Angiogenesis Inhibitors in NSCLC
Abstract
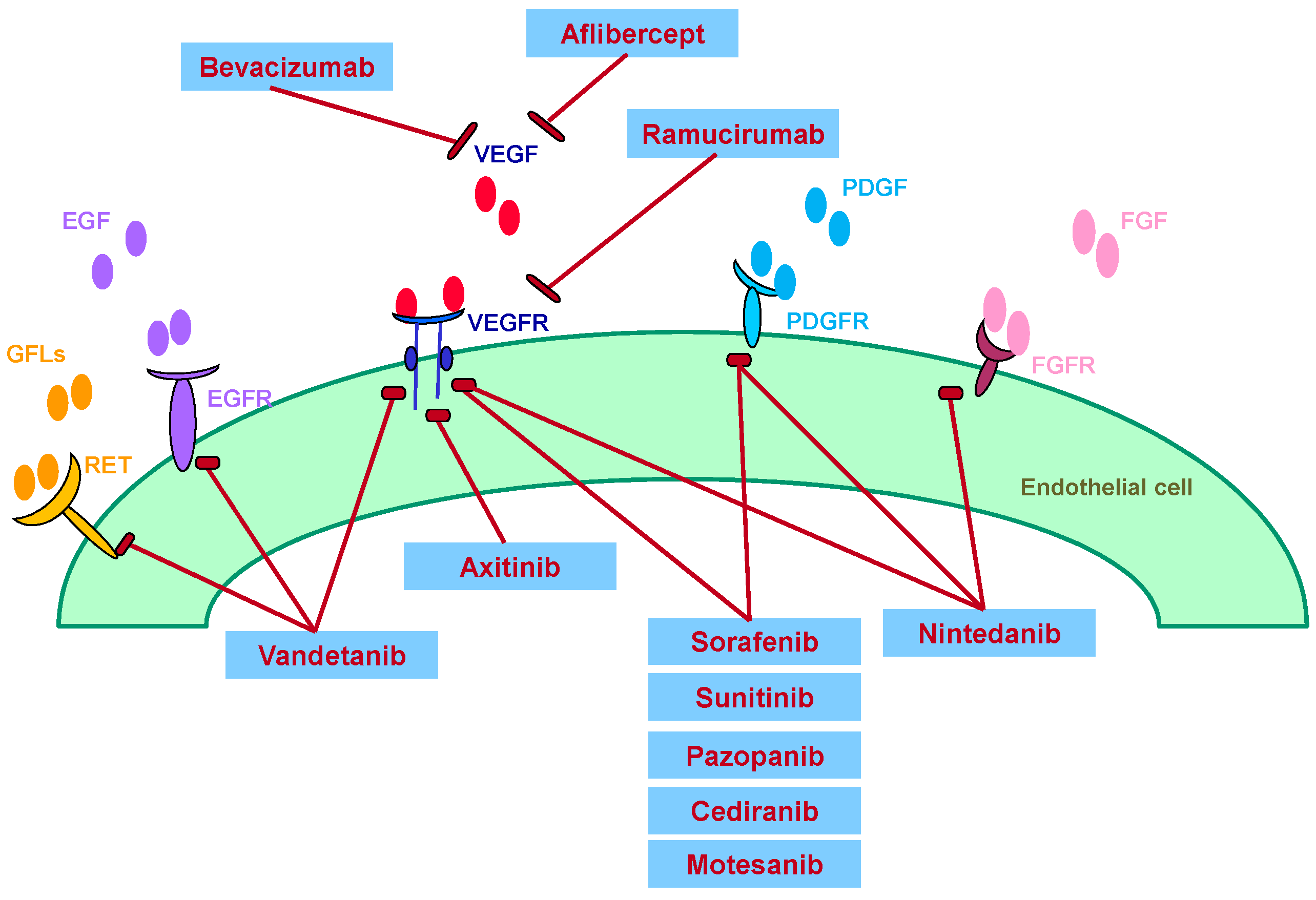
:1. Introduction
2. Bevacizumab
3. Sunitinib, Sorafenib, Vandetanib
4. VEGF Trap: Aflibercept
5. Nintedanib
6. Ramucirumab
7. Discussion and Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, J.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Novello, S.; Barlesi, F.; Califano, R.; Cufer, T.; Ekman, S.; Levra, M.G.; Kerr, K.; Popat, S.; Reck, M.; Senan, S.; et al. ESMO Guidelines Committee.Metastatic Non-Small-Cell Lung Cancer: ESMO Clinical Practice Guidelines. Ann. Oncol. 2016, 27, V1–V27. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J. Tumour angiogenesis: Therapeutic implications. N. Engl. J. Med. 1971, 285, 1182–1186. [Google Scholar] [PubMed]
- Ferrara, N. Molecular and biological properties of vascular endothelial growth factor. J. Mol. Med. 1999, 77, 527–543. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Gerber, H.P.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.R.; Wu, Y.M.; Lin, S.F. The protein tyrosine kinase family of the human genome. Oncogene 2000, 19, 5548–5557. [Google Scholar] [CrossRef] [PubMed]
- Hall, R.D.; Lee, T.M.; Haggstrom, D.E.; Gentzler, R.D. Angiogenesis inhibition as a therapeutic strategy in non-small cell lung cancer (NSCLC). Transl. Lung Cancer Res. 2015, 4, 515–523. [Google Scholar] [PubMed]
- Sandler, A.; Gray, R.; Perry, M.C.; Brahmer, J.; Schiller, J.H.; Dowlati, A.; Lilenbaum, R.; Johnson, D.H. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N. Engl. J. Med. 2006, 355, 2542–2550. [Google Scholar] [CrossRef] [PubMed]
- Sandler, A.B.; Yi, J.; Dahlberg, S.; Kolb, M.M.; Wang, L.; Hambleton, J.; Schiller, J.; Johnson, D.H. Treatment outcomes by tumor histology in Eastern Cooperative Group (ECOG) study E4599 of bevacizumab with paclitaxel/carboplatin for advanced non-small cell lung cancer (NSCLC). J. Thorac. Oncol. 2010, 5, 1416–1423. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; von Pawel, J.; Zatloukal, P.; Ramlau, R.; Gorbounova, V.; Hirsh, V.; Leighl, N.; Mezger, J.; Archer, V.; Moore, N.; et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for non-squamous non- small-cell lung cancer: AVAil. J. Clin. Oncol. 2009, 27, 1227–1234. [Google Scholar] [CrossRef] [PubMed]
- Barlesi, F.; Scherpereel, A.; Rittmeyer, A.; Pazzola, A.; Ferrer Tur, N.; Kim, J.H.; Ahn, M.J.; Aerts, J.G.; Gorbunova, V.; Vikström, A.; et al. Randomized phase III trial of maintenance bevacizuma with or without pemetrexed after first-line induction with bevacizumab, cisplatin, and pemetrexed in advanced Non squamous Non–Small-Cell Lung Cancer: AVAPERL (MO22089). J. Clin. Oncol. 2013, 31, 3004–3011. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.D.; Bonomi, P.; Socinski, M.A.; Govindan, R.; Hong, S.; Obasaju, C.; Pennella, E.J.; Girvan, A.C.; Guba, S.C. Treatment rationale and study design for the PointBreak study: A randomized, open-label phase III study of pemetrexed/carboplatin/bevacizumab followed by maintenance pemetrexed/bevacizumab versus paclitaxel/carboplatin/bevacizumab followed by maintenance bevacizumab in patients with stage IIIB or IV nonsquamous non-small-cell lung cancer. Clin. Lung Cancer 2009, 10, 252–256. [Google Scholar] [PubMed]
