Small GTPases and Stress Responses of vvran1 in the Straw Mushroom Volvariella volvacea
Abstract
:1. Introduction
2. Results
2.1. Identification of V. volvacea Small GTPases
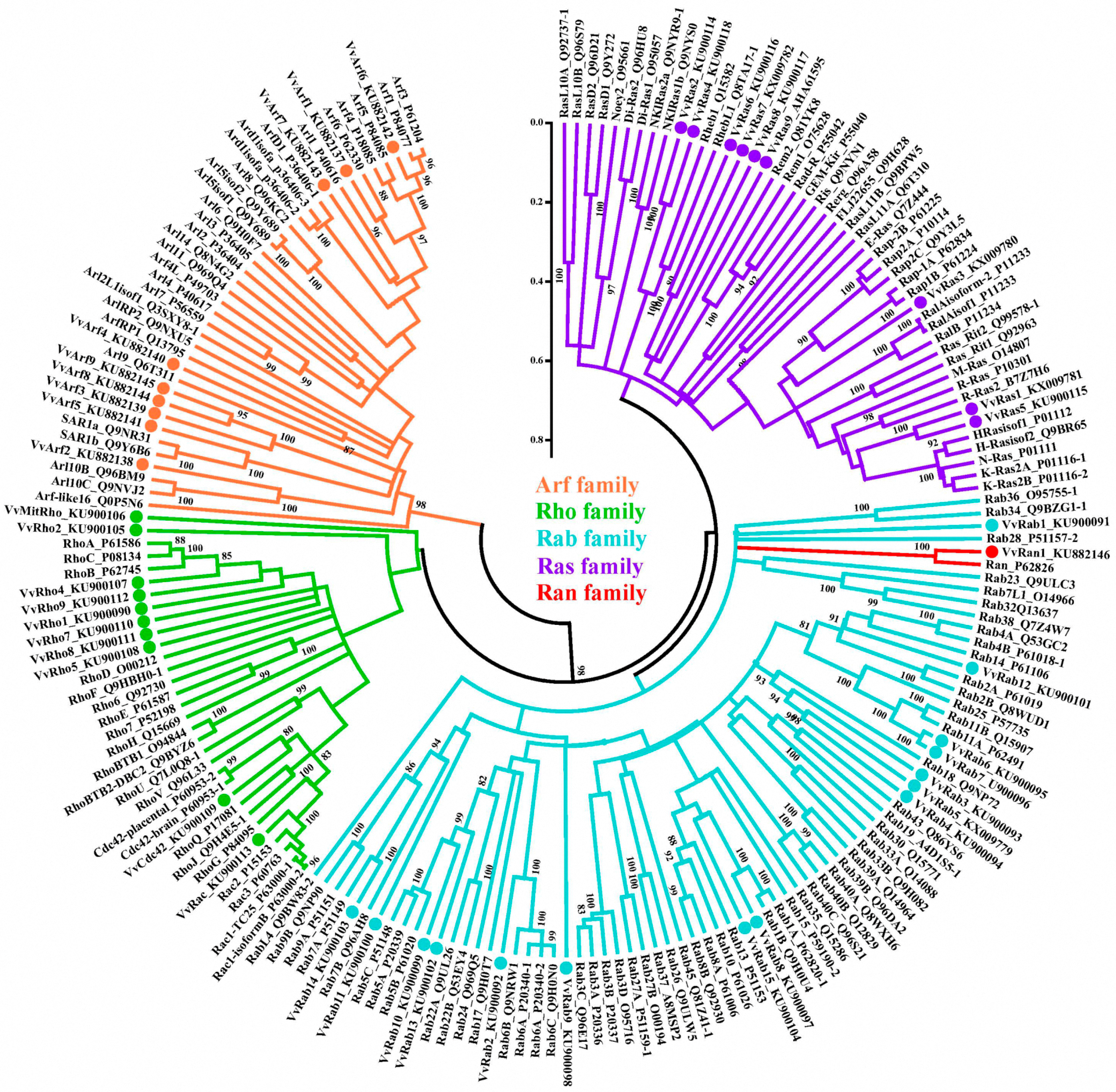
2.2. Classification of V. volvacea Small GTPases
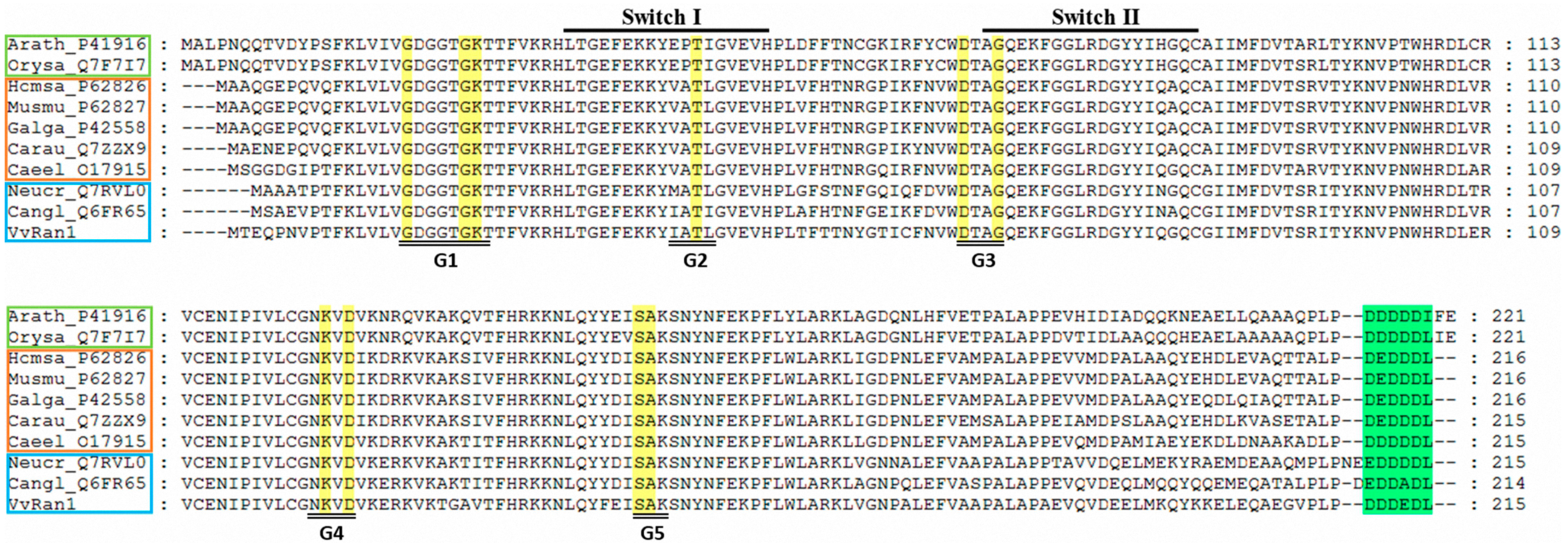
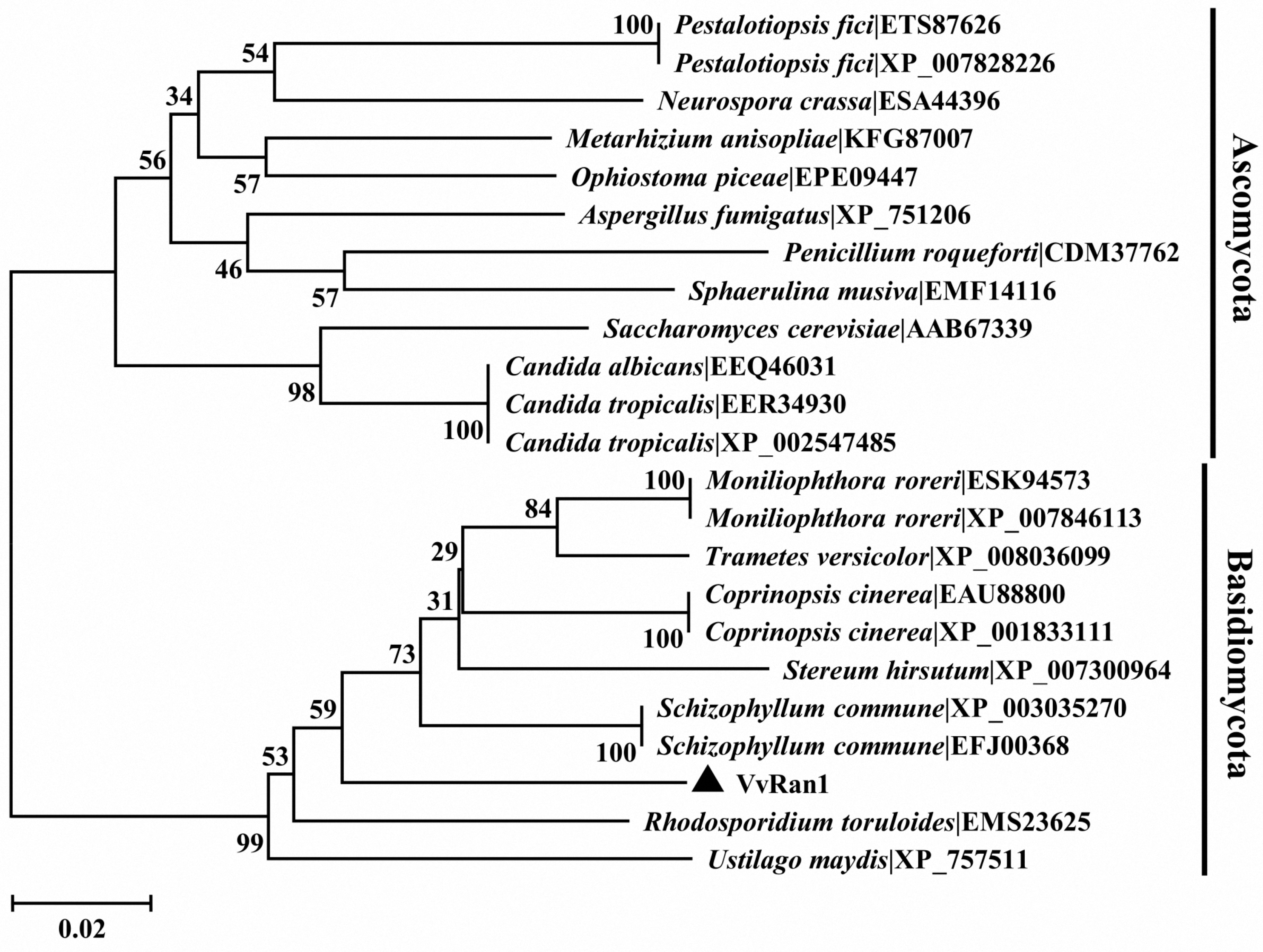
2.3. Phylogenic Analysis of VvRan1
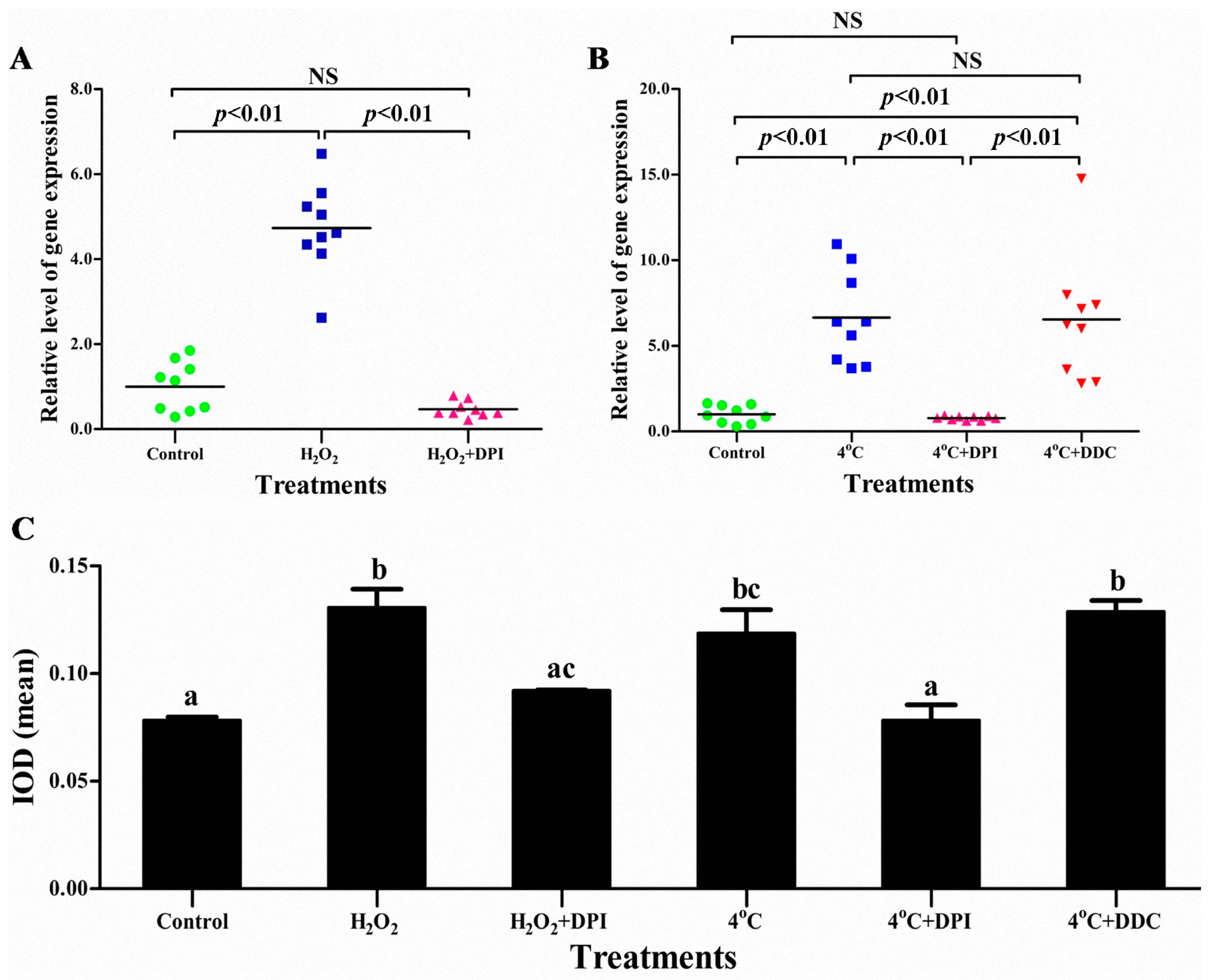
2.4. The Superoxide Anion (O2−) Signal Molecular Triggers vvran1 to Respone Stresses
3. Discussion
4. Materials and Methods
4.1. Strains
4.2. Genome Sequencing, Splicing and Prediction
4.3. Annotation of Small GTPases
4.4. Validation of Sequence Accuracy
4.5. Sequence Homology Comparison and Phylogenetic Tree Construction
4.6. Preparation of Solutions and Stress Treatments
4.7. vvran1 Transcript Analysis
4.8. Histochemical Detection of O2−
4.9. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yang, Z. Small GTPases versatile signaling switches in plants. Plant Cell 2002, 14, S375–S388. [Google Scholar] [PubMed]
- Falchi, R.; Cipriani, G.; Marrazzo, T.; Nonis, A.; Vizzotto, G.; Ruperti, B. Identification and differential expression dynamics of peach small GTPases encoding genes during fruit development and ripening. J. Exp. Bot. 2010, 61, 2829–2842. [Google Scholar] [CrossRef] [PubMed]
- Rojas, A.M.; Fuentes, G.; Rausell, A.; Valencia, A. The Ras protein superfamily: Evolutionary tree and role of conserved amino acids. J. Cell Biol. 2012, 196, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Goitre, L.; Trapani, E.; Trabalzini, L.; Retta, S.F. The Ras superfamily of small GTPases: The unlocked secrets. Methods Mol. Biol. 2014, 1120, 1–18. [Google Scholar] [PubMed]
- Simon, M.N.; de Virgilio, C.; Souza, B.; Pringle, J.R.; Abo, A.; Reed, S.I. Role for the Rho-family GTPase Cdc42 in yeast mating-pheromone signal pathway. Nature 1995, 376, 702–705. [Google Scholar] [CrossRef] [PubMed]
- Arellano, M.; Coll, P.M.; Perez, P. Rho GTPases in the control of cell morphology, cell polarity, and actin localization in fission yeast. Microsc. Res. Tech. 1999, 47, 51–60. [Google Scholar] [CrossRef]
