Divalent Cations Regulate the Ion Conductance Properties of Diverse Classes of Aquaporins
Abstract
1. Introduction
2. Results
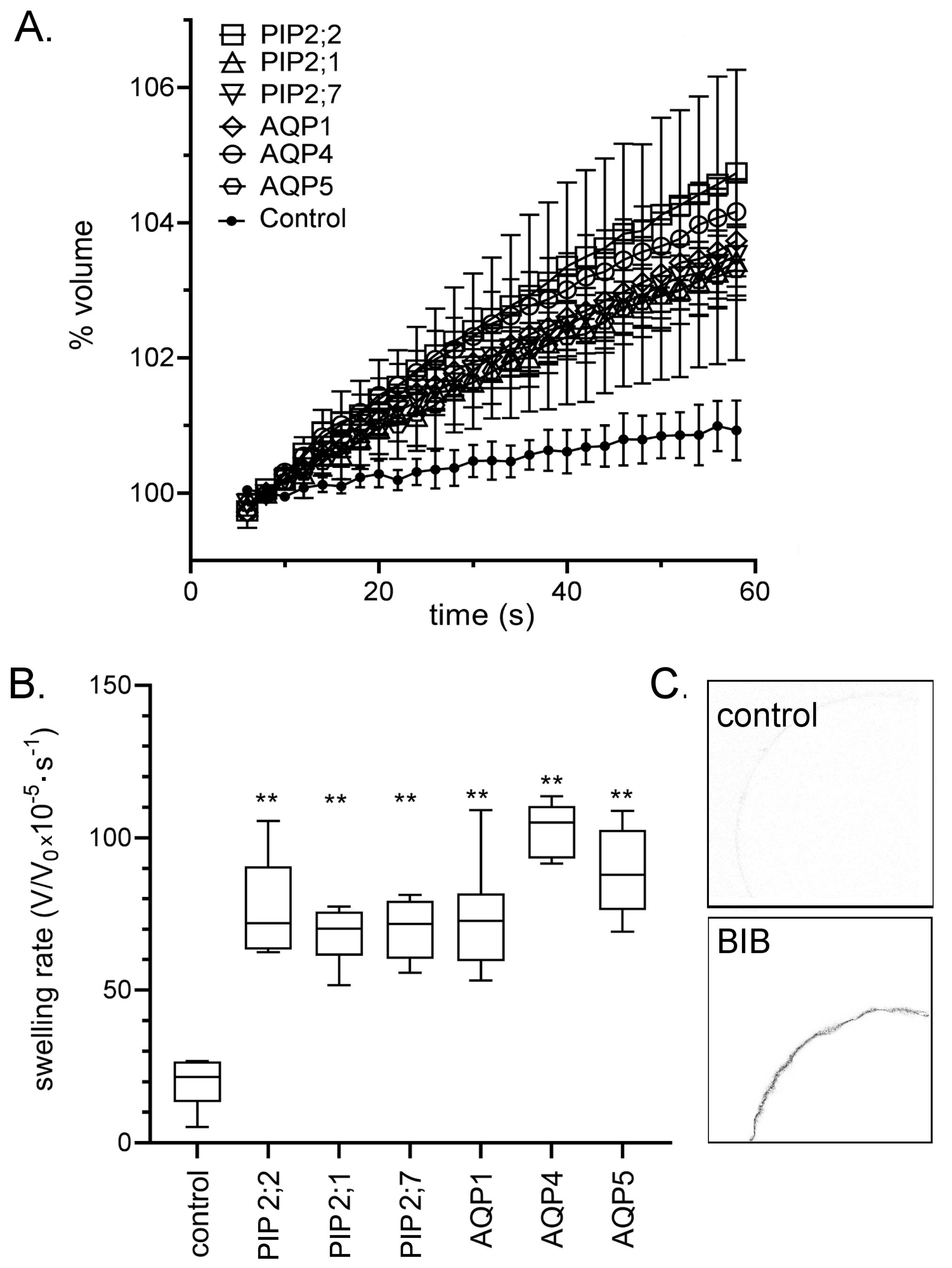
2.1. Expression of Aquaporin (AQP) Channels in Xenopus Oocytes
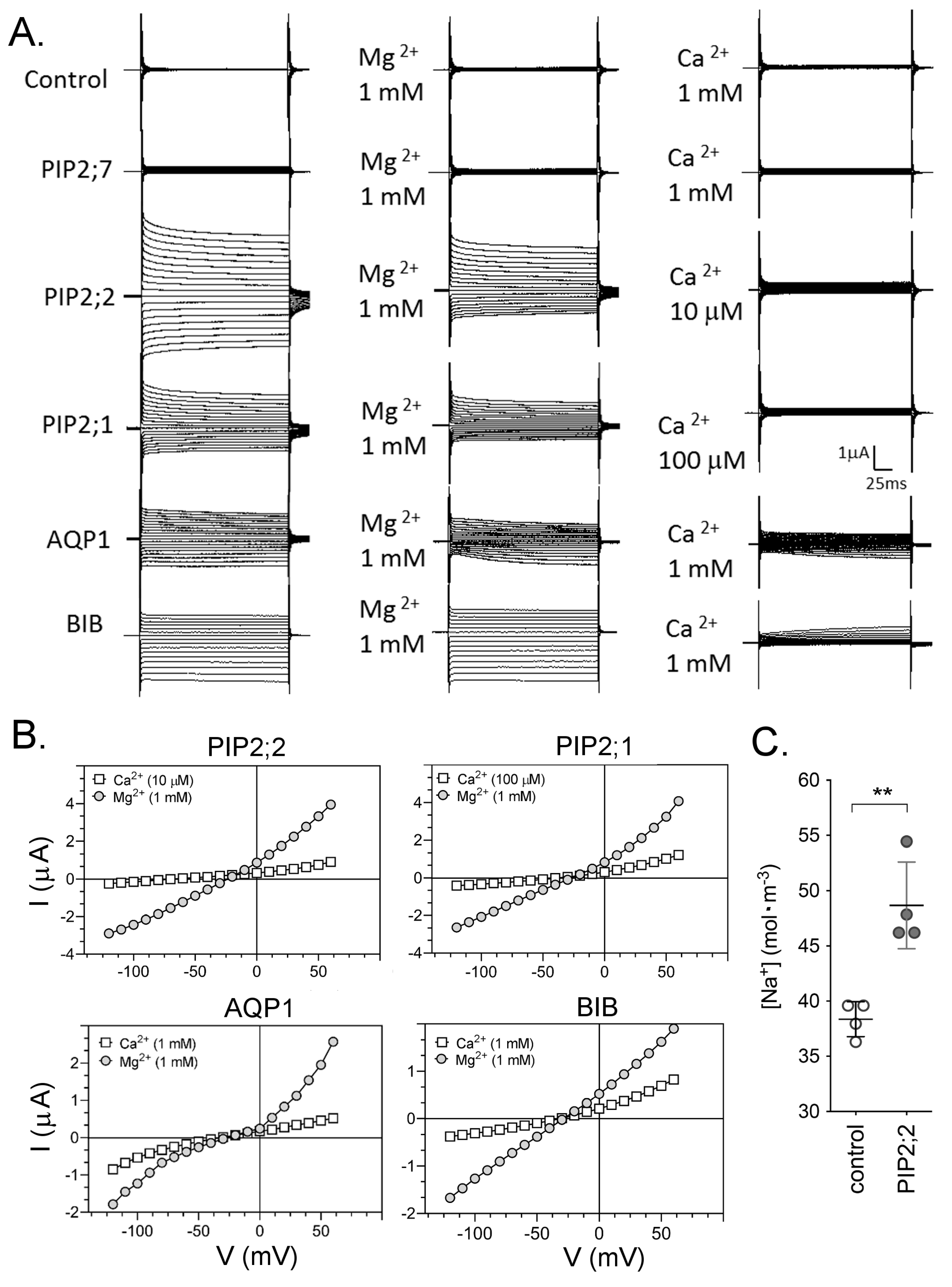
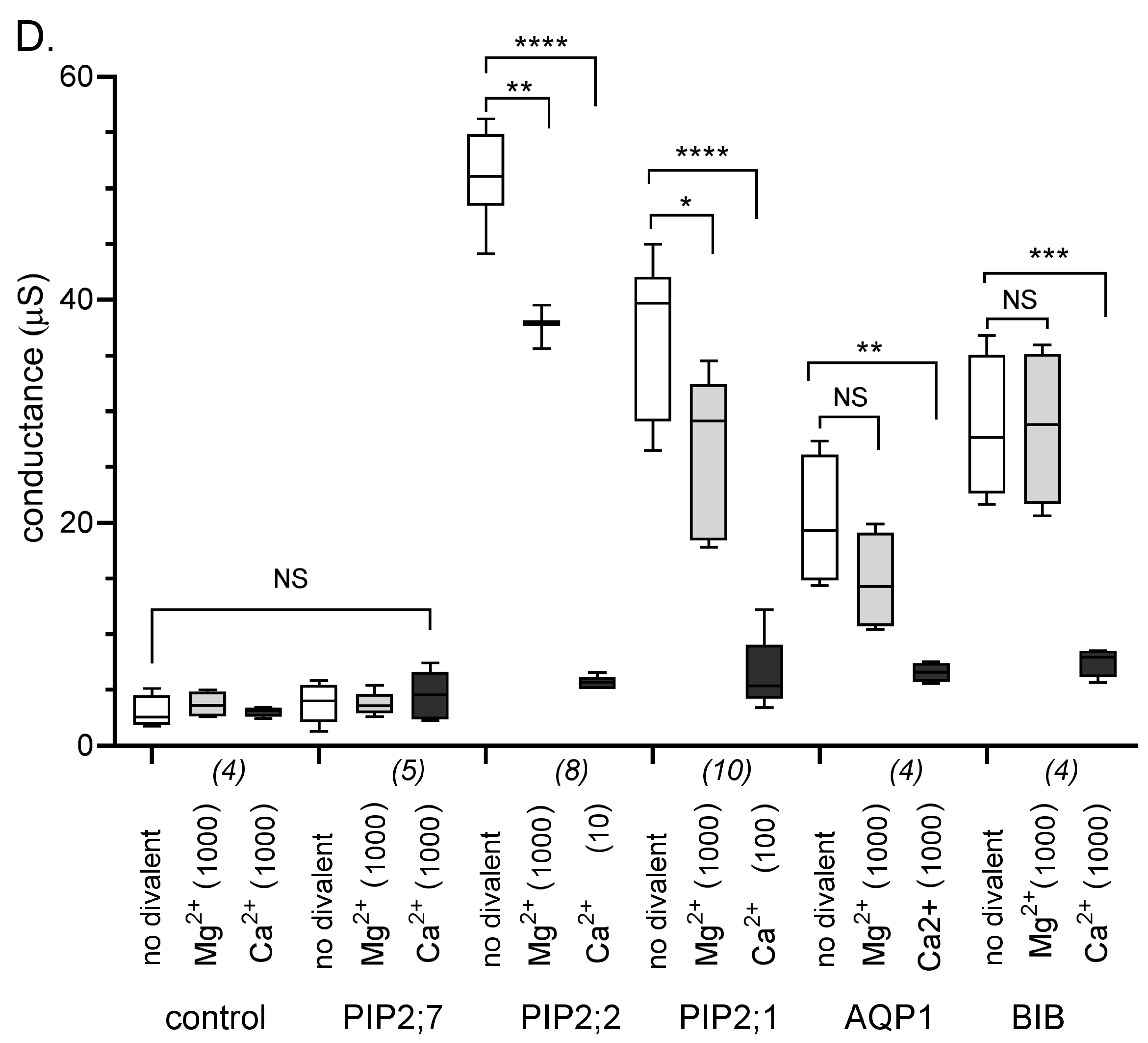
2.2. Differential Sensitivity of AQP Ion Currents to Block by Ca2+
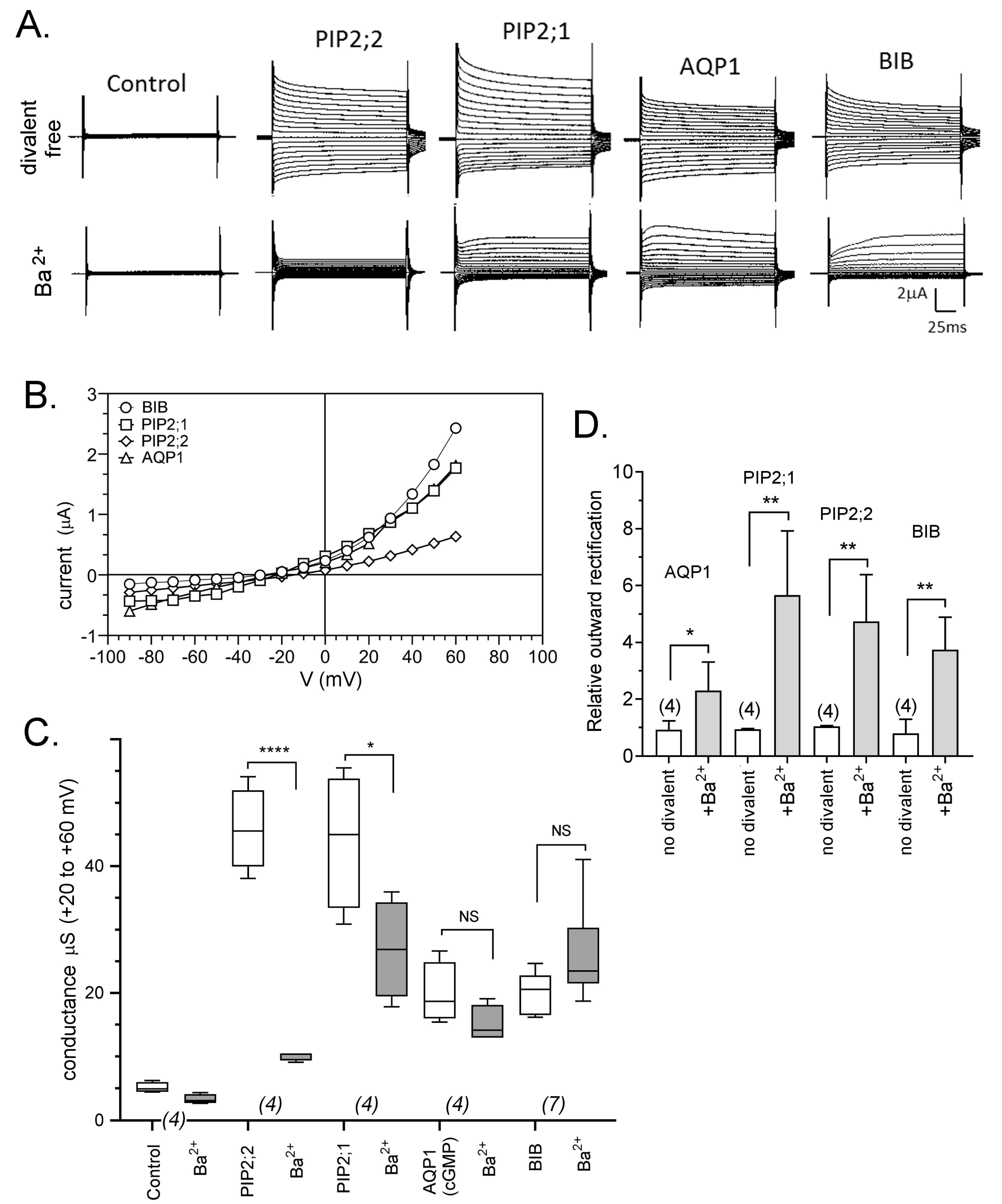
2.3. Voltage-Sensitive Block of AQP Ion Channels by Ba2+
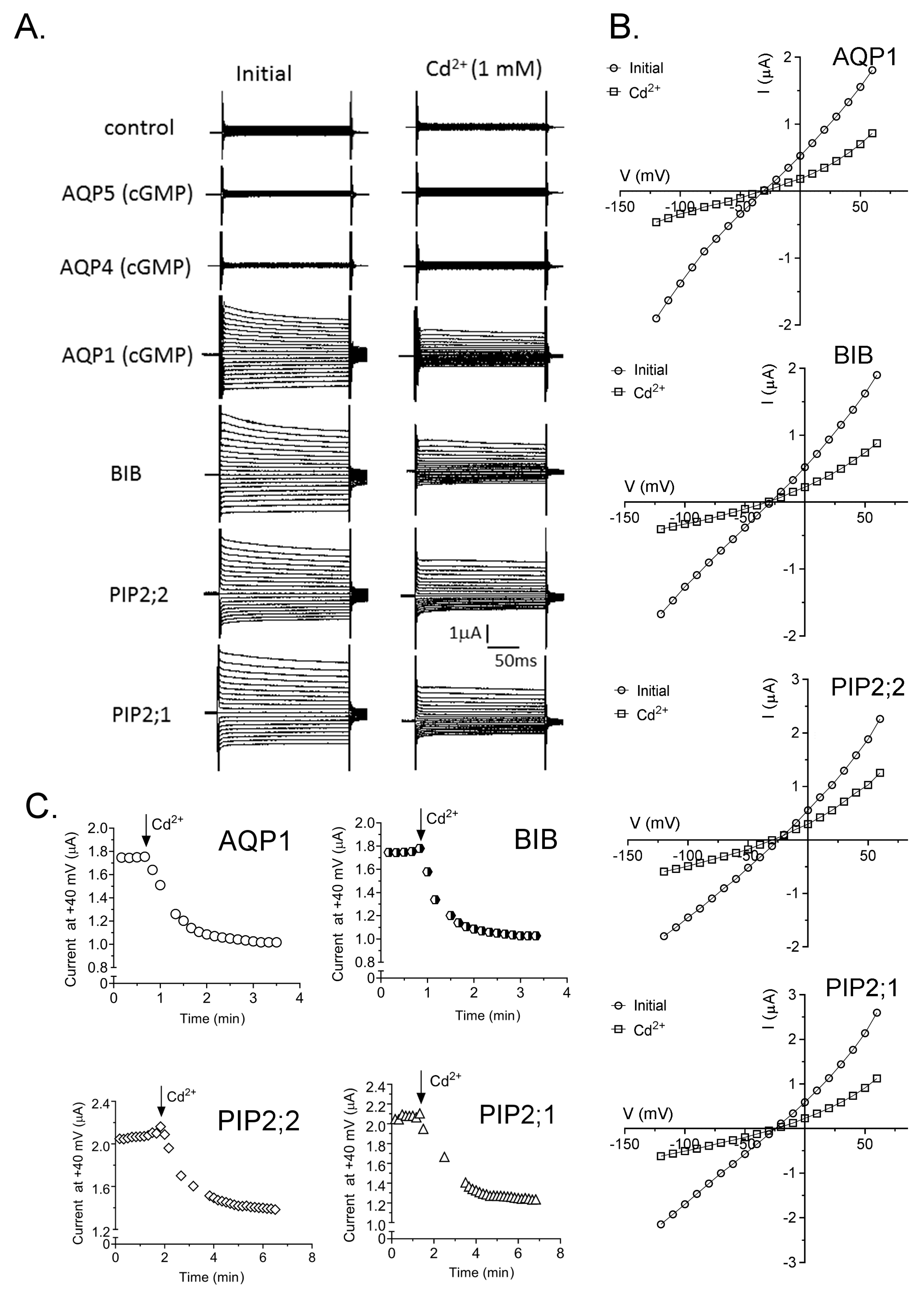
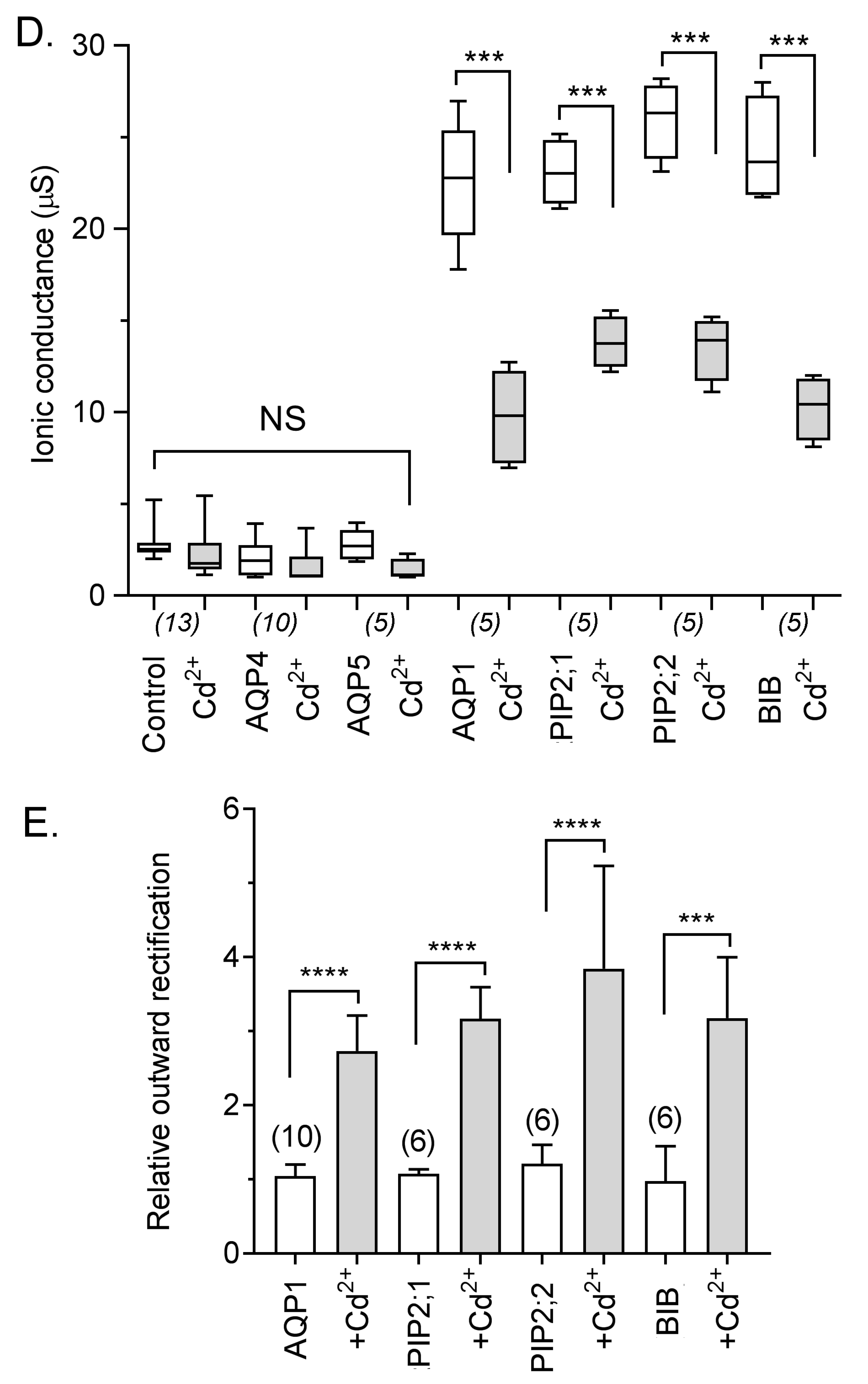
2.4. Inhibition of AQP Ion Channels by Cd2+
2.5. Relief of Ca2+ Inhibition by Addition of Ba2+
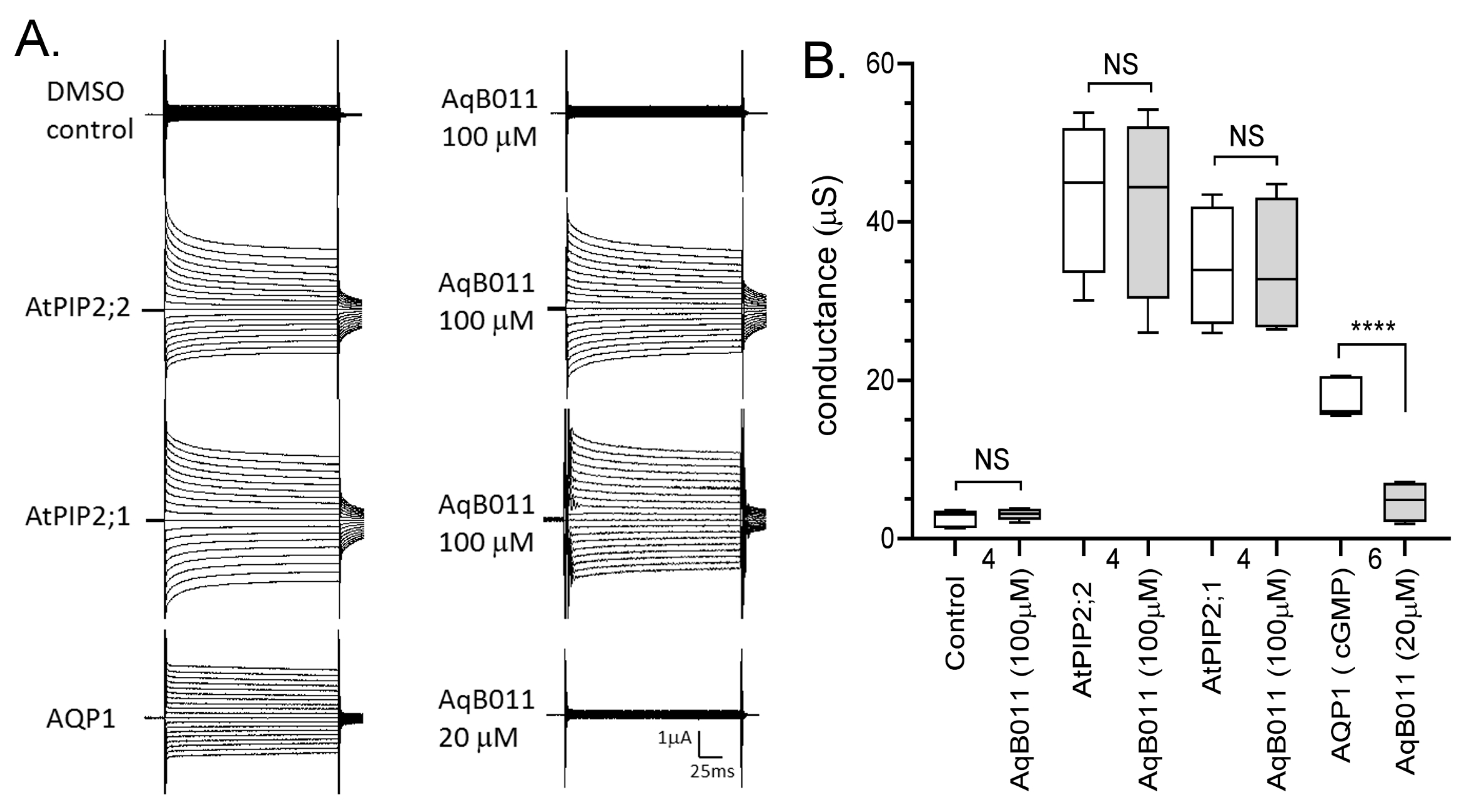
2.6. Pharmacological Effects of the AQP Ion Channel Blocker, AqB011
3. Discussion
4. Materials and Methods
4.1. Preparation and Injection of Xenopus laevis Oocytes
4.2. Osmotic Swelling Assays
4.3. Two Electrode Voltage Clamp Recordings
4.4. Measurement of Oocyte Na+ Accumulation
4.5. Bath Application of Divalent Cations
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Agre, P. Aquaporin water channels (nobel lecture). Angew. Chem. Int. Ed. 2004, 43, 4278–4290. [Google Scholar] [CrossRef] [PubMed]
- Hachez, C.; Chaumont, F. Aquaporins: A family of highly regulated multifunctional channels. Adv. Exp. Med. Biol. 2010, 679, 1–17. [Google Scholar] [PubMed]
- Reizer, J.; Reizer, A.; Saier, M.H., Jr. The MIP family of integral membrane channel proteins: Sequence comparisons, evolutionary relationships, reconstructed pathway of evolution, and proposed functional differentiation of the two repeated halves of the proteins. Crit. Rev. Biochem. Mol. Biol. 1993, 28, 235–257. [Google Scholar] [CrossRef] [PubMed]
- Zampighi, G.A.; Hall, J.E.; Kreman, M. Purified lens junctional protein forms channels in planar lipid films. Proc. Natl. Acad. Sci. USA 1985, 82, 8468–8472. [Google Scholar] [CrossRef] [PubMed]