- Galetta, D.; Cinieri, S.; Pisconti, S.; Gebbia, V.; Morabito, A.; Borsellino, N.; Maiello, E.; Febbraro, A.; Catino, A.; Rizzo, P.; et al. Cisplatin/pemetrexed followed by maintenance pemetrexed versus carboplatin/paclitaxel/bevacizumab followed by maintenance bevacizumab in advanced nonsquamous lungcancer: The GOIM (Gruppo Oncologico Italia Meridionale) ERACLE phase III randomized trial. Clin. Lung Cancer 2015, 16, 262–273. [Google Scholar] [CrossRef] [PubMed]
- Soria, J.C.; Mauguen, A.; Reck, M.; Sandler, A.B.; Saijo, N.; Johnson, D.H.; Burcoveanu, D.; Fukuoka, M.; Bess, B.; Pignon, J.P.; et al. Systematic review and meta-analysis of randomised, phase II/III trials adding bevacizumab to platinum-based chemotherapy as first-line treatment in patients with advanced non-small-cell lung cancer. Ann. Oncol. 2013, 24, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Behera, M.; Pillai, R.M.; Owonikoko, T.K.; Kim, S.; Steuer, C.; Chen, Z.; Saba, N.F.; Belani, C.P.; Khuri, F.R.; Ramalingam, S.S. Bevacizumab in combination with taxane versus non-taxane containing regimens for advanced/metastatic Nonsquamous Non–Small-Cell Lung Cancer: A systematic review. J. Thorac. Oncol. 2015, 10, 1142–1147. [Google Scholar] [CrossRef] [PubMed]
- Dahlberg, S.E.; Ramalingam, S.S.; Belani, C.P.; Schiller, J.H. A randomized phase III study of maintenance therapy with bevacizumab (B), pemetrexed (Pm), or a combination of bevacizumab and pemetrexed (BPm) following carboplatin, paclitaxel and bevacizumab (PCB) for advanced nonsquamous NSCLC: ECOG trial 5508 (NCT01107626). J. Clin. Oncol. 2011, 29, TPS218. [Google Scholar]
- Herbst, R.S.; Ansari, R.; Bustin, F.; Flynn, P.; Hart, L.; Otterson, G.A.; Vlahovic, G.; Soh, C.H.; O′Connor, P.; Hainsworth, J. Efficacy of bevacizumab plus erlotinib versus erlotinib alone in advanced non-small-cell lung cancer after failure of standard first-line chemotherapy (BeTa): A double-blind, placebo-controlled, phase 3 trial. Lancet 2011, 377, 1846–1854. [Google Scholar] [CrossRef]
- Seto, T.; Kato, T.; Nishio, M.; Goto, K.; Atagi, S.; Hosomi, Y.; Yamamoto, N.; Hida, T.; Maemondo, M.; Nakagawa, N.; et al. Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harbouring EGFR mutations (JO25567): An open-label, randomised, multicentre, phase 2 study. Lancet Oncol. 2014, 15, 1236–1244. [Google Scholar] [CrossRef]
- Rosell, R.; Dafni, U.; Felip, E.; Curioni-Fontecedro, A.; Gautschi, O.; Peters, S.; Massutí, B.; Palmero, R.; Aix, S.P.; Carcereny, E.; et al. Erlotinib and bevacizumab in patients with advanced non-small-cell lung cancer and activating EGFR mutations (BELIEF): An international, multicentre, single-arm, phase 2 trial. Lancet Respir. Med. 2017, 5, 435–444. [Google Scholar] [CrossRef]
- Gridelli, C.; Rossi, A.; Ciardiello, F.; de Marinis, F.; Crinò, L.; Morabito, A.; Morgillo, F.; Montanino, A.; Daniele, G.; Piccirillo, M.C.; et al. BEVERLY: Rationale and design of a randomized open-label phase III trial comparing bevacizumab plus erlotinib versus erlotinib alone as first-line treatment of patients with egfr-mutated advanced nonsquamous Non-Small-Cell Lung Cancer. Clin. Lung Cancer 2016, 17, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Grothey, A.; Sugrue, M.M.; Purdie, D.M.; Dong, W.; Sargent, D.; Hedrick, E.; Kozloff, M. Bevacizumab beyond first progression is associated with prolonged overall survival in metastatic colorectal cancer: Results from a large observational cohort study (BRiTE). J. Clin. Oncol. 2008, 26, 5326–5334. [Google Scholar] [CrossRef] [PubMed]