- Wennerberg, K.; Der, C.J. Rho-family GTPases: It’s not only Rac and Rho (and I like it). J. Cell Sci. 2004, 117, 1301–1312. [Google Scholar] [CrossRef] [PubMed]
- Molendijk, A.J.; Ruperti, B.; Palme, K. Small GTPases in vesicle trafficking. Curr. Opin. Plant Biol. 2004, 7, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Hutagalung, A.H.; Novick, P.J. Role of Rab GTPases in membrane traffic and cell physiology. Physiol. Rev. 2011, 91, 119–149. [Google Scholar] [CrossRef] [PubMed]
- Casanova, J.E.; Hsu, V.W.; Jackson, C.L.; Kahn, R.A.; Roy, C.R.; Stow, J.L.; Wandinger-Ness, A.; Sztul, E. Meeting report–Arf and Rab family G proteins. J. Cell Sci. 2013, 126, 5313–5316. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, S.R. Rab GTPase regulation of membrane identity. Curr. Opin. Plant Biol. 2013, 25, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Zhen, Y.; Stenmark, H. Cellular functions of Rab GTPases at a glance. J. Cell Sci. 2015, 128, 3171–3176. [Google Scholar] [CrossRef] [PubMed]
- Avis, J.M.; Clarke, P.R. Ran, a GTPase involved in nuclear processes: Its regulators and effectors. J. Cell Sci. 1996, 109, 2423–2427. [Google Scholar] [PubMed]
- Dasso, M. The Ran GTPase: Theme and variations. Curr. Biol. 2002, 12, R502–R508. [Google Scholar] [CrossRef]
- Arnaoutov, A.; Dasso, M. The Ran GTPase regulates kinetochore function. Dev. Cell 2003, 5, 99–111. [Google Scholar] [CrossRef]
- Garcia-Ranea, J.A.; Valencia, A. Distribution and functional diversification of the Ras superfamily in Saccharomyces cerevisiae. FEBS Lett. 1998, 434, 219–225. [Google Scholar] [CrossRef]
- Rajashekar, B.; Kohler, A.; Johansson, T.; Martin, F.; Tunlid, A.; Ahrén, D. Expansion of signal pathways in the ectomycorrhizal fungus Laccaria bicolor—Evolution of nucleotide sequences and expression patterns in families of protein kinases and Ras small GTPases. New Phytol. 2009, 183, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Raudaskoski, M.; Kothe, E.; Fowler, T.J.; Jung, E.M.; Horton, J.S. Ras and Rho small G proteins: Insights from the Schizophyllum commune genome sequence and comparisons to other fungi. Biotechnol. Genet. Eng. Rev. 2012, 28, 61–100. [Google Scholar] [CrossRef] [PubMed]
