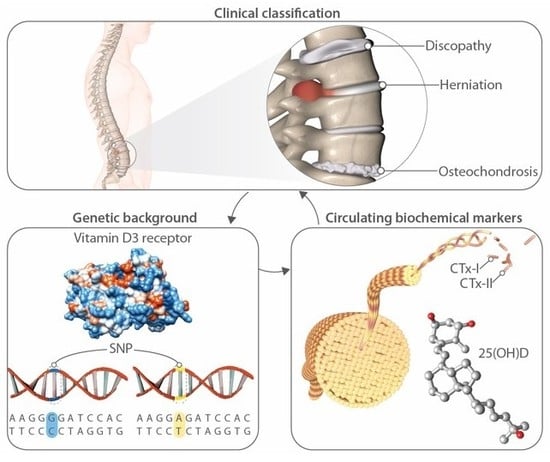
High Levels of Circulating Type II Collagen Degradation Marker (CTx-II) Are Associated with Specific VDR Polymorphisms in Patients with Adult Vertebral Osteochondrosis
Abstract
:1. Introduction
2. Results
2.1. Genotypes and Alleles Frequencies in the Study Population
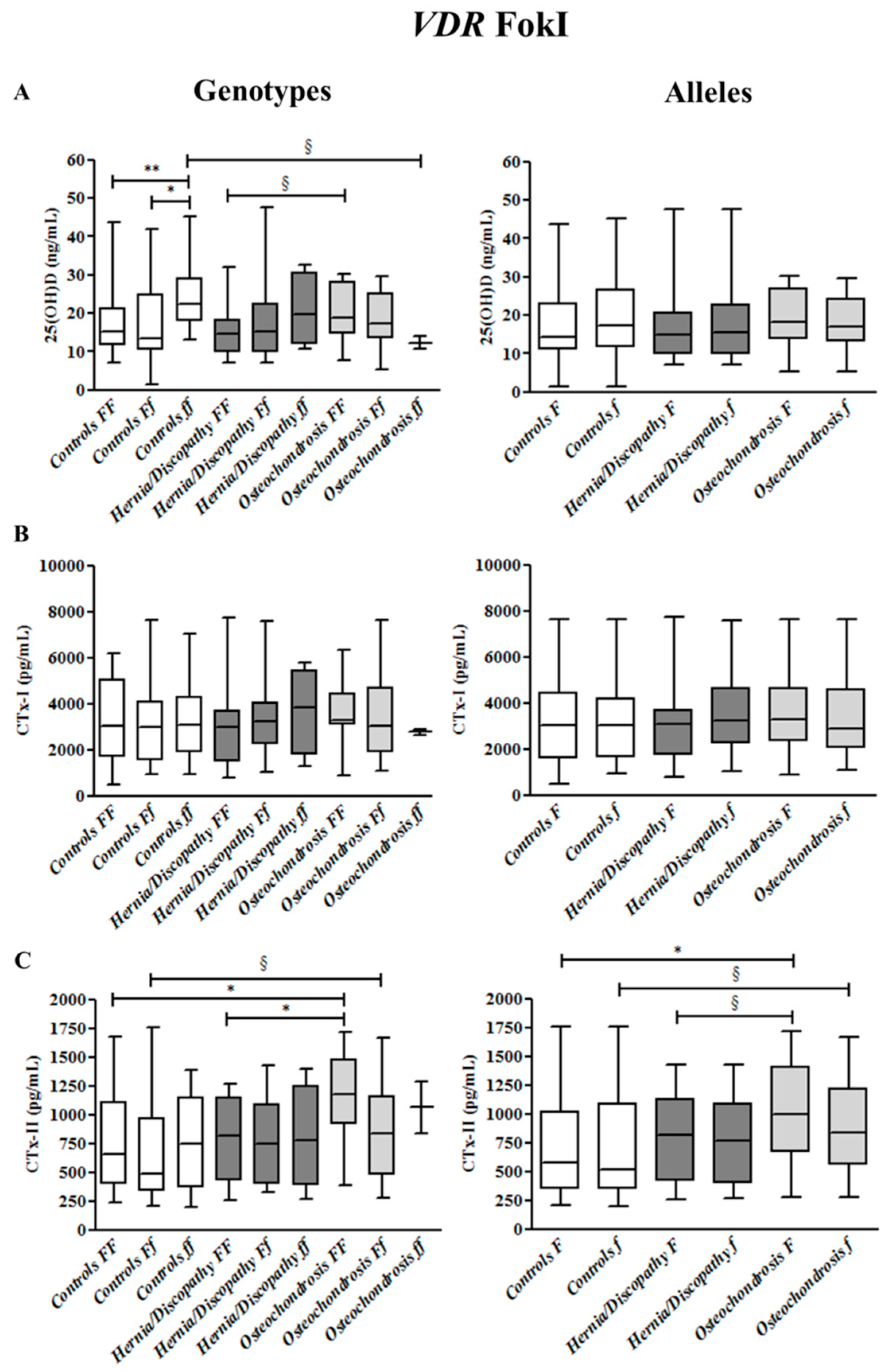
2.2. FokI Polymorphism, and 25(OH)D, CTx-I and CTx-II Circulating Concentrations
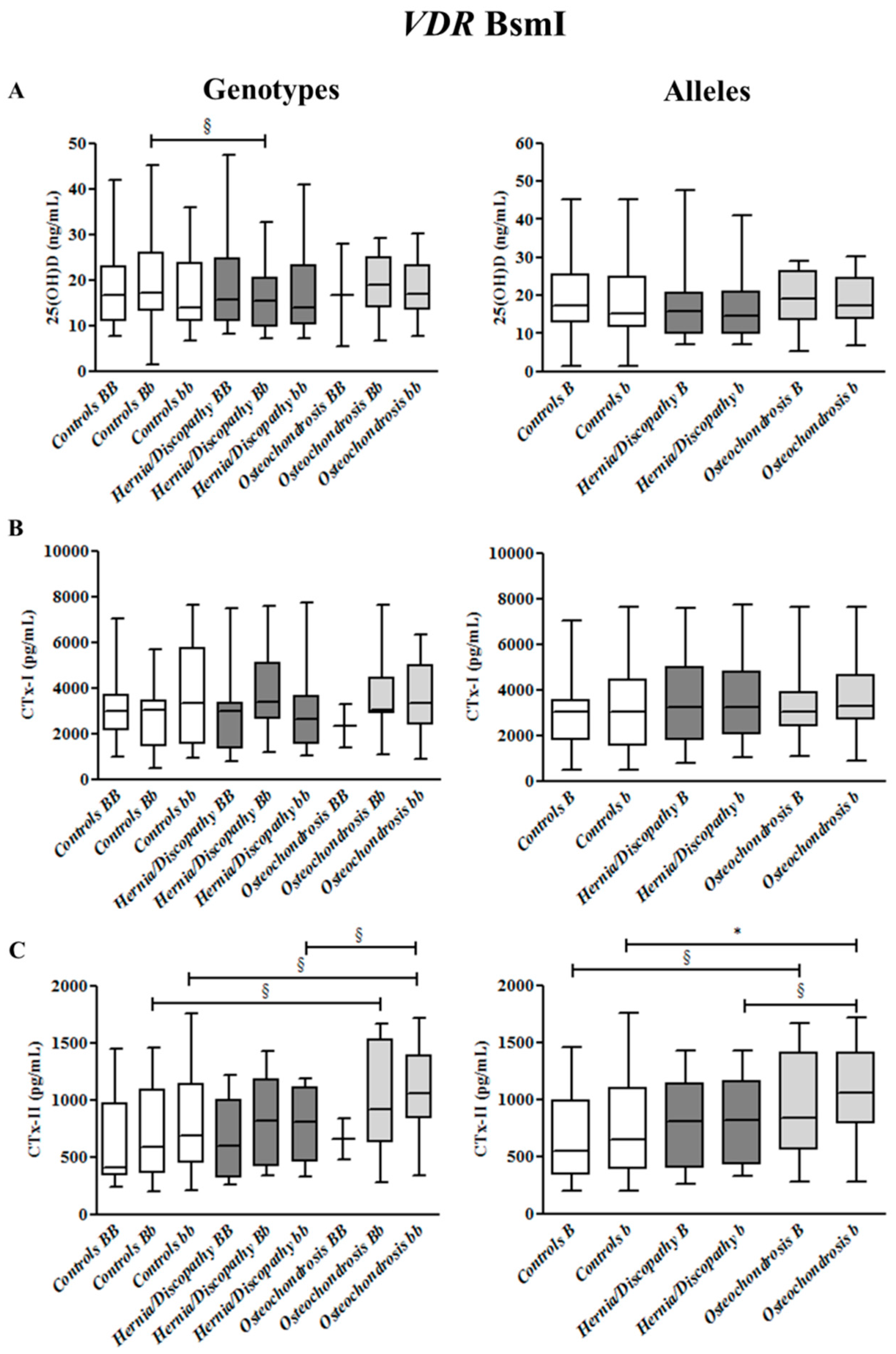
2.3. BsmI Polymorphism, and CTx-I and CTx-II Circulating Concentrations
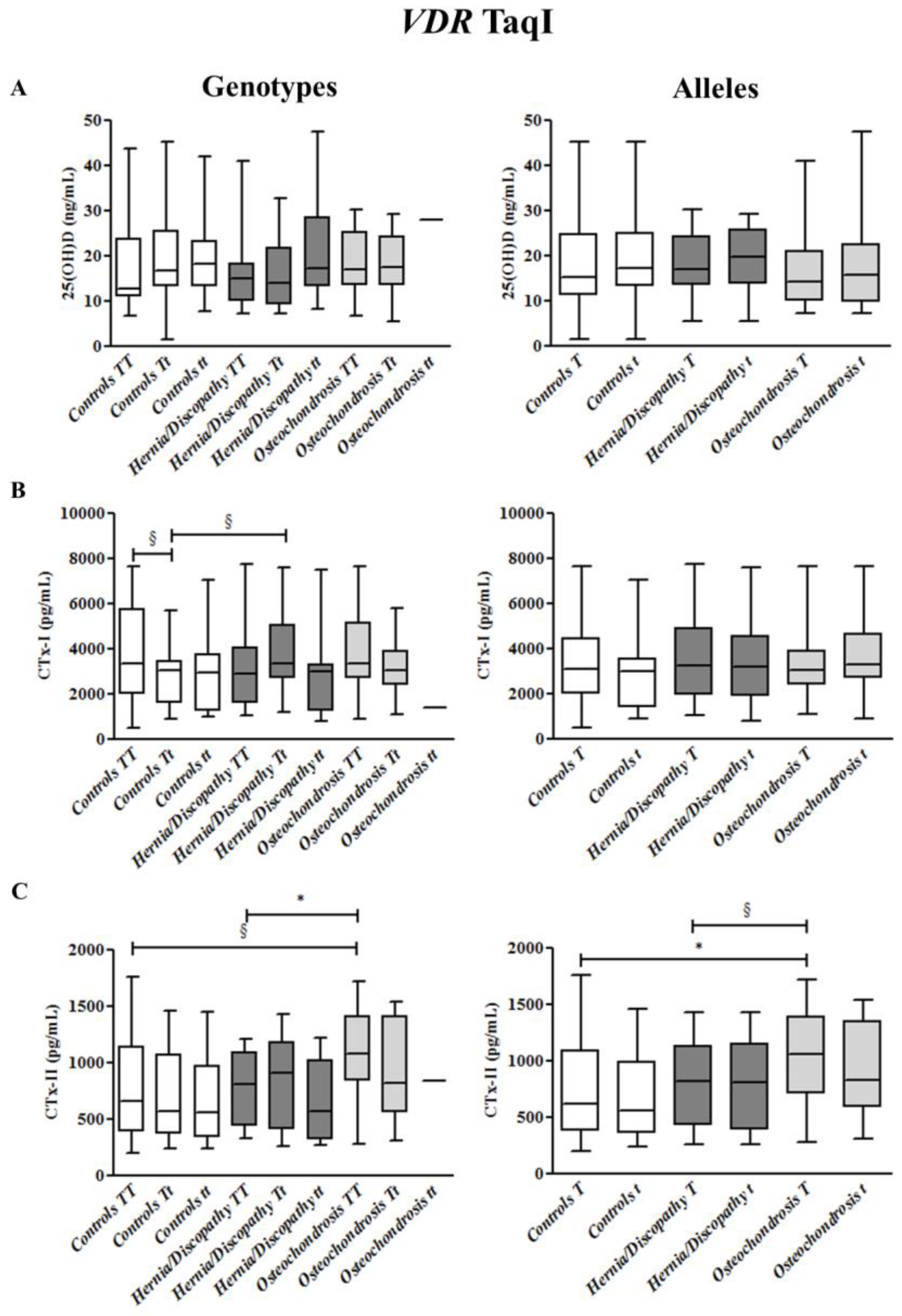
2.4. TaqI Polymorphism, and CTx-I and CTx-II Circulating Concentrations
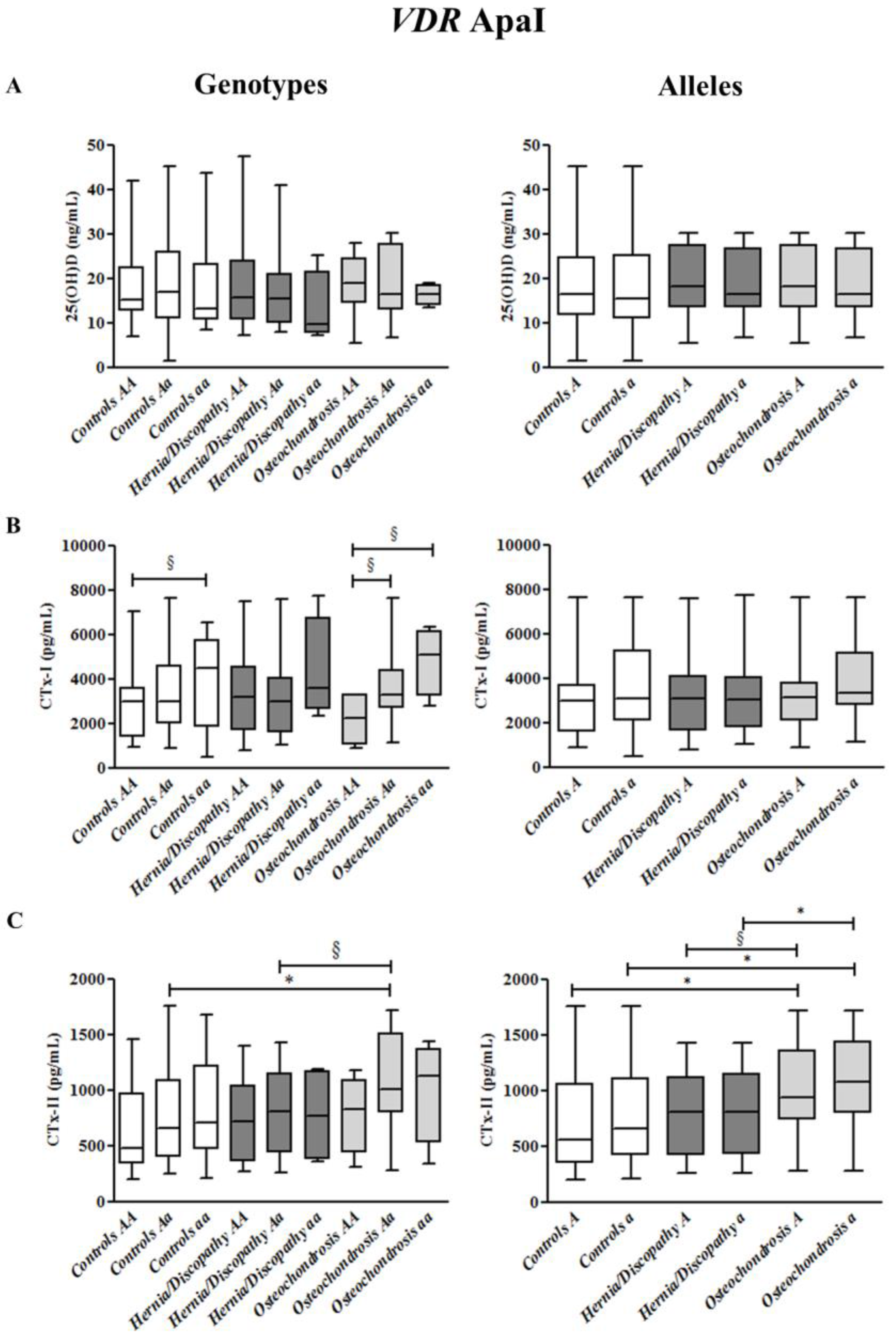
2.5. ApaI Polymorphism, and CTx-I and CTx-II Circulating Concentrations
2.6. Summary for the Association between CTx-II Circulating Concentrations and VDR Polymorphisms
3. Discussion
4. Materials and Methods
4.1. Subjects and Clinical Evaluation
4.2. Samples Collection
4.3. Analysis of Genotypes
4.4. Assessment of Circulating Levels of 25(OH)D, CTx-I and CTx-II
4.5. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Colombini, A.; Brayda-Bruno, M.; Lombardi, G.; Croiset, S.J.; Vrech, V.; Maione, V.; Banfi, G.; Cauci, S. FokI polymorphism in the vitamin D receptor gene (VDR) and its association with lumbar spine pathologies in the Italian population: A case-control study. PLoS ONE 2014, 9, e97027. [Google Scholar] [CrossRef] [PubMed]
- Colombini, A.; Brayda-Bruno, M.; Ferino, L.; Lombardi, G.; Maione, V.; Banfi, G.; Cauci, S. Gender differences in the VDR-FokI polymorphism and conventional non-genetic risk factors in association with lumbar spine pathologies in an Italian case-control study. Int. J. Mol. Sci. 2015, 16, 3722–3739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aufdermaur, M. Juvenile kyphosis (Scheuermann’s disease): Radiography, histology, and pathogenesis. Clin. Orthop. Relat. Res. 1981, 154, 166–174. [Google Scholar]