- Cohn, A.L.; Bekaii-Saab, T.; Bendell, J.C.; Hurwitz, H.; Kozloff, M.; Roach, N.; Tezcan, H.; Feng, S.; Sing, A.; Grothey, A.; et al. on behalf of the ARIES Study Investigators Clinical outcomes in bevacizumab (BV)-treatedpatients (pts) with metastatic colorectal cancer (mCRC): Results from ARIES observational cohort study (OCS) and confirmation of BRiTE data on BV beyond progression (BBP). J. Clin. Oncol. 2010, 28, 3596. [Google Scholar]
- Bennouna, J.; Sastre, J.; Arnold, D.; Österlund, P.; Greil, R.; Van Cutsem, E.; von Moos, R.; Viéitez, J.M.; Bouché, O.; Borg, C.; et al. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): A randomised phase 3 trial. Lancet Oncol. 2013, 14, 29–37. [Google Scholar] [CrossRef]
- Gridelli, C.; Bennouna, J.; de Castro, J.; Griesinger, F.; Grossi, F.; Thatcher, N.; Ohe, Y.; Perez-Moreno, P.D.; Langer, C.J. AvaALL: Open-label randomized phase IIIb trial evaluating the efficacy and safety of standard of care with or without continuous bevacizumab (BV) treatment beyond disease progression in patients (pts) with advanced nonsquamous non-small cell lung cancer (NSCLC) after first-line (1L) treatment with BV plus platinum-doublet chemotherapy (CT). J. Clin. Oncol. 2012. [Google Scholar] [CrossRef]
- Bennouna, J.; de Castro, J.; Gridelli, C.; Griesinger, F.; Grossi, F.; Langer, C.J.; Ohe, Y.; Syrigos, K.N.; Thatcher, N.; Das-Gupta, A.; et al. Efficacy and Safety Results from AvaALL: An Open-label, randomized phase III trial of Standard of Care (SOC) with or without continuous Bevacizumab (Bev) treatment beyond progression (PD) in patients (pts) with Advanced Non-Small Cell Lung cancer (NSCLC) progressing after first-line Bev and chemotherapy (chemo). J. Clin. Oncol. 2017, 35, 9004. [Google Scholar]
- Wozniak, A.J.; Moon, J.; Thomas, C.R., Jr.; Kelly, K.; Mack, P.C.; Gaspar, L.E.; Raben, D.; Fitzgerald, T.J.; Pandya, K.J.; Gandara, D.R. A pilot trial of cisplatin/etoposide/radiotherapy followed by consolidation docetaxel and the combination of bevacizumab (NSC-704865) in patients with inoperable locally advanced stage III non-small-cell lung cancer: SWOG S0533. Clin. Lung Cancer 2015, 16, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Wakelee, H.A.; Dahlberg, S.; Keller, S.M.; Tester, W.J.; Gandara, D.R.; Graziano, S.L.; Adjei, A.A.; Leighl, N.B.; Butts, C.A.; Seena, C.; et al. E1505: Adjuvant chemotherapy +/− bevacizumab for early stage NSCLC—Outcomes based on chemotherapy subsets. J. Clin. Oncol. 2016, 34, 8507. [Google Scholar]
- Herbst, R.S.; Sun, Y.; Eberhardt, W.E.; Germonpré, P.; Saijo, N.; Zhou, C.; Wang, J.; Li, L.; Kabbinavar, F.; Ichinose, Y.; et al. Vandetanib plus docetaxel versus docetaxel as second-line treatment for patients with advanced non-small-cell lung cancer (ZODIAC): A double-blind, randomised, phase 3 trial. Lancet Oncol. 2010, 11, 619–626. [Google Scholar] [CrossRef]
- De Boer, R.H.; Arrieta, Ó.; Yang, C.H.; Gottfried, M.; Chan, V.; Raats, J.; de Marinis, F.; Abratt, R.P.; Wolf, J.; Blackhall, F.H.; et al. Vandetanib plus pemetrexed for the second-line treatment of advanced non-small-cell lung cancer: A randomized, double-blind phase III trial. J. Clin. Oncol. 2011, 29, 1067–1074. [Google Scholar] [CrossRef] [PubMed]