- Ren, A.; Cao, P.; Wu, F.; Li, M.; Jiang, A.; Shi, L.; Zhao, M. Systematic characterization of small GTPases gene family in the model medicinal mushroom Ganoderma lucidum. J. Nanjing Agric. Univ. 2015, 38, 923–929. [Google Scholar]
- Bao, D.; Gong, M.; Zheng, H.; Chen, M.; Zhang, L.; Wang, H.; Jiang, J.; Wu, L.; Zhu, Y.; Zhu, G.; et al. Sequencing and comparative analysis of the straw mushroom (Volvariella volvacea) genome. PLoS ONE 2013, 8, e58294. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Gui, F.; Xie, B.; Deng, Y.; Sun, X.; Lin, M.; Tao, Y.; Li, S. Composition and expression of genes encoding carbohydrate-active enzymes in the straw-degrading mushroom Volvariella volvacea. PLoS ONE 2013, 8, e58780. [Google Scholar] [CrossRef]
- Chinnusamy, V.; Gong, Z.; Zhu, J.K. Nuclear RNA export and its importance in abiotic stress responses of plants. Curr. Top. Microbiol. Immunol. 2008, 326, 235–255. [Google Scholar] [PubMed]
- Yasuda, Y.; Miyamoto, Y.; Saiwaki, T.; Yoneda, Y. Mechanism of the stress-induced collapse of the Ran distribution. Exp. Cell Res. 2006, 312, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Stochaj, U.; Rassadi, R.; Chiu, J. Stress-mediated inhibition of the classical nuclear protein import pathway and nuclear accumulation of the small GTPase Gsp1p. FASEB J. 2000, 14, 2130–2132. [Google Scholar] [CrossRef] [PubMed]
- Kulsum, U.; Singh, V.; Sharma, S.; Srinivasan, A.; Singh, T.P.; Kaur, P. RASOnD—A comprehensive resource and search tool for Ras family oncogenes from various species. BMC Genom. 2011, 12, 341. [Google Scholar] [CrossRef] [PubMed]
- Rose, P.W.; Beran, B.; Bi, C.; Bluhm, W.F.; Dimitropoulos, D.; Goodsell, D.S.; Prlic, A.; Quesada, M.; Quinn, G.B.; Westbrook, J.D.; et al. The RCSB Protein Data Bank: Redesigned web site and web services. Nucleic Acids Res. 2011, 39, D392–D401. [Google Scholar] [CrossRef] [PubMed]
