Clinical Application of Circulating Tumour Cells in Prostate Cancer: From Bench to Bedside and Back
Abstract
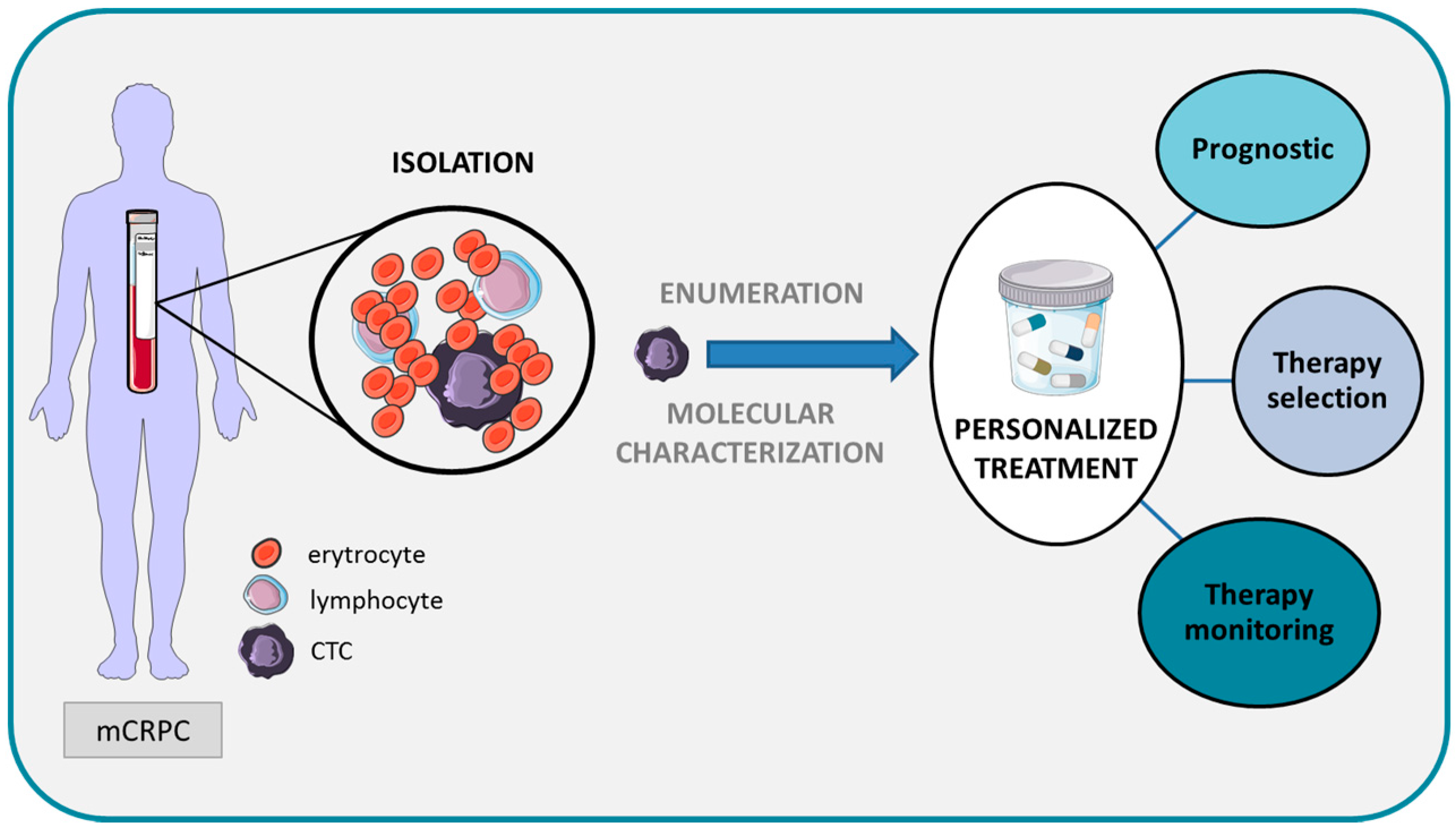
:1. Introduction
2. Current and New Approaches for CTC Detection: Application in PCa
3. Realities and Challenges for CTC Molecular and Functional Characterisation in PCa
3.1. Molecular Profiling
3.2. CTC in Vitro/in Vivo Expansion
4. Clinical Development of CTCs as a Biomarker of PCa
4.1. Requirements for Biomarker Validation
4.2. Obstacles to the Clinical Use of CTCs
4.2.1. Analytical Validation
4.2.2. Clinical Validation
4.2.3. Evidence Supporting the Clinical Use of CTCs in PCa
Localised Disease
Advanced Disease
4.2.4. Challenges to Actual Inclusion of CTC Analyses for Personalising Treatment of PCa
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer statistics, 2013. CA Cancer J. Clin. 2013, 63, 11–30. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, A.; Bellmunt, J.; Bolla, M.; Joniau, S.; Mason, M.; Matveev, V.; Mottet, N.; Schmid, H.P.; van der Kwast, T.; Wiegel, T.; et al. Eau guidelines on prostate cancer. Part 1: Screening, diagnosis, and treatment of clinically localised disease. Eur. Urol. 2011, 59, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Cary, K.C.; Cooperberg, M.R. Biomarkers in prostate cancer surveillance and screening: Past, present, and future. Ther. Adv. Urol. 2013, 5, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Soletormos, G.; Duffy, M.J.; Hayes, D.F.; Sturgeon, C.M.; Barak, V.; Bossuyt, P.M.; Diamandis, E.P.; Gion, M.; Hyltoft-Petersen, P.; Lamerz, R.M.; et al. Design of tumor biomarker-monitoring trials: A proposal by the european group on tumor markers. Clin. Chem. 2013, 59, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.J.; Punt, C.J.; Iannotti, N.; Saidman, B.H.; Sabbath, K.D.; Gabrail, N.Y.; Picus, J.; Morse, M.; Mitchell, E.; Miller, M.C.; et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J. Clin. Oncol. 2008, 26, 3213–3221. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, F.; Yoneda, K.; Kondo, N.; Hashimoto, M.; Takuwa, T.; Matsumoto, S.; Okumura, Y.; Rahman, S.; Tsubota, N.; Tsujimura, T.; et al. Circulating tumor cell as a diagnostic marker in primary lung cancer. Clin. Cancer Res. 2009, 15, 6980–6986. [Google Scholar] [CrossRef] [PubMed]
- Devriese, L.A.; Voest, E.E.; Beijnen, J.H.; Schellens, J.H. Circulating tumor cells as pharmacodynamic biomarker in early clinical oncological trials. Cancer Treat. Rev. 2011, 37, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Danila, D.C.; Heller, G.; Gignac, G.A.; Gonzalez-Espinoza, R.; Anand, A.; Tanaka, E.; Lilja, H.; Schwartz, L.; Larson, S.; Fleisher, M.; et al. Circulating tumor cell number and prognosis in progressive castration-resistant prostate cancer. Clin. Cancer. Res. 2007, 13, 7053–7058. [Google Scholar] [CrossRef] [PubMed]
- De Bono, J.S.; Kristeleit, R.; Tolcher, A.; Fong, P.; Pacey, S.; Karavasilis, V.; Mita, M.; Shaw, H.; Workman, P.; Kaye, S.; et al. Phase I pharmacokinetic and pharmacodynamic study of LAQ824, a hydroxamate histone deacetylase inhibitor with a heat shock protein-90 inhibitory profile, in patients with advanced solid tumors. Clin. Cancer Res. 2008, 14, 6663–6673. [Google Scholar] [CrossRef] [PubMed]
- Scher, H.I.; Jia, X.; de Bono, J.S.; Fleisher, M.; Pienta, K.J.; Raghavan, D.; Heller, G. Circulating tumour cells as prognostic markers in progressive, castration-resistant prostate cancer: A reanalysis of IMMC38 trial data. Lancet Oncol. 2009, 10, 233–239. [Google Scholar] [CrossRef]
- Scher, H.I.; Heller, G.; Molina, A.; Attard, G.; Danila, D.C.; Jia, X.; Peng, W.; Sandhu, S.K.; Olmos, D.; Riisnaes, R.; et al. Circulating tumor cell biomarker panel as an individual-level surrogate for survival in metastatic castration-resistant prostate cancer. J. Clin. Oncol. 2015, 33, 1348–1355. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.J.; Garrett-Mayer, E.; Ou Yang, Y.C.; Carducci, M.A.; Tannock, I.; de Wit, R.; Eisenberger, M. Prostate-specific antigen and pain surrogacy analysis in metastatic hormone-refractory prostate cancer. J. Clin. Oncol. 2007, 25, 3965–3970. [Google Scholar] [CrossRef] [PubMed]