- Ehring, G.R.; Zampighi, G.; Horwitz, J.; Bok, D.; Hall, J.E. Properties of channels reconstituted from the major intrinsic protein of lens fiber membranes. J. Gen. Physiol. 1990, 96, 631–664. [Google Scholar] [CrossRef] [PubMed]
- Ehring, G.R.; Hall, J.E. Single channel properties of lens MIP 28 reconstituted into planar lipid bilayers. Proc. West. Pharmacol. Soc. 1988, 31, 251–253. [Google Scholar] [PubMed]
- Chepelinsky, A.B. Structural function of MIP/aquaporin 0 in the eye lens; genetic defects lead to congenital inherited cataracts. Handb. Exp. Pharmacol. 2009, 265–297. [Google Scholar] [CrossRef]
- Yasui, M.; Kwon, T.H.; Knepper, M.A.; Nielsen, S.; Agre, P. Aquaporin-6: An intracellular vesicle water channel protein in renal epithelia. Proc. Natl. Acad. Sci. USA 1999, 96, 5808–5813. [Google Scholar] [CrossRef] [PubMed]
- Anthony, T.L.; Brooks, H.L.; Boassa, D.; Leonov, S.; Yanochko, G.M.; Regan, J.W.; Yool, A.J. Cloned human aquaporin-1 is a cyclic GMP-gated ion channel. Mol. Pharmacol. 2000, 57, 576–588. [Google Scholar] [PubMed]
- Yu, J.; Yool, A.J.; Schulten, K.; Tajkhorshid, E. Mechanism of gating and ion conductivity of a possible tetrameric pore in aquaporin-1. Structure 2006, 14, 1411–1423. [Google Scholar] [CrossRef] [PubMed]
- Yanochko, G.M.; Yool, A.J. Regulated cationic channel function in Xenopus oocytes expressing Drosophila big brain. J. Neurosci. 2002, 22, 2530–2540. [Google Scholar] [PubMed]
- Yanochko, G.M.; Yool, A.J. Block by extracellular divalent cations of Drosophila big brain channels expressed in xenopus oocytes. Biophys. J. 2004, 86, 1470–1478. [Google Scholar] [CrossRef]