- Wennerberg, K.; Rossman, K.L.; Der, C.J. The Ras superfamily at a glance. J. Cell Sci. 2005, 118, 843–846. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Zhang, Y.; Dusting, G.J. NADPH oxidase-mediated redox signaling: Roles in cellular stress response, stress tolerance, and tissue repair. Pharmacol. Rev. 2011, 63, 218–242. [Google Scholar] [CrossRef] [PubMed]
- Ushio-Fukai, M. Compartmentalization of redox signaling through NADPH oxidase-derived ROS. Antioxid. Redox Signal. 2009, 11, 1289–1299. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, B.V.; Tew, D.G.; Jones, O.T.; England, P.J. Studies on the inhibitory mechanism of iodonium compounds with special reference to neutrophil NADPH oxidase. Biochem. J. 1993, 290, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Cheng, Y.; Kong, X.; Liu, M.; Jiang, F.; Wu, Z. Induction of reactive oxygen species and the potential role of NADPH oxidase in hyperhydricity of garlic plantlets in vitro. Protoplasma 2016. [Google Scholar] [CrossRef] [PubMed]
- Hooijmaijers, C.; Rhee, J.Y.; Kwak, K.J.; Chung, G.C.; Horie, T.; Katsuhara, M.; Kang, H. Hydrogen peroxide permeability of plasma membrane aquaporins of Arabidopsis thaliana. J. Plant Res. 2012, 125, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Siwik, D.A.; Tzortzis, J.D.; Pimental, D.R.; Chang, D.L.; Pagano, P.J.; Singh, K.; Sawyer, D.B.; Colucci, W.S. Inhibition of copper-zinc superoxide dismutase induces cell growth, hypertrophic phenotype, and apoptosis in neonatal rat cardiac myocytes in vitro. Circ. Res. 1999, 85, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Lamarche, N.; Tapon, N.; Stowers, L.; Burbelo, P.D.; Aspenström, P.; Bridges, T.; Chant, J.; Hall, A. Rac and Cdc42 induce actin polymerization and G1 cell cycle progression independently of p65 and the JNK/SAPK MAP kinase cascade. Cell 1996, 87, 519–529. [Google Scholar] [CrossRef]
- Birukov, K.G.; Bochkov, V.N.; Birukova, A.A.; Kawkitinarong, K.; Rios, A.; Leitner, A.; Verin, A.D.; Bokoch, G.M.; Leitinger, N.; Garcia, J.G. Epoxycyclopentenone-containing oxidized phospholipids restore endothelial barrier function via Cdc42 and Rac. Circ. Res. 2004, 95, 892–901. [Google Scholar] [CrossRef] [PubMed]