- Sonpavde, G.; Pond, G.R.; Berry, W.R.; de Wit, R.; Eisenberger, M.A.; Tannock, I.F.; Armstrong, A.J. The association between radiographic response and overall survival in men with metastatic castration-resistant prostate cancer receiving chemotherapy. Cancer 2011, 117, 3963–3971. [Google Scholar] [CrossRef] [PubMed]
- De la Taille, A.; Colombel, M.; Muscatelli, B.; Abbou, C.C.; Jouault, H.; Amsallem, S.; Chopin, D. Detection of circulating epithelial cells in prostate cancer. Chirurgie 1997, 122, 268–273. [Google Scholar] [PubMed]
- Helo, P.; Cronin, A.M.; Danila, D.C.; Wenske, S.; Gonzalez-Espinoza, R.; Anand, A.; Koscuiszka, M.; Vaananen, R.M.; Pettersson, K.; Chun, F.K.; et al. Circulating prostate tumor cells detected by reverse transcription-PCR in men with localized or castration-refractory prostate cancer: Concordance with cellsearch assay and association with bone metastases and with survival. Clin. Chem. 2009, 55, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Franklin, W.A.; Glaspy, J.; Pflaumer, S.M.; Jones, R.B.; Hami, L.; Martinez, C.; Murphy, J.R.; Shpall, E.J. Incidence of tumor-cell contamination in leukapheresis products of breast cancer patients mobilized with stem cell factor and granulocyte colony-stimulating factor (G-CSF) or with G-CSF alone. Blood 1999, 94, 340–347. [Google Scholar] [PubMed]
- Shaffer, D.R.; Leversha, M.A.; Danila, D.C.; Lin, O.; Gonzalez-Espinoza, R.; Gu, B.; Anand, A.; Smith, K.; Maslak, P.; Doyle, G.V.; et al. Circulating tumor cell analysis in patients with progressive castration-resistant prostate cancer. Clin. Cancer Res. 2007, 13, 2023–2029. [Google Scholar] [CrossRef] [PubMed]
- Nagrath, S.; Sequist, L.V.; Maheswaran, S.; Bell, D.W.; Irimia, D.; Ulkus, L.; Smith, M.R.; Kwak, E.L.; Digumarthy, S.; Muzikansky, A.; et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature 2007, 450, 1235–1239. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.M.; Xu, S.C.; Li, J.; Han, K.Q.; Pi, H.F.; Zheng, L.; Zuo, G.H.; Huang, X.B.; Li, H.Y.; Zhao, H.Z.; et al. Epithelial-mesenchymal transition markers expressed in circulating tumor cells in hepatocellular carcinoma patients with different stages of disease. Cell Death Dis. 2013, 4, e831. [Google Scholar] [CrossRef] [PubMed]
- Ruscetti, M.; Quach, B.; Dadashian, E.L.; Mulholland, D.J.; Wu, H. Tracking and functional characterization of epithelial-mesenchymal transition and mesenchymal tumor cells during prostate cancer metastasis. Cancer Res. 2015, 75, 2749–2759. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Chen, Z.; Dong, J.; Wei, P.; Hu, R.; Zhou, C.; Sun, N.; Luo, M.; Yang, W.; Yao, R.; et al. Folate receptor-positive circulating tumor cells as a novel diagnostic biomarker in non-small cell lung cancer. Transl. Oncol. 2013, 6, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Cann, G.M.; Gulzar, Z.G.; Cooper, S.; Li, R.; Luo, S.; Tat, M.; Stuart, S.; Schroth, G.; Srinivas, S.; Ronaghi, M.; et al. mRNA-Seq of single prostate cancer circulating tumor cells reveals recapitulation of gene expression and pathways found in prostate cancer. PLoS ONE 2012, 7, e49144. [Google Scholar] [CrossRef] [PubMed]
- Capoun, O.; Mikulova, V.; Jancikova, M.; Honova, H.; Kolostova, K.; Sobotka, R.; Michael, P.; Zima, T.; Hanus, T.; Soukup, V. Prognosis of castration-resistant prostate cancer patients—Use of the ADNAtest system for detection of circulating tumor cells. Anticancer Res. 2016, 36, 2019–2026. [Google Scholar] [PubMed]
- Allard, W.J.; Matera, J.; Miller, M.C.; Repollet, M.; Connelly, M.C.; Rao, C.; Tibbe, A.G.; Uhr, J.W.; Terstappen, L.W. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin. Cancer Res. 2004, 10, 6897–6904. [Google Scholar] [CrossRef] [PubMed]