- Scheuermann, H.W. The classic: Kyphosis dorsalis juvenilis. Clin. Orthop. Relat. Res. 1977, 128, 5–7. [Google Scholar] [CrossRef]
- Lesoin, F.; Leys, D.; Rousseaux, M.; Dubois, F.; Villette, L.; Pruvo, J.P.; Petit, H.; Jomin, M. Thoracic disk herniation and Scheuermann’s disease. Eur. Neurol. 1987, 26, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, S.L.; Roach, J.; Herring, J.A. Lumbar Scheuermann’s. A clinical series and classification. Spine 1987, 12, 929–932. [Google Scholar] [CrossRef] [PubMed]
- Tallroth, K.; Schlenzka, D. Spinal stenosis subsequent to juvenile lumbar osteochondrosis. Skelet. Radiol. 1990, 19, 203–205. [Google Scholar] [CrossRef]
- Trotta, A.; Corrado, A.; Soragnese, M.F.; Santoro, N.; Cantatore, F.P. Adult Scheuermann’s disease as cause of mechanic dorsalgia. Reumatismo 2008, 60, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Tribus, C.B. Scheuermann’s kyphosis in adolescents and adults: Diagnosis and management. J. Am. Acad. Orthop. Surg. 1998, 6, 36–43. [Google Scholar] [CrossRef] [PubMed]
- McFadden, K.D.; Taylor, J.R. End-plate lesions of the lumbar spine. Spine 1989, 14, 867–869. [Google Scholar] [CrossRef] [PubMed]
- Lowe, T.G. Scheuermann disease. J. Bone Jt. Surg. Am. Vol. 1990, 72, 940–945. [Google Scholar] [CrossRef]
- Liu, N.; Guo, X.; Chen, Z.; Qi, Q.; Li, W.; Guo, Z.; Zeng, Y.; Sun, C.; Liu, Z. Radiological signs of Scheuermann disease and low back pain: Retrospective categorization of 188 hospital staff members with 6-year follow-up. Spine 2014, 39, 1666–1675. [Google Scholar] [CrossRef] [PubMed]