- Araujo-Palomares, C.L.; Richthammer, C.; Seiler, S.; Castro-Longoria, E. Functional characterization and cellular dynamics of the CDC-42–RAC–CDC-24 module in Neurospora crassa. PLoS ONE 2011, 6, e27148. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Cai, W. RAN1 is involved in plant cold resistance and development in rice (Oryza sativa). J. Exp. Bot. 2014, 65, 3277–3287. [Google Scholar] [CrossRef] [PubMed]
- Kuźniak, E.; Urbanek, H. The involvement of hydrogen peroxide in plant responses to stresses. Acta Physiol. Plant. 2000, 22, 195–203. [Google Scholar] [CrossRef]
- Yan, Y.; Weaver, V.M.; Blair, I.A. Analysis of protein expression during oxidative stress in breast epithelial cells using a stable isotope labeled proteome internal standard. J. Proteome Res. 2005, 4, 2007–2014. [Google Scholar] [CrossRef] [PubMed]
- Heo, J. Redox regulation of Ran GTPase. Biochem. Biophys. Res. Commun. 2008, 376, 568–572. [Google Scholar] [CrossRef] [PubMed]
- Kose, S.; Imamoto, N. Nucleocytoplasmic transport under stress conditions and its role in HSP70 chaperone systems. BBA-Gen. Subj. 2014, 1840, 2953–2960. [Google Scholar] [CrossRef] [PubMed]
- Mariani, D.; Mathias, C.J.; da Silva, C.G.; Herdeiro Rda, S.; Pereira, R.; Panek, A.D.; Eleutherio, E.C.; Pereira, M.D. Involvement of glutathione transferases, Gtt1 and Gtt2, with oxidative stress response generated by H2O2 during growth of Saccharomyces cerevisiae. Redox Rep. 2008, 13, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ma, Y.; Huang, C.; Li, J.; Wan, Q.; Bi, Y. Involvement of glucose-6-phosphate dehydrogenase in reduced glutathione maintenance and hydrogen peroxide signal under salt stress. Plant Signal. Behav. 2008, 3, 394–395. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Mittler, R. Reactive oxygen species-dependent wound responses in animals and plants. Free Radic. Biol. Med. 2012, 53, 2269–2276. [Google Scholar] [CrossRef] [PubMed]