- Alix-Panabieres, C.; Brouillet, J.P.; Fabbro, M.; Yssel, H.; Rousset, T.; Maudelonde, T.; Choquet-Kastylevsky, G.; Vendrell, J.P. Characterization and enumeration of cells secreting tumor markers in the peripheral blood of breast cancer patients. J. Immunol. Methods 2005, 299, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Farace, F.; Massard, C.; Vimond, N.; Drusch, F.; Jacques, N.; Billiot, F.; Laplanche, A.; Chauchereau, A.; Lacroix, L.; Planchard, D.; et al. A direct comparison of Cellsearch and ISET for circulating tumour-cell detection in patients with metastatic carcinomas. Br. J. Cancer 2011, 105, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Kolostova, K.; Broul, M.; Schraml, J.; Cegan, M.; Matkowski, R.; Fiutowski, M.; Bobek, V. Circulating tumor cells in localized prostate cancer: Isolation, cultivation in vitro and relationship to T-stage and gleason score. Anticancer Res. 2014, 34, 3641–3646. [Google Scholar] [PubMed]
- Gupta, V.; Jafferji, I.; Garza, M.; Melnikova, V.O.; Hasegawa, D.K.; Pethig, R.; Davis, D.W. ApostreamTM, a new dielectrophoretic device for antibody independent isolation and recovery of viable cancer cells from blood. Biomicrofluidics 2012, 6, 24133. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Lin, H.K.; Lu, B.; Williams, A.; Datar, R.; Cote, R.J.; Tai, Y.C. 3D microfilter device for viable circulating tumor cell (CTC) enrichment from blood. Biomed. Microdevices 2011, 13, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Peeters, D.J.; de Laere, B.; van den Eynden, G.G.; van Laere, S.J.; Rothe, F.; Ignatiadis, M.; Sieuwerts, A.M.; Lambrechts, D.; Rutten, A.; van Dam, P.A.; et al. Semiautomated isolation and molecular characterisation of single or highly purified tumour cells from cellsearch enriched blood samples using dielectrophoretic cell sorting. Br. J. Cancer 2013, 108, 1358–1367. [Google Scholar] [CrossRef] [PubMed]
- Saucedo-Zeni, N.; Mewes, S.; Niestroj, R.; Gasiorowski, L.; Murawa, D.; Nowaczyk, P.; Tomasi, T.; Weber, E.; Dworacki, G.; Morgenthaler, N.G.; et al. A novel method for the in vivo isolation of circulating tumor cells from peripheral blood of cancer patients using a functionalized and structured medical wire. Int. J. Oncol. 2012, 41, 1241–1250. [Google Scholar] [PubMed]
- Paterlini-Brechot, P.; Benali, N.L. Circulating tumor cells (CTC) detection: Clinical impact and future directions. Cancer Lett. 2007, 253, 180–204. [Google Scholar] [CrossRef] [PubMed]
- Paris, P.L.; Kobayashi, Y.; Zhao, Q.; Zeng, W.; Sridharan, S.; Fan, T.; Adler, H.L.; Yera, E.R.; Zarrabi, M.H.; Zucker, S.; et al. Functional phenotyping and genotyping of circulating tumor cells from patients with castration resistant prostate cancer. Cancer Lett. 2009, 277, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Fusi, A.; Klopocki, E.; Schmittel, A.; Tinhofer, I.; Nonnenmacher, A.; Keilholz, U. Negative enrichment by immunomagnetic nanobeads for unbiased characterization of circulating tumor cells from peripheral blood of cancer patients. J. Transl. Med. 2011, 9. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Lu, Y.T.; Li, F.; Wu, K.; Hou, S.; Yu, J.; Shen, Q.; Wu, D.; Song, M.; OuYang, W.H.; et al. High-purity prostate circulating tumor cell isolation by a polymer nanofiber-embedded microchip for whole exome sequencing. Adv. Mater. 2013, 25, 2897–2902. [Google Scholar] [CrossRef] [PubMed]
- Kirby, B.J.; Jodari, M.; Loftus, M.S.; Gakhar, G.; Pratt, E.D.; Chanel-Vos, C.; Gleghorn, J.P.; Santana, S.M.; Liu, H.; Smith, J.P.; et al. Functional characterization of circulating tumor cells with a prostate-cancer-specific microfluidic device. PLoS ONE 2013, 7, e35976. [Google Scholar] [CrossRef] [PubMed]