- Natale, R.B.; Thongprasert, S.; Greco, F.A.; Thomas, M.; Tsai, C.M.; Sunpaweravong, P.; Ferry, D.; Mulatero, C.; Whorf, R.; Thompson, J.; et al. Phase III trial of vandetanib compared with erlotinib in patients with previously treated advanced non-small-cell lung cancer. J. Clin. Oncol. 2011, 29, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Hirsh, V.; Park, K.; Qin, S.; Blajman, C.R.; Perng, R.P.; Chen, Y.M.; Emerson, L.; Langmuir, P.; Manegold, C. Vandetanib Versus placebo in patients with advanced non-small-cell lung cancer after prior therapy with an epidermal growth factor receptor tyrosine kinase inhibitor: A randomized, double-blind phase III trial (ZEPHYR). J. Clin. Oncol. 2012, 30, 1114–1121. [Google Scholar] [CrossRef] [PubMed]
- Scagliotti, G.; Novello, S.; von Pawel, J.; Reck, M.; Rodrigues Pereira, J.; Thomas, M.; Abrano Miziara, J.M.; Balint, B.; de Marinis, F.; Keller, A.; et al. Phase III study of carboplatin and paclitaxel alone or with sorafenib in advanced non-small-cell lung cancer. J. Clin. Oncol. 2010, 28, 1835–1842. [Google Scholar] [CrossRef] [PubMed]
- Paz-Ares, L.G.; Biesma, B.; Heigener, D.; von Pawel, J.; Eisen, T.; Bennouna, J.; Zhang, L.; Liao, M.; Sun, Y.; Gans, S.; et al. Phase III, randomized, double-blind, placebo-controlled trial of gemcitabine/cisplatin alone or with sorafenib for the first-line treatment of advanced, nonsquamous non-small-cell lung cancer. J. Clin. Oncol. 2012, 30, 3084–3092. [Google Scholar] [CrossRef] [PubMed]
- Paz-Ares, L.; Hirsh, V.; Zhang, L.; de Marinis, F.; Yang, J.C.; Wakelee, H.A.; Seto, T.; Wu, Y.L.; Novello, S.; Juhász, E.; et al. Monotherapy Administration of Sorafenib in Patients with Non-Small Cell Lung Cancer (MISSION) Trial: A Phase III, Multicenter, Placebo-Controlled Trial of Sorafenib in Patients with Relapsed or Refractory Predominantly Nonsquamous Non-Small-Cell Lung Cancer after 2 or 3 Previous Treatment Regimens. J. Thorac. Oncol. 2015, 10, 1745–1753. [Google Scholar] [PubMed]
- Scagliotti, G.V.; Krzakowski, M.; Szczesna, A.; Strausz, J.; Makhson, A.; Reck, M.; Wierzbicki, R.F.; Albert, I.; Thomas, M.; Miziara, J.E.; et al. Sunitinib plus erlotinib versus placebo plus erlotinib in patients with previously treated advanced non-small-cell lung cancer: A phase III trial. J. Clin. Oncol. 2012, 30, 2070–2078. [Google Scholar] [CrossRef] [PubMed]
- Laurie, S.A.; Solomon, B.J.; Seymour, L.; Ellis, P.M.; Goss, G.D.; Shepherd, F.A.; Boyer, M.J.; Arnold, A.M.; Clingan, P.; Laberge, F.; et al. Randomised, double-blind trial of carboplatin and paclitaxel with daily oral cediranib or placebo in patients with advanced non-small cell lung cancer: NCIC Clinical Trials Group study BR29. Eur. J. Cancer. 2014, 50, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Scagliotti, G.V.; Vynnychenko, I.; Park, K.; Ichinose, Y.; Kubota, K.; Blackhall, F.; Pirker, R.; Galiulin, R.; Ciuleanu, T.E.; Sydorenko, O.; et al. International, randomized, placebo-controlled, double-blind phase III study of motesanib plus carboplatin/paclitaxel in patients with advanced nonsquamous non-small-cell lung cancer: MONET1. J. Clin. Oncol. 2012, 30, 2829–2836. [Google Scholar] [CrossRef] [PubMed]
- Ramlau, R.; Gorbunova, V.; Ciuleanu, T.E.; Novello, S.; Ozguroglu, M.; Goksel, T.; Baldotto, C.; Bennouna, J.; Shepherd, F.A.; Le-Guennec, S.; et al. Aflibercept and Docetaxel versus Docetaxel alone after platinum failure in patients with advanced or metastatic non-small-cell lung cancer: A randomized, controlled phase III trial. J. Clin. Oncol. 2012, 30, 3640–3647. [Google Scholar] [CrossRef] [PubMed]