- Byrt, C.S.; Zhao, M.; Kourghi, M.; Bose, J.; Henderson, S.W.; Qiu, J.; Gilliham, M.; Schultz, C.; Schwarz, M.; Ramesh, S.A.; et al. Non-selective cation channel activity of aquaporin AtPIP2;1 regulated by Ca2+ and pH. Plant Cell Environ. 2017, 40, 802–815. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Hojo, S.; Sekine, S.; Sawada, S.; Okumura, T.; Nagata, T.; Shimada, Y.; Tsukada, K. Expression of aquaporin-1 is a poor prognostic factor for stage II and III colon cancer. Mol. Clin. Oncol. 2013, 1, 953–958. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y. Aquaporin-1 activity of plasma membrane affects HT20 colon cancer cell migration. IUBMB Life 2009, 61, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Verkman, A.S. Increased migration and metastatic potential of tumor cells expressing aquaporin water channels. FASEB J. 2006, 20, 1892–1894. [Google Scholar] [CrossRef] [PubMed]
- El Hindy, N.; Rump, K.; Lambertz, N.; Zhu, Y.; Frey, U.H.; Bankfalvi, A.; Siffert, W.; Sure, U.; Peters, J.; Adamzik, M.; et al. The functional aquaporin 1-783G/C-polymorphism is associated with survival in patients with glioblastoma multiforme. J. Surg. Oncol. 2013, 108, 492–498. [Google Scholar] [CrossRef] [PubMed]
- El Hindy, N.; Bankfalvi, A.; Herring, A.; Adamzik, M.; Lambertz, N.; Zhu, Y.; Siffert, W.; Sure, U.; Sandalcioglu, I.E. Correlation of aquaporin-1 water channel protein expression with tumor angiogenesis in human astrocytoma. Anticancer Res. 2013, 33, 609–613. [Google Scholar] [PubMed]
- Kourghi, M.; Pei, J.V.; De Ieso, M.L.; Flynn, G.; Yool, A.J. Bumetanide derivatives AQB007 and AQB011 selectively block the aquaporin-1 ion channel conductance and slow cancer cell migration. Mol. Pharmacol. 2016, 89, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Dorward, H.S.; Du, A.; Bruhn, M.A.; Wrin, J.; Pei, J.V.; Evdokiou, A.; Price, T.J.; Yool, A.J.; Hardingham, J.E. Pharmacological blockade of aquaporin-1 water channel by AQB013 restricts migration and invasiveness of colon cancer cells and prevents endothelial tube formation in vitro. J. Exp. Clin. Cancer Res. 2016, 35, 36. [Google Scholar] [CrossRef] [PubMed]