- Ozkumur, E.; Shah, A.M.; Ciciliano, J.C.; Emmink, B.L.; Miyamoto, D.T.; Brachtel, E.; Yu, M.; Chen, P.I.; Morgan, B.; Trautwein, J.; et al. Inertial focusing for tumor antigen-dependent and -independent sorting of rare circulating tumor cells. Sci. Ttransl. Med. 2013, 5. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.J.; Marengo, M.S.; Oltean, S.; Kemeny, G.; Bitting, R.L.; Turnbull, J.D.; Herold, C.I.; Marcom, P.K.; George, D.J.; Garcia-Blanco, M.A. Circulating tumor cells from patients with advanced prostate and breast cancer display both epithelial and mesenchymal markers. Mol. Cancer Res. 2012, 9, 997–1007. [Google Scholar] [CrossRef] [PubMed]
- Gorges, T.M.; Penkalla, N.; Schalk, T.; Joosse, S.A.; Riethdorf, S.; Tucholski, J.; Lucke, K.; Wikman, H.; Jackson, S.; Brychta, N.; et al. Enumeration and molecular characterization of tumor cells in lung cancer patients using a novel in vivo device for capturing circulating tumor cells. Clin. Cancer Res. 2016, 22, 2197–2206. [Google Scholar] [CrossRef] [PubMed]
- Vona, G.; Estepa, L.; Beroud, C.; Damotte, D.; Capron, F.; Nalpas, B.; Mineur, A.; Franco, D.; Lacour, B.; Pol, S.; et al. Impact of cytomorphological detection of circulating tumor cells in patients with liver cancer. Hepatology 2004, 39, 792–797. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.K.; Zheng, S.; Williams, A.J.; Balic, M.; Groshen, S.; Scher, H.I.; Fleisher, M.; Stadler, W.; Datar, R.H.; Tai, Y.C.; et al. Portable filter-based microdevice for detection and characterization of circulating tumor cells. Clin. Cancer Res. 2010, 16, 5011–5018. [Google Scholar] [CrossRef] [PubMed]
- Vona, G.; Sabile, A.; Louha, M.; Sitruk, V.; Romana, S.; Schutze, K.; Capron, F.; Franco, D.; Pazzagli, M.; Vekemans, M.; et al. Isolation by size of epithelial tumor cells: A new method for the immunomorphological and molecular characterization of circulating tumor cells. Am. J. Pathol. 2000, 156, 57–63. [Google Scholar] [CrossRef]
- Rosenberg, R.; Gertler, R.; Friederichs, J.; Fuehrer, K.; Dahm, M.; Phelps, R.; Thorban, S.; Nekarda, H.; Siewert, J.R. Comparison of two density gradient centrifugation systems for the enrichment of disseminated tumor cells in blood. Cytometry 2002, 49, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.K.; He, M.; Wilson, T.; Liu, X.; Zhang, K.; Carmichael, C.; Torres, A.; Hernandez, S.; Lau, C.; Agarwal, N.; et al. Detection and phenotyping of circulating tumor cells in high-risk localized prostate cancer. Clin. Genitourin. Cancer 2015, 13, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Adams, A.A.; Okagbare, P.I.; Feng, J.; Hupert, M.L.; Patterson, D.; Gottert, J.; McCarley, R.L.; Nikitopoulos, D.; Murphy, M.C.; Soper, S.A. Highly efficient circulating tumor cell isolation from whole blood and label-free enumeration using polymer-based microfluidics with an integrated conductivity sensor. J. Am. Chem. Soc. 2008, 130, 8633–8641. [Google Scholar] [CrossRef] [PubMed]
- Dharmasiri, U.; Njoroge, S.K.; Witek, M.A.; Adebiyi, M.G.; Kamande, J.W.; Hupert, M.L.; Barany, F.; Soper, S.A. High-throughput selection, enumeration, electrokinetic manipulation, and molecular profiling of low-abundance circulating tumor cells using a microfluidic system. Anal. Chem. 2011, 83, 2301–2309. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.T.; Zhao, L.; Shen, Q.; Garcia, M.A.; Wu, D.; Hou, S.; Song, M.; Xu, X.; Ouyang, W.H.; Ouyang, W.W.; et al. Nanovelcro chip for CTC enumeration in prostate cancer patients. Methods 2013, 64, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Cristofanilli, M.; Budd, G.T.; Ellis, M.J.; Stopeck, A.; Matera, J.; Miller, M.C.; Reuben, J.M.; Doyle, G.V.; Allard, W.J.; Terstappen, L.W.; et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N. Engl. J. Med. 2004, 351, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Friedlander, T.W.; Fong, L. The end of the beginning: Circulating tumor cells as a biomarker in castration-resistant prostate cancer. J. Clin. Oncol. 2014, 32, 1104–1106. [Google Scholar] [CrossRef] [PubMed]