- Stoddard, A.; Osborn, J.F. Scheuermann’s disease or spinal osteochondrosis: Its frequency and relationship with spondylosis. J. Bone Jt. Surg. Br. Vol. 1979, 61, 56–58. [Google Scholar]
- Aufdermaur, M.; Spycher, M. Pathogenesis of osteochondrosis juvenilis Scheuermann. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 1986, 4, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Dommisse, G.F. The vulnerable, rapidly growing thoracic spine of the adolescent. S. Afr. Med. J. S.-Afr. Tydskr. Geneeskd. 1990, 78, 211–213. [Google Scholar]
- Graat, H.C.; van Rhijn, L.W.; Schrander-Stumpel, C.T.; van Ooij, A. Classical Scheuermann disease in male monozygotic twins: Further support for the genetic etiology hypothesis. Spine 2002, 27, E485–E487. [Google Scholar] [CrossRef] [PubMed]
- Damborg, F.; Engell, V.; Andersen, M.; Kyvik, K.O.; Thomsen, K. Prevalence, concordance, and heritability of Scheuermann kyphosis based on a study of twins. J. Bone Jt. Surg. Am. Vol. 2006, 88, 2133–2136. [Google Scholar]
- Halal, F.; Gledhill, R.B.; Fraser, C. Dominant inheritance of Scheuermann’s juvenile kyphosis. Am. J. Dis. Child. 1978, 132, 1105–1107. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, L.; Sillence, D. Familial Scheuermann disease: A genetic and linkage study. J. Med. Genet. 1992, 29, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Colombini, A.; Cauci, S.; Lombardi, G.; Lanteri, P.; Croiset, S.; Brayda-Bruno, M.; Banfi, G. Relationship between vitamin D receptor gene (VDR) polymorphisms, vitamin D status, osteoarthritis and intervertebral disc degeneration. J. Steroid Biochem. Mol. Biol. 2013, 138, 24–40. [Google Scholar] [CrossRef] [PubMed]