- Migliati, E.; Meurice, N.; DuBois, P.; Fang, J.S.; Somasekharan, S.; Beckett, E.; Flynn, G.; Yool, A.J. Inhibition of aquaporin-1 and aquaporin-4 water permeability by a derivative of the loop diuretic bumetanide acting at an internal pore-occluding binding site. Mol. Pharmacol. 2009, 76, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.V.; Kourghi, M.; De Ieso, M.L.; Campbell, E.M.; Dorward, H.S.; Hardingham, J.E.; Yool, A.J. Differential inhibition of water and ion channel activities of mammalian aquaporin-1 by two structurally related bacopaside compounds derived from the medicinal plant bacopa monnieri. Mol. Pharmacol. 2016, 90, 496–507. [Google Scholar] [CrossRef] [PubMed]
- Doherty, D.; Jan, L.Y.; Jan, Y.N. The Drosophila neurogenic gene big brain, which encodes a membrane-associated protein, acts cell autonomously and can act synergistically with Notch and Delta. Development 1997, 124, 3881–3893. [Google Scholar] [PubMed]
- Rao, Y.; Bodmer, R.; Jan, L.Y.; Jan, Y.N. The big brain gene of Drosophila functions to control the number of neuronal precursors in the peripheral nervous system. Development 1992, 116, 31–40. [Google Scholar] [PubMed]
- Soto, G.; Alleva, K.; Amodeo, G.; Muschietti, J.; Ayub, N.D. New insight into the evolution of aquaporins from flowering plants and vertebrates: Orthologous identification and functional transfer is possible. Gene 2012, 503, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Monneuse, J.M.; Sugano, M.; Becue, T.; Santoni, V.; Hem, S.; Rossignol, M. Towards the profiling of the Arabidopsis thaliana plasma membrane transportome by targeted proteomics. Proteomics 2011, 11, 1789–1797. [Google Scholar] [CrossRef] [PubMed]