- Neill, S.J.; Desikan, R.; Clarke, A.; Hurst, R.D.; Hancock, J.T. Hydrogen peroxide and nitric oxide as signalling molecules in plants. J. Exp. Bot. 2002, 53, 1237–1247. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.A.; Xu, Y.; Wang, X.; Du, C.; Du, J.; Yuan, M.; Xu, Z.; Chong, K. OsRAN2, essential for mitosis, enhances cold tolerance in rice by promoting export of intranuclear tubulin and maintaining cell division under cold stress. Plant Cell Environ. 2011, 34, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.; Prutzman, K.C.; Mocanu, V.; Campbell, S.L. Mechanism of free radical nitric oxide-mediated Ras guanine nucleotide dissociation. J. Mol. Biol. 2005, 346, 1423–1440. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhu, H.; Ruan, J.; Qian, W.; Fang, X.; Shi, Z.; Li, Y.; Li, S.; Shan, G.; Kristiansen, K.; et al. De novo assembly of human genomes with massively parallel short read sequencing. Genome Res. 2010, 20, 265–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ter-Hovhannisyan, V.; Lomsadze, A.; Chernoff, Y.O.; Borodovsky, M. Gene prediction in novel fungal genomes using an ab initio algorithm with unsupervised training. Genome Res. 2008, 18, 1979–1990. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Yan, J.; Xie, B.; Li, Y.; Chen, B.; Liu, S.; Li, D.; Yang, Z.; Zeng, X.; Deng, Y.; Jiang, Y. Genes encoding FAD-binding proteins in Volvariella volvacea exhibit differential expression in homokaryons and heterokaryons. Microbiol. Res. 2013, 168, 533–546. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lin, H.; Ma, B. ZOOM Lite: Next-generation sequencing data mapping and visualization software. Nucleic Acids Res. 2010, 38, W743–W748. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Guo, L.; Zhao, J.; Xie, B. Sequence characterization and differential expression of a glutathione S-transferase gene vv-gto1 from Volvariella volvacea. Acta Microbiol. Sin. 2014, 54, 71–78. (In Chinese) [Google Scholar]
- Burge, C.B.; Karlin, S. Finding the genes in genomic DNA. Curr. Opin. Struct. Biol. 1998, 8, 346–354. [Google Scholar] [CrossRef]