- Brayda-Bruno, M.; Vigano, M.; Cauci, S.; Vitale, J.A.; de Girolamo, L.; De Luca, P.; Lombardi, G.; Banfi, G.; Colombini, A. Plasma vitamin D and osteo-cartilaginous markers in Italian males affected by intervertebral disc degeneration: Focus on seasonal and pathological trend of type II collagen degradation. Clin. Chim. Acta Int. J. Clin. Chem. 2017, 471, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Amizuka, N.; Ozawa, H. Intracellular localization and translocation of 1 alpha, 25-dihydroxyvitamin D3 receptor in osteoblasts. Arch. Histol. Cytol. 1992, 55, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Gruber, H.E.; Hoelscher, G.; Ingram, J.A.; Chow, Y.; Loeffler, B.; Hanley, E.N., Jr. 1,25(OH)2-vitamin D3 inhibits proliferation and decreases production of monocyte chemoattractant protein-1, thrombopoietin, VEGF, and angiogenin by human annulus cells in vitro. Spine 2008, 33, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Colombini, A.; Lanteri, P.; Lombardi, G.; Grasso, D.; Recordati, C.; Lovi, A.; Banfi, G.; Bassani, R.; Brayda-Bruno, M. Metabolic effects of vitamin D active metabolites in monolayer and micromass cultures of nucleus pulposus and annulus fibrosus cells isolated from human intervertebral disc. Int. J. Biochem. Cell Biol. 2012, 44, 1019–1030. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhao, S.; Niu, F.; Bi, G.B. Association between vitamin D receptor gene polymorphisms and intervertebral disc degeneration: A meta-analysis. J. Orthop. Sci. 2017, 22, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Qin, Z.L.; Zong, S.H.; He, M.L.; Zhan, X.L.; Xiao, Z.M.; Wei, Q.J. Vitamin D receptor gene polymorphisms and