Serum Calcium and the Risk of Breast Cancer: Findings from the Swedish AMORIS Study and a Meta-Analysis of Prospective Studies
Abstract
:1. Introduction
2. Results
2.1. The AMORIS Study
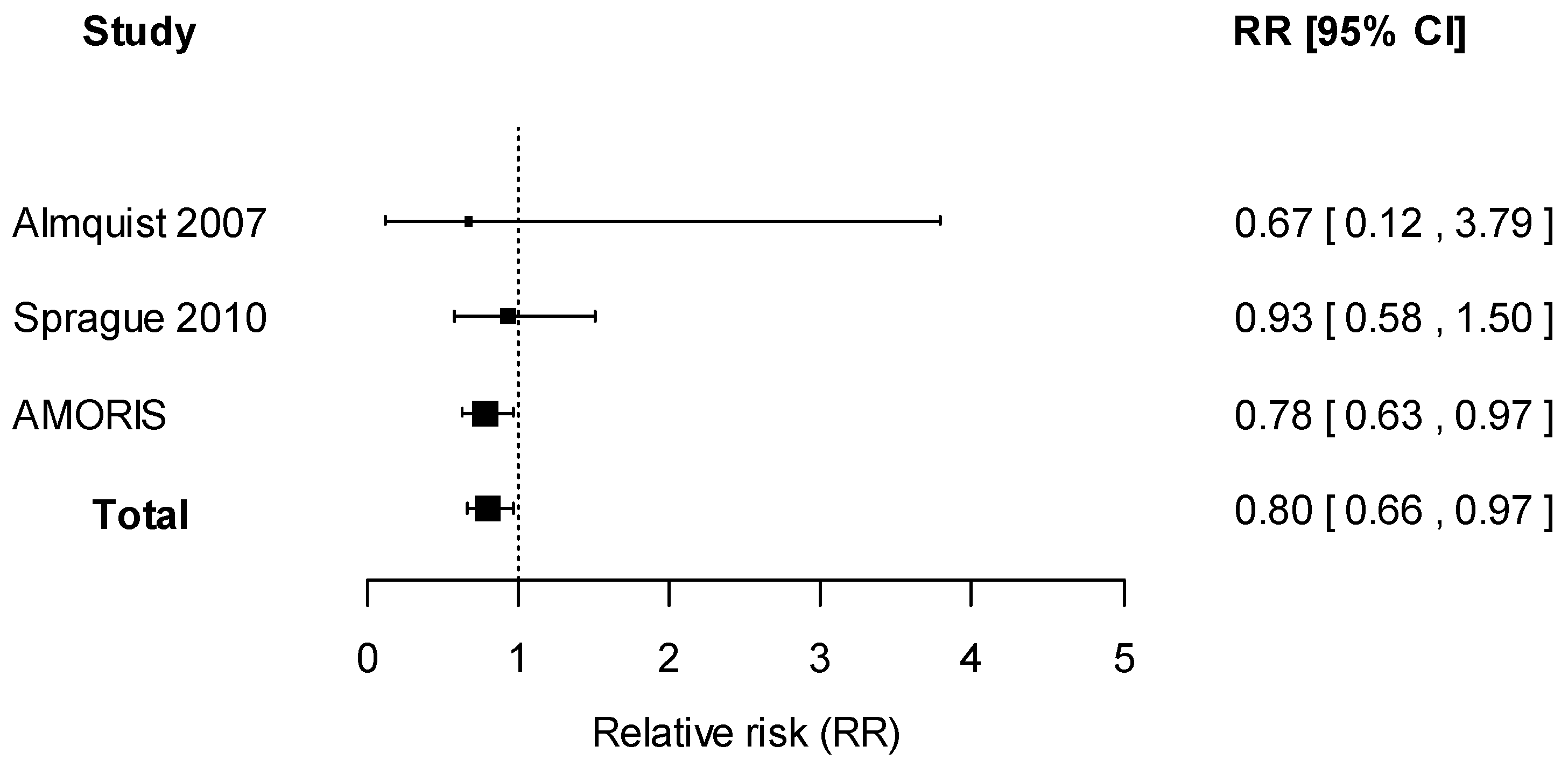
2.2. Systematic Review
2.2.1. Methodological Quality
2.2.2. Data Synthesis
3. Discussion
4. Methods
4.1. The AMORIS Study
4.1.1. Study Population
4.1.2. Statistical Analysis
4.2. Systematic Review
4.2.1. Search Strategies
4.2.2. Data Extraction
4.2.3. Methodological Quality
4.2.4. Data Synthesis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, P.; Hu, P.; Xie, D.; Qin, Y.; Wang, F.; Wang, H. Meta-analysis of vitamin D, calcium and the prevention of breast cancer. Breast Cancer Res. Treat. 2010, 121, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Rohan, T.E. Vitamin D, calcium, and breast cancer risk: A review. Cancer Epidemiol. Biomark. Prev. 2006, 15, 1427–1437. [Google Scholar] [CrossRef] [PubMed]
- Mantell, D.J.; Owens, P.E.; Bundred, N.J.; Mawer, E.B.; Canfield, A.E. 1α,25-Dihydroxyvitamin D(3) inhibits angiogenesis in vitro and in vivo. Circ. Res. 2000, 87, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Colston, K.W.; Berger, U.; Coombes, R.C. Possible role for vitamin D in controlling breast cancer cell proliferation. Lancet 1989, 1, 188–191. [Google Scholar] [CrossRef]
- Wang, D.; Vélez de-la-Paz, O.I.; Zhai, J.-X.; Liu, D.-W. Serum 25-hydroxyvitamin D and breast cancer risk: A meta-analysis of prospective studies. Tumor Biol. 2013, 34, 3509–3517. [Google Scholar] [CrossRef] [PubMed]
- Sprague, B.L.; Skinner, H.G.; Trentham-Dietz, A.; Lee, K.E.; Klein, B.E.K.; Klein, R. Serum calcium and breast cancer risk in a prospective cohort study. Ann. Epidemiol. 2010, 20, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Almquist, M.; Bondeson, A.-G.; Bondeson, L.; Malm, J.; Manjer, J. Serum levels of vitamin D, PTH and calcium and breast cancer risk—A prospective nested case-control study. Int. J. Cancer 2010, 127, 2159–2168. [Google Scholar] [CrossRef] [PubMed]