- Leversha, M.A.; Han, J.; Asgari, Z.; Danila, D.C.; Lin, O.; Gonzalez-Espinoza, R.; Anand, A.; Lilja, H.; Heller, G.; Fleisher, M.; et al. Fluorescence in situ hybridization analysis of circulating tumor cells in metastatic prostate cancer. Clin. Cancer Res. 2009, 15, 2091–2097. [Google Scholar] [CrossRef] [PubMed]
- Attard, G.; Swennenhuis, J.F.; Olmos, D.; Reid, A.H.; Vickers, E.; A’Hern, R.; Levink, R.; Coumans, F.; Moreira, J.; Riisnaes, R.; et al. Characterization of ERG, AR and PTEN gene status in circulating tumor cells from patients with castration-resistant prostate cancer. Cancer Res. 2009, 69, 2912–2918. [Google Scholar] [CrossRef] [PubMed]
- Dittamore, R.; Louw, J.; Krupa, R.; Anand, A.; Danila, D.C.; Arslan, Z. Molecular characterization of circulating tumor cells (CTC) and CTC subpopulations in progressive metastatic castration resistant prostate cancer (PCRPC). Clin. Oncol. 2014, 32. abstract 132. [Google Scholar]
- Antonarakis, E.S.; Lu, C.; Wang, H.; Luber, B.; Nakazawa, M.; Roeser, J.C.; Chen, Y.; Mohammad, T.A.; Chen, Y.; Fedor, H.L.; et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N. Engl. J. Med. 2014, 371, 1028–1038. [Google Scholar] [CrossRef] [PubMed]
- Scher, H.I.; Lu, D.; Schreiber, N.A.; Louw, J.; Graf, R.P.; Vargas, H.A.; Johnson, A.; Jendrisak, A.; Bambury, R.; Danila, D.; et al. Association of AR-V7 on circulating tumor cells as a treatment-specific biomarker with outcomes and survival in castration-resistant prostate cancer. JAMA Oncol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, M.; Lu, C.; Chen, Y.; Paller, C.J.; Carducci, M.A.; Eisenberger, M.A.; Luo, J.; Antonarakis, E.S. Serial blood-based analysis of AR-V7 in men with advanced prostate cancer. Ann. Oncol. 2015, 26, 1859–1865. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, D.T.; Zheng, Y.; Wittner, B.S.; Lee, R.J.; Zhu, H.; Broderick, K.T.; Desai, R.; Fox, D.B.; Brannigan, B.W.; Trautwein, J.; et al. RNA-Seq of single prostate CTCs implicates noncanonical Wnt signaling in antiandrogen resistance. Science 2015, 349, 1351–1356. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Lu, Y.T.; Ho, H.; Li, B.; Chen, J.F.; Lin, M.; Li, F.; Wu, K.; Wu, H.; Lichterman, J.; et al. A comparison of isolated circulating tumor cells and tissue biopsies using whole-genome sequencing in prostate cancer. Oncotarget 2015, 6, 44781–44793. [Google Scholar] [PubMed]
- Zhang, Y.; Ma, Q.; Liu, T.; Ke, S.; Jiang, K.; Wen, Y.; Ma, B.; Zhou, Y.; Fan, Q.; Qiu, X. Tumor self-seeding by circulating tumor cells in nude mouse models of human osteosarcoma and a preliminary study of its mechanisms. J. Cancer Res. Clin. Oncol. 2014, 140, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Vela, I.; Sboner, A.; Iaquinta, P.J.; Karthaus, W.R.; Gopalan, A.; Dowling, C.; Wanjala, J.N.; Undvall, E.A.; Arora, V.K.; et al. Organoid cultures derived from patients with advanced prostate cancer. Cell 2014, 159, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Helzer, K.T.; Barnes, H.E.; Day, L.; Harvey, J.; Billings, P.R.; Forsyth, A. Circulating tumor cells are transcriptionally similar to the primary tumor in a murine prostate model. Cancer Res. 2009, 69, 7860–7866. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.S.; Rodriguez-Bravo, V.; Chippada-Venkata, U.; De Ia Iglesia-Vicente, J.; Gong, Y.; Galsky, M.; Oh, W.; Cordon-Cardo, C.; Domingo-Domenech, J. Generation of prostate cancer patient derived xenograft models from circulating tumor cells. J. Vis. Exp. 2015. [Google Scholar] [CrossRef] [PubMed]
- Rossi, E.; Rugge, M.; Facchinetti, A.; Pizzi, M.; Nardo, G.; Barbieri, V.; Manicone, M.; De Faveri, S.; Chiara Scaini, M.; Basso, U.; et al. Retaining the long-survive capacity of circulating tumor cells (CTCs) followed by xeno-transplantation: Not only from metastatic cancer of the breast but also of prostate cancer patients. Oncoscience 2014, 1, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Altman, D.G.; McShane, L.M.; Sauerbrei, W.; Taube, S.E. Reporting recommendations for tumor marker prognostic studies (REMARK): Explanation and elaboration. BMC Med. 2012, 10. [Google Scholar] [CrossRef] [PubMed]