lumbar disc degeneration: A systematic review and meta-analysis. Eur. Spine J. 2017, 26, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Colombini, A.; Brayda-Bruno, M.; Lombardi, G.; Croiset, S.J.; Ceriani, C.; Buligan, C.; Barbina, M.; Banfi, G.; Cauci, S. BsmI, ApaI and TaqI Polymorphisms in the Vitamin D Receptor Gene (VDR) and Association with Lumbar Spine Pathologies: An Italian Case-Control Study. PLoS ONE 2016, 11, e0155004. [Google Scholar] [CrossRef] [PubMed]
- Colombini, A.; Lombardi, G.; Corsi, M.M.; Banfi, G. Pathophysiology of the human intervertebral disc. Int. J. Biochem. Cell Biol. 2008, 40, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Collins, F.S.; Varmus, H. A new initiative on precision medicine. N. Engl. J. Med. 2015, 372, 793–795. [Google Scholar] [CrossRef] [PubMed]
- Rescigno, T.; Micolucci, L.; Tecce, M.F.; Capasso, A. Bioactive nutrients and nutrigenomics in age-related diseases. Molecules 2017, 22, 105. [Google Scholar] [CrossRef] [PubMed]
- Lanteri, P.; Lombardi, G.; Colombini, A.; Banfi, G. Vitamin D in exercise: Physiologic and analytical concerns. Clin. Chim. Acta Int. J. Clin. Chem. 2013, 415, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Uitterlinden, A.G.; Fang, Y.; Van Meurs, J.B.; Pols, H.A.; Van Leeuwen, J.P. Genetics and biology of vitamin D receptor polymorphisms. Gene 2004, 338, 143–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marques, V.V.; Aguiar, J.P.N.; Donizetti, S.T.; de Oliveira, J.; Marques, P.C.A.; Vitor, F.A.; Manoukian, F.N. Genetic polymorphisms of vitamin D metabolism genes and serum level of vitamin D in colorectal cancer. Int. J. Biol. Markers 2017. [Google Scholar] [CrossRef] [PubMed]