- Natale, R.B.; Bodkin, D.; Govindan, R.; Sleckman, B.G.; Rizvi, N.A.; Capó, A.; Germonpré, P.; Eberhardt, W.E.; Stockman, P.K.; Kennedy, S.J.; et al. Vandetanib versus gefitinib in patients with advanced non-small-cell lung cancer: Results from a two-part, double-blind, randomized phase II study. J. Clin. Oncol. 2009, 27, 2523–2539. [Google Scholar] [CrossRef] [PubMed]
- Socinski, M.A.; Wang, X.F.; Baggstrom, M.Q.; Gu, L.; Stinchcombe, T.E.; Edelman, M.J.; Baker, S.J.R.; Mannuel, H.D.; Crawford, J.; Vokes, E.E.; et al. Sunitinib switch maintenance in advanced non-small cell lung cancer (NSCLC): An ALLIANCE (CALGB 30607), randomized, placebo-controlled phase III trial. J. Clin. Oncol. 2014, 32, 8040. [Google Scholar] [CrossRef]
- Spigel, D.; Burris, H.A.; Greco, F.A.; Shih, K.C.; Lipman, A.J.; Flora, D.B.; Daniel, D.B.; Waterhouse, D.M.; Haymach, J.V.; Hainsworth, J.D. A randomized phase II study of pazopanib or placebo in combination with erlotinib in patients with advanced non-small-cell lung cancer. J. Thorac. Oncol. 2012, 7, S208. [Google Scholar] [CrossRef] [PubMed]
- Belani, C.P.; Yamamoto, N.; Bondarenko, I.M.; Poltoratskiy, A.; Novello, S.; Tang, J.; Bycott, P.; Niethammer, A.G.; Ingrosso, A.; Kim, S.; et al. Randomized phase II study of pemetrexed/cisplatin with or without axitinib for non-squamous non-small-cell lung cancer. BMC Cancer 2014, 14, 290. [Google Scholar] [CrossRef] [PubMed]
- Twelves, C.; Chmielowska, E.; Havel, L.; Popat, S.; Swieboda-Sadlej, A.; Sawrycki, P.; Bycott, P.; Ingrosso, A.; Kim, S.; Williamset, J.A.; et al. Randomised phase II study of axitinib or bevacizumab combined with paclitaxel/carboplatin as first-line therapy for patients with advanced non-small-cell lung cancer. Ann. Oncol. 2014, 25, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Leighl, N.B.; Raez, L.E.; Besse, B.; Rosen, P.J.; Barlesi, F.; Massarelli, E.; Gabrail, N.; Hart, L.L.; Albain, K.S.; Berkowitz, L.; et al. A multicenter, phase 2 study of vascular endothelial growth factor trap (Aflibercept) in platinum- and erlotinib-resistant adenocarcinoma of the lung. J. Thorac. Oncol. 2010, 5, 1054–1059. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Modiano, M.R.; Neal, J.W.; Brahmer, J.R.; Rigas, J.R.; Jotte, R.M.; Leighl, N.B.; Riess, J.W.; Kuo, C.J.; Liu, L.; et al. A phase II multicentre study of ziv-aflibercept in combination with cisplatin and pemetrexed in patients with previously untreated advanced/metastatic non-squamous non-small cell lung cancer. Br. J. Cancer 2014, 110, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Hilberg, F.; Roth, G.J.; Krssak, M.; Kautschitsch, S.; Sommergruber, W.; Tontsch-Grunt, U.; Garin-Chesa, P.; Bader, G.; Zoephel, A.; et al. BIBF 1120: Triple angiokinase inhibitor with sustained receptor blockade and good antitumor efficacy. Cancer Res. 2008, 68, 4774–4782. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.J.; Heckel, A.; Colbatzky, F.; Handschuh, S.; Kley, J.; Lehmann-Lintz, T.; Lotz, R.; Tontsch-Grunt, U.; Walter, R.; Hilberg, F. Design, synthesis, and evaluation of indolinones as triple angiokinase inhibitors and the discovery of a highly specific 6-methoxycarbonyl-substituted indolinone (BIBF 1120). J. Med. Chem. 2009, 52, 4466–4480. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Kaiser, R.; Mellemgaard, A.; Douillard, J.Y.; Orlov, S.; Krzakowski, M.; von Pawel, J.; Gottfried, M.; Bondarenko, I.; Liao, M.; et al. Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): A phase 3, double-blind, randomised controlled trial. Lancet Oncol. 2014, 2, 143–155. [Google Scholar] [CrossRef]