- Khleif, S.N.; Doroshow, J.H.; Hait, W.N. AACR-FDA-NCI cancer biomarkers collaborative consensus report: Advancing the use of biomarkers in cancer drug development. Clin. Cancer Res. 2010, 16, 3299–3318. [Google Scholar] [CrossRef] [PubMed]
- Teutsch, S.M.; Bradley, L.A.; Palomaki, G.E.; Haddow, J.E.; Piper, M.; Calonge, N.; Dotson, W.D.; Douglas, M.P.; Berg, A.O. The evaluation of genomic applications in practice and prevention (EGAPP) initiative: Methods of the EGAPP working group. Genet. Med. 2009, 11, 3–14. [Google Scholar] [CrossRef] [PubMed]
- McShane, L.M.; Hayes, D.F. Publication of tumor marker research results: The necessity for complete and transparent reporting. J. Clin. Oncol. 2012, 30, 4223–4232. [Google Scholar] [CrossRef] [PubMed]
- Maestro, L.M.; Sastre, J.; Rafael, S.B.; Veganzones, S.B.; Vidaurreta, M.; Martin, M.; Olivier, C.; de La Orden, V.B.; Garcia-Saenz, J.A.; Alfonso, R.; et al. Circulating tumor cells in solid tumor in metastatic and localized stages. Anticancer Res. 2009, 29, 4839–4843. [Google Scholar] [PubMed]
- Lalmahomed, Z.S.; Kraan, J.; Gratama, J.W.; Mostert, B.; Sleijfer, S.; Verhoef, C. Circulating tumor cells and sample size: The more, the better. J. Clin. Oncol. 2010, 28, e288–e290. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Ang, R.R.; Duffy, S.P.; Bazov, J.; Chi, K.N.; Black, P.C.; Ma, H. Morphological differences between circulating tumor cells from prostate cancer patients and cultured prostate cancer cells. PLoS ONE 2014, 9, e85264. [Google Scholar] [CrossRef] [PubMed]
- Kraan, J.; Sleijfer, S.; Strijbos, M.H.; Ignatiadis, M.; Peeters, D.; Pierga, J.Y.; Farace, F.; Riethdorf, S.; Fehm, T.; Zorzino, L.; et al. External quality assurance of circulating tumor cell enumeration using the Cellsearch system: A feasibility study. Cytom. B Clin. Cytom. 2011, 80, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.; Morris, K.; Zhou, C.; Sloane, R.; Lancashire, M.; Morris, D.; Bramley, S.; Krebs, M.; Khoja, L.; Dive, C. Method validation of circulating tumour cell enumeration at low cell counts. BMC Cancer 2013, 13. [Google Scholar] [CrossRef] [PubMed]
- Doyen, J.; Alix-Panabieres, C.; Hofman, P.; Parks, S.K.; Chamorey, E.; Naman, H.; Hannoun-Levi, J.M. Circulating tumor cells in prostate cancer: A potential surrogate marker of survival. Crit. Rev. Oncol. Hematol. 2012, 81, 241–256. [Google Scholar] [CrossRef] [PubMed]
- Coumans, F.A.; Doggen, C.J.; Attard, G.; de Bono, J.S.; Terstappen, L.W. All circulating EPCAM+CK+CD45− objects predict overall survival in castration-resistant prostate cancer. Ann. Oncol. 2010, 21, 1851–1857. [Google Scholar] [CrossRef] [PubMed]
- Larson, C.J.; Moreno, J.G.; Pienta, K.J.; Gross, S.; Repollet, M.; O’Hara, S.M.; Russell, T.; Terstappen, L.W. Apoptosis of circulating tumor cells in prostate cancer patients. Cytom. A 2004, 62, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.G.; Miller, M.C.; Gross, S.; Allard, W.J.; Gomella, L.G.; Terstappen, L.W. Circulating tumor cells predict survival in patients with metastatic prostate cancer. Urology 2005, 65, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Gewanter, R.M.; Katz, A.E.; Olsson, C.A.; Benson, M.C.; Singh, A.; Schiff, P.B.; Ennis, R.D. RT-PCR for PSA as a prognostic factor for patients with clinically localized prostate cancer treated with radiotherapy. Urology 2003, 61, 967–971. [Google Scholar] [CrossRef]
- Meyer, C.P.; Pantel, K.; Tennstedt, P.; Stroelin, P.; Schlomm, T.; Heinzer, H.; Riethdorf, S.; Steuber, T. Limited prognostic value of preoperative circulating tumor cells for early biochemical recurrence in patients with localized prostate cancer. Urol. Oncol. 2016, 34, 235.e11–235.e16. [Google Scholar] [CrossRef] [PubMed]