- Videman, T.; Gibbons, L.E.; Battie, M.C.; Maravilla, K.; Vanninen, E.; Leppavuori, J.; Kaprio, J.; Peltonen, L. The relative roles of intragenic polymorphisms of the vitamin d receptor gene in lumbar spine degeneration and bone density. Spine 2001, 26, E7–E12. [Google Scholar] [CrossRef] [PubMed]
- La Marra, F.; Stinco, G.; Buligan, C.; Chiriaco, G.; Serraino, D.; Di Loreto, C.; Cauci, S. Immunohistochemical evaluation of vitamin D receptor (VDR) expression in cutaneous melanoma tissues and four VDR gene polymorphisms. Cancer Biol. Med. 2017, 14, 162–175. [Google Scholar] [PubMed]
- Sugiyama, T.; Kusuhara, S.; Chung, T.K.; Yonekura, H.; Azem, E.; Hayakawa, T. Effects of 25-hydroxy-cholecalciferol on the development of osteochondrosis in swine. Anim. Sci. J. 2013, 84, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Long, M.D.; Campbell, M.J. Integrative genomic approaches to dissect clinically-significant relationships between the VDR cistrome and gene expression in primary colon cancer. J. Steroid Biochem. Mol. Biol. 2017, 173, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.K.; van den Berg, P.R.; Long, M.D.; Vreugdenhil, A.; Grieshober, L.; Ochs-Balcom, H.M.; Wang, J.; Delcambre, S.; Heikkinen, S.; Carlberg, C.; et al. Integration of VDR genome wide binding and GWAS genetic variation data reveals co-occurrence of VDR and NF-kappaB binding that is linked to immune phenotypes. BMC Genom. 2017, 18, 132. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef] [PubMed]