- Javot, H.; Lauvergeat, V.; Santoni, V.; Martin-Laurent, F.; Guclu, J.; Vinh, J.; Heyes, J.; Franck, K.I.; Schaffner, A.R.; Bouchez, D.; et al. Role of a single aquaporin isoform in root water uptake. Plant Cell 2003, 15, 509–522. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, O.; Reshetnyak, G.; Grondin, A.; Saijo, Y.; Leonhardt, N.; Maurel, C.; Verdoucq, L. Aquaporins facilitate hydrogen peroxide entry into guard cells to mediate ABA- and pathogen-triggered stomatal closure. Proc. Natl. Acad. Sci. USA 2017, 114, 9200–9205. [Google Scholar] [CrossRef] [PubMed]
- Pou, A.; Jeanguenin, L.; Milhiet, T.; Batoko, H.; Chaumont, F.; Hachez, C. Salinity-mediated transcriptional and post-translational regulation of the Arabidopsis aquaporin PIP2;7. Plant Mol. Biol. 2016, 92, 731–744. [Google Scholar] [CrossRef] [PubMed]
- Boursiac, Y.; Chen, S.; Luu, D.T.; Sorieul, M.; van den Dries, N.; Maurel, C. Early effects of salinity on water transport in Arabidopsis roots. Molecular and cellular features of aquaporin expression. Plant Physiol. 2005, 139, 790–805. [Google Scholar] [CrossRef] [PubMed]
- Ascher, P.; Nowak, L. The role of divalent cations in the N-methyl-d-aspartate responses of mouse central neurones in culture. J. Physiol. 1988, 399, 247–266. [Google Scholar] [CrossRef] [PubMed]
- Gerbeau, P.; Amodeo, G.; Henzler, T.; Santoni, V.; Ripoche, P.; Maurel, C. The water permeability of Arabidopsis plasma membrane is regulated by divalent cations and ph. Plant J. 2002, 30, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, J.C.; Furman, R.E. Divalent effects on cGMP-activated currents in excised patches from amphibian photoreceptors. J. Membr. Biol. 1993, 131, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Jan, Y.N.; Jan, L.Y. Control of rectification and permeation by residues in two distinct domains in an inward rectifier K+ channel. Neuron 1995, 14, 1047–1054. [Google Scholar] [CrossRef]