- Strijbos, M.H.; Gratama, J.W.; Schmitz, P.I.; Rao, C.; Onstenk, W.; Doyle, G.V.; Miller, M.C.; de Wit, R.; Terstappen, L.W.; Sleijfer, S. Circulating endothelial cells, circulating tumour cells, tissue factor, endothelin-1 and overall survival in prostate cancer patients treated with docetaxel. Eur. J. Cancer 2010, 46, 2027–2035. [Google Scholar] [CrossRef] [PubMed]
- Petrylak, D.P.; Vogelzang, N.J.; Budnik, N.; Wiechno, P.J.; Sternberg, C.N.; Doner, K.; Bellmunt, J.; Burke, J.M.; de Olza, M.O.; Choudhury, A.; et al. Docetaxel and prednisone with or without lenalidomide in chemotherapy-naive patients with metastatic castration-resistant prostate cancer (MAINSAIL): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet Oncol. 2015, 16, 417–425. [Google Scholar] [CrossRef]
- Vogelzang, N.J.; Petrylak, D.; Fizazi, K. Analysis of circulating tumor cells (CTCs) in a phase 3 study of docetaxel and prednisone (DP) with or without lenalidomide (LEN) in patients (PTs) with castrate-resistant prostate cancer (CRPC): The mainsail trial. In Proceedings of the European Cancer Congress 2013, Amsterdam, The Netherlands, 27 September–1 October 2013.
- Goldkorn, A.; Ely, B.; Quinn, D.I.; Tangen, C.M.; Fink, L.M.; Xu, T.; Twardowski, P.; van Veldhuizen, P.J.; Agarwal, N.; Carducci, M.A.; et al. Circulating tumor cell counts are prognostic of overall survival in SWOG S0421: A phase III trial of docetaxel with or without atrasentan for metastatic castration-resistant prostate cancer. J. Clin. Oncol. 2014, 32, 1136–1142. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Xiao, Z.; Li, X.; Wang, F.; Zhang, J.; Zhou, R.; Wang, J.; Liu, L. Prognostic role of circulating tumor cells and disseminated tumor cells in patients with prostate cancer: A systematic review and meta-analysis. Tumor Biol. 2014, 35, 5551–5560. [Google Scholar] [CrossRef] [PubMed]
- Olmos, D.; Baird, R.D.; Yap, T.A.; Massard, C.; Pope, L.; Sandhu, S.K.; Attard, G.; Dukes, J.; Papadatos-Pastos, D.; Grainger, P.; et al. Baseline circulating tumor cell counts significantly enhance a prognostic score for patients participating in phase I oncology trials. Clin. Cancer Res. 2011, 17, 5188–5196. [Google Scholar] [CrossRef] [PubMed]
- Onstenk, W.; Sieuwerts, A.M.; Kraan, J.; Van, M.; Nieuweboer, A.J.; Mathijssen, R.H.; Hamberg, P.; Meulenbeld, H.J.; De Laere, B.; Dirix, L.Y.; et al. Efficacy of cabazitaxel in castration-resistant prostate cancer is independent of the presence of AR-V7 in circulating tumor cells. Eur. Urol. 2015, 68, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Antonarakis, E.S.; Lu, C.; Luber, B.; Wang, H.; Chen, Y.; Nakazawa, M.; Nadal, R.; Paller, C.J.; Denmeade, S.R.; Carducci, M.A.; et al. Androgen receptor splice variant 7 and efficacy of taxane chemotherapy in patients with metastatic castration-resistant prostate cancer. JAMA Oncol. 2015, 1, 582–591. [Google Scholar] [CrossRef] [PubMed]
- Todenhöfer, T.; Azad, A.; Stewart, C.; Gao, J.; Eigl, B.J.; Gleave, M.E.; Joshua, A.M.; Black, P.C.; Chi, K.N. AR-V7 transcripts in whole blood RNA of patients with metastatic castration resistant prostate cancer correlate with response to abiraterone acetate. J. Urol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Bernemann, C.; Schnoeller, T.J.; Luedeke, M.; Steinestel, K.; Boegemann, M.; Schrader, A.J.; Steinestel, J. Expression of AR-V7 in circulating tumour cells does not preclude response to next generation androgen deprivation therapy in patients with castration resistant prostate cancer. Eur. Urol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Steinestel, J.; Luedeke, M.; Arndt, A.; Schnoeller, T.J.; Lennerz, J.K.; Wurm, C.; Maier, C.; Cronauer, M.V.; Steineste, I.K.; Schrader, A.J. Detecting predictive androgen receptor modifications in circulating prostate cancer cells. Oncotarget 2015. [Google Scholar] [CrossRef]
- Dancey, J.E.; Dobbin, K.K.; Groshen, S.; Jessup, J.M.; Hruszkewycz, A.H.; Koehler, M.; Parchment, R.; Ratain, M.J.; Shankar, L.K.; Stadler, W.M.; et al. Guidelines for the development and incorporation of biomarker studies in early clinical trials of novel agents. Clin. Cancer Res. 2010, 16, 1745–1755. [Google Scholar] [CrossRef] [PubMed]