- Hoff, K.J.; Stanke, M. WebAUGUSTUS—A web service for training AUGUSTUS and predicting genes in eukaryotes. Nucleic Acids Res. 2013, 41, W123–W128. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, K.B.; Nicholas, H.B., Jr.; Deerfield, D.W., II. GeneDoc: Analysis and visualization of genetic variation. Embnet. News 1997, 4, 1–4. [Google Scholar]
- Yan, J.; Zhang, Y.; Zhang, L.; Xie, B.; Qiu, Q.; Xie, B. Abiotic stress tolerance ability of Volvariella volvacea strain H1521 generated by a cross between two genome sequencing strains. J. Fujian Agric. For. Univ. 2015, 44, 512–515. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Shinogi, T.; Suzuki, T.; Kurihara, T.; Narusaka, Y.; Park, P. Microscopic detection of reactive oxygen species generation in the compatible and incompatible interactions of Alternaria alternata Japanese pear pathotype and host plants. J. Gen. Plant Pathol. 2003, 69, 7–16. [Google Scholar] [CrossRef]

| Name | Gene ID_Predicted Method | GenBank ID | Name | Gene ID_Predicted Method | GenBank ID |
|---|---|---|---|---|---|
| VvArf1 | GME959_T | KU882137 | VvRab4 | GME5663_g | KU900094 |
| VvArf2 | GME2124_T | KU882138 | VvRab5 | GME5664_g | KX009779 |
| VvArf3 | GME4741_g | KU882139 | VvRab6 | GME7647_g | KU900095 |
| VvArf4 | GME4841_g | KU882140 | VvRab7 | GME7649_g | KU900096 |
| VvArf5 | GME5296_g | KU882141 | VvRab8 | GME7988_g | KU900097 |
| VvArf6 | GME8402_T | KU882142 | VvRab9 | GME8752_g | KU900098 |
| VvArf7 | GME8740_g | KU882143 | VvRab10 | GME9500_g | KU900099 |
| VvArf8 | GME10621_g | KU882144 | VvRab11 | GME10910_g | KU900100 |
| VvArf9 | GME10622_g | KU882145 | VvRab12 | GME11319_T | KU900101 |
| VvRho1 | GME749_g | KU900090 | VvRab13 | GME11465_g | KU900102 |
| VvRho2 | GME1391_g | KU900105 | VvRab14 | GME11526_g | KU900103 |
| VvMitRho | GME1938_g | KU900106 | VvRab15 | GME11774_T | KU900104 |
| VvRho4 | GME3984_G | KU900107 | VvRas1 | GME267_T | KX009781 |
| VvRho5 | GME4319_g | KU900108 | VvRas2 | GME5033_g | KU900114 |
| VvCdc42 | GME7713_T | KU900109 | VvRas3 | GME8078_g | KX009780 |