- Hanna, N.; Kaiser, R.; Sullivan, R.N.; Aren, O.R.; Ahn, M.J.; Tiangco, B.; Zvirbule, Z.; Barrios, C.H.; Demirkazik, A.; Gaschler-Markefski, B.; et al. A multicenter, randomized, double-blind, phase III study of nintedanib plus pemetrexed versus placebo plus pemetrexed in patients with advanced nonsquamous non-small cell lung cancer (NSCLC) after failure of first-line chemotherapy (LUME-Lung 2). J. Clin. Oncol. 2013, 31, 8034. [Google Scholar]
- Garon, E.B.; Ciuleanu, T.E.; Arrieta, O.; Prabhash, K.; Syrigos, K.N.; Goksel, T.; Park, K.; Gorbunova, V.; Kowalyszyn, R.D.; Pikiel, J.; et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): A multicentre, double-blind, randomized phase 3 trial. Lancet 2014, 84, 665–673. [Google Scholar] [CrossRef]
- Spratlin, J.L.; Cohen, R.B.; Eadens, M.; Gore, L.; Camidge, D.R.; Diab, S.; Leong, S.; O′Bryant, C.; Chow, L.Q.M.; Serkova, N.J.; et al. Phase I pharmacologic and biologic study of ramucirumab (IMC-1121B), a fully human immunoglobulin G1 monoclonal antibody targeting the vascular endothelial growth factor receptor-2. J. Clin. Oncol. 2010, 28, 780–787. [Google Scholar] [CrossRef] [PubMed]
- Camidge, D.R.; Berge, E.M.; Doebele, R.C.; Ballas, M.S.; Jahan, T.; Haigentz, M., Jr.; Hoffman, D.; Spicer, J.; West, H.; Lee, P.; et al. A phase II, open-label study of ramucirumab in combination with paclitaxel and carboplatin as first-line therapy in patients with stage IIIB/IV non-small-cell lung cancer. J. Thorac. Oncol. 2014, 9, 1532–1539. [Google Scholar] [CrossRef] [PubMed]
- Doebele, R.C.; Spigel, D.; Tehfe, M.; Thomas, S.; Reck, M.; Verma, S.; Eakle, J.; Bustin, F.; Goldschmidt, J., Jr.; et al. Phase 2, randomized, open-label study of ramucirumab in combination with first-line pemetrexed and platinum chemotherapy in patients with nonsquamous, advanced/metastatic non-small cell lung cancer. Cancer 2015, 121, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Dowlati, A.; Gray, R.; Sandler, A.B.; Schiller, J.H.; Johnson, D.H. Cell adhesion molecules, vascular endothelial growth factor, and basic fibroblast growth factor in patients with non-small cell lung cancer treated with chemotherapy with or without bevacizumab-an Eastern Cooperative Oncology Group Study. Clin. Cancer Res. 2008, 14, 1407–1412. [Google Scholar] [CrossRef] [PubMed]
- Chaft, J.E.; Rusch, V.; Ginsberg, M.S.; Paik, P.K.; Finley, D.J.; Kris, M.G.; Price, K.A.; Azzoli, C.G.; Fury, M.G.; Riely, G.J.; et al. Phase II trial of neoadjuvant bevacizumab plus chemotherapy and adjuvant bevacizumab in patients with resectable nonsquamous Non-Small-Cell lung Cancers. J. Thorac. Oncol. 2013, 8, 1085–1090. [Google Scholar] [CrossRef] [PubMed]
- Manegold, C.; Dingemans, A.C.; Gray, J.E.; Nakagawa, K.; Nicolson, M.; Peters, S.; Reck, M.; Wu, Y.L.; Brustugun, O.T.; Crinò, L.; et al. The Potential of Combined Immunotherapy and Antiangiogenesis for the Synergistic Treatment of Advanced NSCLC. J. Thorac. Oncol. 2017, 12, 194–207. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, N.A.; Antonia, S.J.; Shepherd, F.A.; Chow, L.Q.; Goldman, J.; Shen, Y.; Chen, A.C.; Gettinger, S. Nivolumab (Anti-PD-1; BMS-936558, ONO-4538) maintenance as monotherapy or in combination with bevacizumab (BEV) for Non-Small Cell Lung Cancer (NSCLC) previously treated with chemotherapy. Radiat. Oncol. 2014, 90, S32. [Google Scholar] [CrossRef]