- Armstrong, C.M.; Cota, G. Calcium block of Na+ channels and its effect on closing rate. Proc. Natl. Acad. Sci. USA 1999, 96, 4154–4157. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Lagunas, F.; Melishchuk, A.; Armstrong, C.M. Block of shaker potassium channels by external calcium ions. Proc. Natl. Acad. Sci. USA 2003, 100, 347–351. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, J.F.; Nowak, L.M. Mechanisms of blockade of excitatory amino acid receptor channels. Trends Pharmacol. Sci. 1990, 11, 167–172. [Google Scholar] [CrossRef]
- Nichols, C.G.; Lopatin, A.N. Inward rectifier potassium channels. Annu. Rev. Physiol. 1997, 59, 171–191. [Google Scholar] [CrossRef] [PubMed]
- Boassa, D.; Stamer, W.D.; Yool, A.J. Ion channel function of aquaporin-1 natively expressed in choroid plexus. J. Neurosci. 2006, 26, 7811–7819. [Google Scholar] [CrossRef] [PubMed]
- Verdoucq, L.; Grondin, A.; Maurel, C. Structure-function analysis of plant aquaporin AtPIP2;1 gating by divalent cations and protons. Biochem. J. 2008, 415, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Demidchik, V.; Tester, M. Sodium fluxes through nonselective cation channels in the plasma membrane of protoplasts from Arabidopsis roots. Plant Physiol. 2002, 128, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, K. New members of mammalian aquaporins: AQP10-AQP12. In Aquaporins; Beitz, E., Ed.; Springer: Berlin, Germany, 2009; pp. 251–262. [Google Scholar]
- Finn, R.N.; Chauvigne, F.; Hlidberg, J.B.; Cutler, C.P.; Cerda, J. The lineage-specific evolution of aquaporin gene clusters facilitated tetrapod terrestrial adaptation. PLoS ONE 2014, 9, e113686. [Google Scholar] [CrossRef] [PubMed]
