |
| Found in multiple species of plants | Monocrotaline | CYP3A4-dependent hepatotoxicity in mice [168]. |
| Dehydromonocrotaline, dehydrorectronecine | Toxic to human hepatoma cell lines [168,169]. | |
| Garcinia cambogia | Extract | Hepatotoxic to humans [170]. |
| Mistletoe (Viscum coloratum) | Extract | Hepatotoxic to humans [171]. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brewer, C.T.; Chen, T. Hepatotoxicity of Herbal Supplements Mediated by Modulation of Cytochrome P450. Int. J. Mol. Sci. 2017, 18, 2353. https://doi.org/10.3390/ijms18112353
Brewer CT, Chen T. Hepatotoxicity of Herbal Supplements Mediated by Modulation of Cytochrome P450. International Journal of Molecular Sciences. 2017; 18(11):2353. https://doi.org/10.3390/ijms18112353
Chicago/Turabian StyleBrewer, Christopher Trent, and Taosheng Chen. 2017. "Hepatotoxicity of Herbal Supplements Mediated by Modulation of Cytochrome P450" International Journal of Molecular Sciences 18, no. 11: 2353. https://doi.org/10.3390/ijms18112353