- De Bono, J.S.; Attard, G.; Adjei, A.; Pollak, M.N.; Fong, P.C.; Haluska, P.; Roberts, L.; Melvin, C.; Repollet, M.; Chianese, D.; et al. Potential applications for circulating tumor cells expressing the Insulin-like growth factor-I receptor. Clin. Cancer Res. 2007, 13, 3611–3616. [Google Scholar] [CrossRef] [PubMed]
- Pantel, K.; Alix-Panabieres, C. The potential of circulating tumor cells as a liquid biopsy to guide therapy in prostate cancer. Cancer Discov. 2012, 2, 974–975. [Google Scholar] [CrossRef] [PubMed]
| System | Isolation Strategy | Identification | Referene |
|---|---|---|---|
| CellSearch | Immunocapture (EpCAM) | IF for CK and CD45, and DAPI | [24] |
| MagSweeper | Immunocapture (EpCAM, CD45) | PCR for PSA, KLK3, TMPRSS2, CD45 | [22] |
| EPISPOT assay | Non-EpCAM-based immunocapture of CD45- and CXCR4-positive cells | Secretion of proteins: CK19, MUC1, PSA | [25] |
| ISET | Cell size | ICC for CK | [26] |
| Metacell | Cell size | ICC or IF for CK | [27] |
| ApoStream | Dielectrophoretic field | ICC for EpCAM and CK | [28] |
| CTC membrane microfilter | Cell size | IF for CK | [29] |
| DEPArray | Microfluidic and dielectrophoretic field | Image-based selection | [30] |
| CellCollector | In vivo immunoisolation (EpCAM) | IF for CK, EpCAM, CD45 | [31] |
| Ficoll-Paque | Cell density | ICC for CK, PSA PCR | [32] |
| Vita-Assay | Marker-independent functional collagen adhesion matrix | ICC or flow cytometry (EpCAM, CK, CD44, CD34, CD45, vimentin) | [33] |
| RosetteSep | Immunodepletion of CD45-positive cells | IF for CK, EpCAM, CD45 | [34] |
| AdnaTest | Immunocapture (EpCAM or EMT markers) | qRT-PCR | [23] |
| NanoVelcro CTC Chip | Microfluidics and immunocapture | IF for CK, EpCAM, CD45 | [35] |
| GEDI microfluidic device | Microfluidics/immunocapture (PSMA) | ICC for CD45, PSMA, EpCAM | [36] |
| CTC-iChip | Microfluidic and immunocapture | Immunofluorescence, cytopathology, FISH | [37] |
| Ref. | Treatment | AR-V7 Prevalence | PSA Response in AR-V7+ vs. AR-V7− Patients | AR-V7 Assay |
|---|---|---|---|---|
| Antonarakis et al. [53] | Abiraterone, enzalutamide | 19% 39% | 0% vs. 68% (p < 0.01) 0% vs. 53% (p < 0.01) | CTC-derived mRNA |
| Steinestel et al. [88] | Abiraterone or enzalutamide | 64% | 7% vs. 63% (p < 0.01) | CTC-derived mRNA |
| Todenhöfer et al. [86] | Abiraterone | 11% | 0% vs. 42% (p < 0.01) | Whole-blood mRNA |
| Antonarakis et al. [85] | Docetaxel or cabazitaxel | 46% | 41% vs. 65% (p < 0.01) | CTC-derived mRNA |
| Onstenk et al. [84] | Cabazitaxel | 55% | 8% vs. 22% (p < 0.01) | CTC-derived mRNA |
| Scher et al. [54] | Abiraterone, enzalutamide or taxanes | 18% | 0% vs. 64% 33% vs. 44% | CTC-derived mRNA |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
León-Mateos, L.; Vieito, M.; Anido, U.; López López, R.; Muinelo Romay, L. Clinical Application of Circulating Tumour Cells in Prostate Cancer: From Bench to Bedside and Back. Int. J. Mol. Sci. 2016, 17, 1580. https://doi.org/10.3390/ijms17091580
León-Mateos L, Vieito M, Anido U, López López R, Muinelo Romay L. Clinical Application of Circulating Tumour Cells in Prostate Cancer: From Bench to Bedside and Back. International Journal of Molecular Sciences. 2016; 17(9):1580. https://doi.org/10.3390/ijms17091580
Chicago/Turabian StyleLeón-Mateos, Luis, María Vieito, Urbano Anido, Rafael López López, and Laura Muinelo Romay. 2016. "Clinical Application of Circulating Tumour Cells in Prostate Cancer: From Bench to Bedside and Back" International Journal of Molecular Sciences 17, no. 9: 1580. https://doi.org/10.3390/ijms17091580
APA StyleLeón-Mateos, L., Vieito, M., Anido, U., López López, R., & Muinelo Romay, L. (2016). Clinical Application of Circulating Tumour Cells in Prostate Cancer: From Bench to Bedside and Back. International Journal of Molecular Sciences, 17(9), 1580. https://doi.org/10.3390/ijms17091580