- Soto, A.M.; Longo, G.; Noble, D. Preface to from the century of the genome to the century of the organism: New theoretical approaches. Prog. Biophys. Mol. Biol. 2016, 122, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Pani, M.A.; Knapp, M.; Donner, H.; Braun, J.; Baur, M.P.; Usadel, K.H.; Badenhoop, K. Vitamin D receptor allele combinations influence genetic susceptibility to type 1 diabetes in Germans. Diabetes 2000, 49, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Lutsey, P.L.; Parrinello, C.M.; Misialek, J.R.; Hoofnagle, A.N.; Henderson, C.M.; Laha, T.J.; Michos, E.D.; Eckfeldt, J.H.; Selvin, E. Short-term Variability of Vitamin D-Related Biomarkers. Clin. Chem. 2016, 62, 1647–1653. [Google Scholar] [CrossRef] [PubMed]
| VDR | Healthy Controls | All LBP Cases | Hernia/Discopathy | Osteochondrosis | |
|---|---|---|---|---|---|
| n = 79 (%) | n = 79 (%) | n = 53 (%) | n = 26 (%) | ||
| genotypes | FF | 32 (40.5) | 36 (45.6) | 27 (50.9) | 9 (34.6) |
| Ff | 34 (43.0) | 37 (46.8) | 22 (41.5) | 15 (57.7) | |
| ff | 13 (16.5) | 6 (7.6) | 4 (7.5) | 2 (7.7) | |
| BB | 14 (17.7) | 14 (17.7) | 12 (22.6) | 2 (7.7) | |
| Bb | 39 (49.4) | 36 (45.6) | 25 (47.2) | 11 (42.3) | |
| bb | 26 (32.9) | 29 (36.7) | 16 (30.2) | 13 (50.0) | |
| TT | 26 (32.9) | 38 (48.1) | 22 (41.5) | 16 (61.5) | |
| Tt | 40 (50.6) | 31 (39.2) | 22 (41.5) | 9 (34.6) | |
| tt | 13 (16.5) | 10 (12.7) | 9 (17.0) | 1 (3.8) | |
| AA | 32 (40.5) | 25 (31.7) | 19 (35.8) | 6 (23.1) | |
| Aa | 34 (43.0) ^ | 46 (58.2) | 30 (56.6) | 16 (61.5) ^ | |
| aa | 13 (16.5) | 8 (10.1) | 4 (7.5) | 4 (15.3) | |
| alleles | F | 98/158 (62.0) | 109/158 (69.0) | 76/106 (71.7) | 33/52 (63.5) |
| f | 60/158 (38.0) | 49/158 (31.0) | 30/106 (28.3) | 19/52 (36.5) | |
| B | 67/158 (42.4) ^ | 64/158 (40.5) | 49/101 (48.5) | 15/52 (28.8) ^ | |
| b | 91/158 (57.6) ^ | 94/158 (59.5) | 52/101 (51.5) | 37/52 (71.2) ^ | |
| T | 92/158 (58.2) | 107/158 (67.7) | 66/106 (62.3) | 41/52 (78.8) | |