- Campbell, E.M.; Birdsell, D.N.; Yool, A.J. The activity of human aquaporin 1 as a cGMP-gated cation channel is regulated by tyrosine phosphorylation in the carboxyl-terminal domain. Mol. Pharmacol. 2012, 81, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Weaver, C.D.; Shomer, N.H.; Louis, C.F.; Roberts, D.M. Nodulin 26, a nodule-specific symbiosome membrane protein from soybean, is an ion channel. J. Biol. Chem. 1994, 269, 17858–17862. [Google Scholar] [PubMed]
- Anthony, T.L.; Fujino, H.; Pierce, K.L.; Yool, A.J.; Regan, J.W. Differential regulation of Ca2+-dependent Cl− currents by FP prostanoid receptor isoforms in Xenopus oocytes. Biochem. Pharmacol. 2002, 63, 1797–1806. [Google Scholar] [CrossRef]
- Miledi, R. A calcium-dependent transient outward current in Xenopus laevis oocytes. Proc. R. Soc. Lond B Biol. Sci. 1982, 215, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Leonetti, M.D.; Pico, A.R.; Hsiung, Y.; MacKinnon, R. Structure of the human BK channel Ca2+-activation apparatus at 3.0 Å resolution. Science 2010, 329, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Tornroth-Horsefield, S.; Wang, Y.; Hedfalk, K.; Johanson, U.; Karlsson, M.; Tajkhorshid, E.; Neutze, R.; Kjellbom, P. Structural mechanism of plant aquaporin gating. Nature 2006, 439, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Preston, G.M.; Jung, J.S.; Guggino, W.B.; Agre, P. The mercury-sensitive residue at cysteine-189 in the chip28 water channel. J. Biol. Chem. 1993, 268, 17–20. [Google Scholar] [PubMed]
- Preston, G.M.; Carroll, T.P.; Guggino, W.B.; Agre, P. Appearance of water channels in Xenopus oocytes expressing red cell chip28 protein. Science 1992, 256, 385–387. [Google Scholar] [CrossRef] [PubMed]
- Byrt, C.S.; Xu, B.; Krishnan, M.; Lightfoot, D.J.; Athman, A.; Jacobs, A.K.; Watson-Haigh, N.S.; Plett, D.; Munns, R.; Tester, M.; et al. The Na+ transporter, TAHKT1;5-D, limits shoot Na+ accumulation in bread wheat. Plant J. 2014, 80, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Findlay, G.P. Membranes and the electrophysiology of turgor regulation. Funct. Plant Physiol. 2001, 28, 619–636. [Google Scholar] [CrossRef]