- Herbst, R.B.; Bendell, J.C.; Isambert, N.; Calvo, E.; Santana-Davila, R.; Cassier, P.; Perez-Gracia, J.L.; Yang, J.; Rege, J.; Ferry, D.; et al. A phase 1 study of ramucirumab (R) plus pembrolizumab (P) in patients (pts) with advanced gastric or gastroesophageal junction (G/GEJ) adenocarcinoma, nonsmall cell lung cancer (NSCLC), or urothelial carcinoma (UC): Phase 1a results. J. Clin. Oncol. 2016, 34, 3056. [Google Scholar]
| Author and Publication Year | Trial | Setting | Pts | Systemic Treatment | Results |
|---|---|---|---|---|---|
| Sandler, 2006 [8] | E4599 | 1st line Advanced NSCLC | 878 | Bevacizumab + carboplatin/paclitaxel vs. carboplatin/paclitaxe | OS: 12.3 vs. 10.3 months HR 0.79, p = 0.003 PFS: 6.2 vs. 4.5 months HR 0.66, p < 0.001 |
| Reck, 2009 [10] | AVAIL | 1st line Advanced NSCLC | 1043 | Bevacizumab 7,5 + cisplatin/gemcitabine vs. bevacizumab15 + cisplatin/gemcitabine vs. cisplatin/gemcitabine | PFS Beva7.5: 6.8 vs. 6.2 HR 0.75, p = 0.003 Beva 15: 6.6 months HR 0.82, p = 0.03 |
| Barlesi, 2013 [11] | AVAPERL | Maintenance Advanced NSCLC | 253 | Bevacizumab + pemetrexed vs. bevacizumab, maintenance | PFS: 7.4 vs. 3.7 months HR 0.57, p < 0.0001 |
| Patel, 2009 [12] | POINT BREAK | Maintenance Advanced NSCLC | 939 | Pemetrexed + carboplatin + bevacizumab followed by maintenance pemetrexed + bevacizumab vs. paclitaxel + carboplatin + bevacizumab followed by maintenance bevacizumab | OS:12.6 vs. 13.4 months HR 1.00 (p = 0.949) PFS 6.0 vs. 5.6 months HR 0.83, p = 0.012 |
| Galetta, 2015 [13] | ERACLE | Maintenance Advanced NSCLC | 118 | Cisplatin + pemetrexed followed by maintenance pemetrexed vs. paclitaxel + carboplatin and bevacizumab followed by maintenance bevacizumab | QoL HR 0.137, p = 0.078 HR 0.97, p = 0.41 |
| Agent | Trial | Setting | Pts | Systemic Treatment | Results |
|---|---|---|---|---|---|
| Vandetanib | ZODIAC Herbst, 2010 [28] | 2nd line Advanced NSCLC | 1391 | Vandetanib + docetaxel vs. Docetaxel | PFS: 4 vs. 3.2 months HR 0.79, p < 0.0001 RR%: 17 vs. 10, p < 0.001 OS HR 0.91, p = 0.196 |
| ZEAL de Boer, 2011 [29] | 2nd line Advanced NSCLC | 534 | Vendetanib + pemetrexed vs. Pemetrexed | PFS 0.86, p = 0.108 OS HR 0.86, p = 0.219, p < 0.001 | |
| ZEST Natale, 2011 [30] | ≥2nd line Advanced NSCLC | 1240 | Vandetanib vs. erlotinib | PFS: HR 0.98 p = 0.721 OS HR 1.01, p = 0.830 | |
| ZEPHYR Lee, 2012 [31] | ≥2nd line (prior EGFR TKI) Advanced NSCLC | 924 | Vandetanib vs. placebo | OS: 8.5 vs. 7.8 months HR 0.95, p = 0.527 | |
| Sorafenib | ESCAPE Scagliotti 2010 [32] | 1st line Advanced NSCLC | 926 | Sorafenib + carboplatin/paclitaxel vs. carboplatin/paclitaxel | OS: 10.7 vs. 10.6 months HR 1.15, p = 0.915 |
| NEXUS Paz-ares, 2012 [33] | 1st line Advanced NSCLC | 904 | Gemcitabine + cisplatin with Or without sorafenib | OS: 12.4 vs. 12.5 months HR 0.98, p = 0.401 PFS: 6.0 vs. 5.5 months HR 0.83, p = 0.008 | |
| MISSION Paz-ares, 2015 [34] | 3rd or 4th line Advanced NSCLC | 703 | Sorafenib vs. placebo | OS: 8.2 vs. 8.3 months HR 0.99, p = 0.4687 PFS: 2.8 vs. 1.4 months; HR 0.61, p < 0.0001 | |
| Sunitinib | SUN1087 Scagliotti 2012 [35] | Advanced refractory NSCLC | 956 | Sunitinib + erlotinib vs. erlotinib | OS: 9 vs. 8.5 months HR 0.922, p = 0.1388 PFS: 3.6 vs. 2 months HR 0.807, p = 0.0023 RR%: 10.6 vs. 6.9 |