| VvRho7 | GME7714_g | KU900110 | VvRas4 | GME8593_A | KU900118 |
| VvRho8 | GME9067_g | KU900111 | VvRas5 | GME10486_g | KU900115 |
| VvRho9 | GME9847_g | KU900112 | VvRas6 | GME11128_g | KU900116 |
| VvRac | GME11424_T | KU900113 | VvRas7 | GME11133_T | KX009782 |
| VvRab1 | GME3051_g | KU900091 | VvRas8 | GME11134_g | KU900117 |
| VvRab2 | GME5340_g | KU900092 | VvRas9 | GME11562_T | AHA61595 |
| VvRab3 | GME5662_g | KU900093 | VvRan1 | GME5409_T | KU882146 |
| Species | Number of Proteins | ||||||
|---|---|---|---|---|---|---|---|
| Arf | Rho | Ras | Rab | Ran | Total | Reference Number | |
| Schizophyllum commune | 7 | 7 | 5 | 14 | 1 | 34 | 34 a |
| Laccaria bicolor | 9 | 21 | 12 | 10 | 1 | 53 | 55 b |
| Pchrysosporium chrysosporium | 8 | 7 | 4 | 14 | 1 | 34 | 27 b |
| Coprinopsis cinerea | 8 | 11 | 5 | 14 | 1 | 39 | 29 b |
| Volvariella volvacea | 9 | 10 | 9 | 15 | 1 | 44 | This study |
| Primer | Sequence (5′-3′) |
|---|---|
| Ran1-F | AGTTCGTCGCTGCTCCTGCTCT |
| Ran1-R | ACCCTCAGCCTGTTCCAGTTCCTT |
| GAPDH-F | CATCTTCCACTGGTGCGGCTAAG |
| GAPDH-R | GGCTTCTCAAGGCGAACGACAA |
| 18S rRNA-F | TCTTGTGAAACTCTGTCGTGCTGGG |
| 18S rRNA-R | TTGCCCACACCCCAAAGCTAATTCG |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, J.-J.; Xie, B.; Zhang, L.; Li, S.-J.; Van Peer, A.F.; Wu, T.-J.; Chen, B.-Z.; Xie, B.-G. Small GTPases and Stress Responses of vvran1 in the Straw Mushroom Volvariella volvacea. Int. J. Mol. Sci. 2016, 17, 1527. https://doi.org/10.3390/ijms17091527
Yan J-J, Xie B, Zhang L, Li S-J, Van Peer AF, Wu T-J, Chen B-Z, Xie B-G. Small GTPases and Stress Responses of vvran1 in the Straw Mushroom Volvariella volvacea. International Journal of Molecular Sciences. 2016; 17(9):1527. https://doi.org/10.3390/ijms17091527
Chicago/Turabian StyleYan, Jun-Jie, Bin Xie, Lei Zhang, Shao-Jie Li, Arend F. Van Peer, Ta-Ju Wu, Bing-Zhi Chen, and Bao-Gui Xie. 2016. "Small GTPases and Stress Responses of vvran1 in the Straw Mushroom Volvariella volvacea" International Journal of Molecular Sciences 17, no. 9: 1527. https://doi.org/10.3390/ijms17091527