- Grondin, A.; Rodrigues, O.; Verdoucq, L.; Merlot, S.; Leonhardt, N.; Maurel, C. Aquaporins contribute to ABA-triggered stomatal closure through OST1-mediated phosphorylation. Plant Cell 2015, 27, 1945–1954. [Google Scholar] [CrossRef] [PubMed]
- MacRobbie, E.A. Control of volume and turgor in stomatal guard cells. J. Membr. Biol. 2006, 210, 131–142. [Google Scholar] [CrossRef] [PubMed]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kourghi, M.; Nourmohammadi, S.; Pei, J.V.; Qiu, J.; McGaughey, S.; Tyerman, S.D.; Byrt, C.S.; Yool, A.J. Divalent Cations Regulate the Ion Conductance Properties of Diverse Classes of Aquaporins. Int. J. Mol. Sci. 2017, 18, 2323. https://doi.org/10.3390/ijms18112323
Kourghi M, Nourmohammadi S, Pei JV, Qiu J, McGaughey S, Tyerman SD, Byrt CS, Yool AJ. Divalent Cations Regulate the Ion Conductance Properties of Diverse Classes of Aquaporins. International Journal of Molecular Sciences. 2017; 18(11):2323. https://doi.org/10.3390/ijms18112323
Chicago/Turabian StyleKourghi, Mohamad, Saeed Nourmohammadi, Jinxin V. Pei, Jiaen Qiu, Samantha McGaughey, Stephen D. Tyerman, Caitlin S. Byrt, and Andrea J. Yool. 2017. "Divalent Cations Regulate the Ion Conductance Properties of Diverse Classes of Aquaporins" International Journal of Molecular Sciences 18, no. 11: 2323. https://doi.org/10.3390/ijms18112323
APA StyleKourghi, M., Nourmohammadi, S., Pei, J. V., Qiu, J., McGaughey, S., Tyerman, S. D., Byrt, C. S., & Yool, A. J. (2017). Divalent Cations Regulate the Ion Conductance Properties of Diverse Classes of Aquaporins. International Journal of Molecular Sciences, 18(11), 2323. https://doi.org/10.3390/ijms18112323