| Cediranib | BR29 Laurie, 2014 [36] | 1st line Advanced NSCLC | 306 | Cediranib + carboplatin/paclitaxel vs. carboplatin/paclitaxel | OS 0.94, p = 0.72 RR%: 52 vs. 34, p = 0.001 |
| Motesanib | MONET-1 Scagliotti JCO 2012 [37] | 1st line Advanced NSCLC nonsquamous | 1090 | Motesanib + carboplatin/paclitaxel vs. carboplatin/paclitaxel | OS: 13 vs. 11 months HR 0.90, p = 0.14 PFS 5.6 vs. 5.4 months, p < 0.001 RR%: 40 vs. 26, p < 0.001 |
| Aflibercept | VITAL Ramlau, 2012 [38] | 2nd line Advanced NSCLC, any histology | 913 | Aflibercept + docetaxel vs. docetaxel | OS: 10.1 vs. 10.4 months, HR 1.01, p = 0.8985 PFS: 5.2 vs. 4.1 months, HR 0.82, p = 0.0035 RR%: 23.3 vs. 8.9, p < 0.001 |
| Agent | Trial | Setting | Pts | Systemic Treatment | Results |
|---|---|---|---|---|---|
| Nintedanib | LUME- Lung-1 Reck, 2014 [48] | 2nd line Advanced NSCLC any histology | 1314 | Docetaxel + nintedanib vs. docetaxel | RR%: 4.7 vs. 3.6 DCR%: 60.2 vs. 44, p < 0.0001 PFS: 3.4 vs. 2.7 months, HR 0.79, p = 0.0019 OS:10.1 vs. 9.1 months *, HR 0.94, p = 0.27 |
| Nintedanib | LUME- Lung-2 Hanna, 2013 [49] | 2nd line Advanced NSCLC Non-squamous histology | 713 | Docetaxel + nintedanib vs. docetaxel | RR%: 9.1 vs. 8.3 DCR%: 60.9 vs. 53.3, p = 0.039 PFS: 4.4 vs. 3.6 months, HR: 0.83, p = 0.04 OS:12.2 vs. 12.7 months, HR: 1.03, p = 0.79 |
| Ramucirumab | REVEL Garon, 2014 [50] | 2nd line Advanced NSCLC, any histology | 1253 | Ramucirumab + docetaxel vs. docetaxel | OS: 10.5 vs. 9.1 months HR 0.86, p = 0.023 PFS: 4.5 vs. 3.0 months HR 0.76, p < 0.0001 |
| Agent | Trial | Author | Setting | Experimental Treatment | Results | Approval |
|---|---|---|---|---|---|---|
| Bevacizumab | E4599 | Sandler, 2006 [8] | 1st line, advanced non-squamous NSCLC | Bevacizumab + carboplatin and paclitaxel | Improvement in OS and PFS | 1st line, advanced, non-squamous NSCLC |
| Nintedanib | LUME- Lung-1 | Reck, 2014 [48] | 2nd line, advanced NSCLC, any histology | Nintedanib + docetaxel | Improvement in PFS (any histology) and OS (non-squamous histology) | 2nd line, advanced non-squamous NSCLC |
| Ramucirumab | REVEL | Garon, 2014 [50] | 2nd line, advanced NSCLC, any histology | Ramucirumab + docetaxel | Improvement in OS and PFS | 2nd line, advanced NSCLC, any histology |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manzo, A.; Montanino, A.; Carillio, G.; Costanzo, R.; Sandomenico, C.; Normanno, N.; Piccirillo, M.C.; Daniele, G.; Perrone, F.; Rocco, G.; et al. Angiogenesis Inhibitors in NSCLC. Int. J. Mol. Sci. 2017, 18, 2021. https://doi.org/10.3390/ijms18102021
Manzo A, Montanino A, Carillio G, Costanzo R, Sandomenico C, Normanno N, Piccirillo MC, Daniele G, Perrone F, Rocco G, et al. Angiogenesis Inhibitors in NSCLC. International Journal of Molecular Sciences. 2017; 18(10):2021. https://doi.org/10.3390/ijms18102021
Chicago/Turabian StyleManzo, Anna, Agnese Montanino, Guido Carillio, Raffaele Costanzo, Claudia Sandomenico, Nicola Normanno, Maria Carmela Piccirillo, Gennaro Daniele, Francesco Perrone, Gaetano Rocco, and et al. 2017. "Angiogenesis Inhibitors in NSCLC" International Journal of Molecular Sciences 18, no. 10: 2021. https://doi.org/10.3390/ijms18102021
APA StyleManzo, A., Montanino, A., Carillio, G., Costanzo, R., Sandomenico, C., Normanno, N., Piccirillo, M. C., Daniele, G., Perrone, F., Rocco, G., & Morabito, A. (2017). Angiogenesis Inhibitors in NSCLC. International Journal of Molecular Sciences, 18(10), 2021. https://doi.org/10.3390/ijms18102021