| t | 66/158 (41.2) | 51/158 (32.3) | 40/106 (37.7) | 11/52 (21.2) | |
| A | 98/158 (62.0) | 96/158 (60.8) | 68/106 (64.1) | 28/52 (53.8) | |
| a | 60/158 (38.0) | 62/158 (39.2) | 38/106 (35.9) | 24/52 (46.2) | |
| VDR Polymorphisms | Genotypes | Alleles | |||
|---|---|---|---|---|---|
| FokI | FF * | Ff § | ff # | F *,^ | f § |
| BsmI | BB | Bb § | bb §,^ | B § | b * |
| TaqI | TT §,^ | Tt | tt # | T *,^ | t # |
| ApaI | AA # | Aa *,^ | aa | A * | a * |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cauci, S.; Viganò, M.; De Girolamo, L.; De Luca, P.; Perucca Orfei, C.; Banfi, G.; Lombardi, G.; Brayda-Bruno, M.; Colombini, A. High Levels of Circulating Type II Collagen Degradation Marker (CTx-II) Are Associated with Specific VDR Polymorphisms in Patients with Adult Vertebral Osteochondrosis. Int. J. Mol. Sci. 2017, 18, 2073. https://doi.org/10.3390/ijms18102073
Cauci S, Viganò M, De Girolamo L, De Luca P, Perucca Orfei C, Banfi G, Lombardi G, Brayda-Bruno M, Colombini A. High Levels of Circulating Type II Collagen Degradation Marker (CTx-II) Are Associated with Specific VDR Polymorphisms in Patients with Adult Vertebral Osteochondrosis. International Journal of Molecular Sciences. 2017; 18(10):2073. https://doi.org/10.3390/ijms18102073
Chicago/Turabian StyleCauci, Sabina, Marco Viganò, Laura De Girolamo, Paola De Luca, Carlotta Perucca Orfei, Giuseppe Banfi, Giovanni Lombardi, Marco Brayda-Bruno, and Alessandra Colombini. 2017. "High Levels of Circulating Type II Collagen Degradation Marker (CTx-II) Are Associated with Specific VDR Polymorphisms in Patients with Adult Vertebral Osteochondrosis" International Journal of Molecular Sciences 18, no. 10: 2073. https://doi.org/10.3390/ijms18102073