- Peacock, M. Calcium metabolism in health and disease. Clin. J. Am. Soc. Nephrol. 2010, 5, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Almquist, M.; Manjer, J.; Bondeson, L.; Bondeson, A.-G. Serum calcium and breast cancer risk: Results from a prospective cohort study of 7847 women. Cancer Causes Control 2007, 18, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Rohan, T.E.; Negassa, A.; Chlebowski, R.T.; Ceria-Ulep, C.D.; Cochrane, B.B.; Lane, D.S.; Ginsberg, M.; Wassertheil-Smoller, S.; Page, D.L. A randomized controlled trial of calcium plus vitamin D supplementation and risk of benign proliferative breast disease. Breast Cancer Res. Treat. 2009, 116, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Chlebowski, R.T.; Johnson, K.C.; Kooperberg, C.; Pettinger, M.; Wactawski-Wende, J.; Rohan, T.; Rossouw, J.; Lane, D.; O’Sullivan, M.J.; Yasmeen, S.; et al. Calcium plus vitamin D supplementation and the risk of breast cancer. J. Natl. Cancer Inst. 2008, 100, 1581–1591. [Google Scholar] [CrossRef] [PubMed]
- Hjartåker, A.; Thoresen, M.; Engeset, D.; Lund, E. Dairy consumption and calcium intake and risk of breast cancer in a prospective cohort: The Norwegian Women and Cancer study. Cancer Causes Control 2010, 21, 1875–1885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, M.-H.; Holmes, M.D.; Hankinson, S.E.; Wu, K.; Colditz, G.; Willett, W.C. Intake of dairy products, calcium, and vitamin D and risk of breast cancer. J. Natl. Cancer Inst. 2002, 94, 1301–1311. [Google Scholar] [CrossRef] [PubMed]
- McCullough, M.L.; Rodriguez, C.; Diver, W.R.; Feigelson, H.S.; Stevens, V.L.; Thun, M.J.; Calle, E.E. Dairy, calcium, and vitamin D intake and postmenopausal breast cancer risk in the Cancer Prevention Study II Nutrition Cohort. Cancer Epidemiol. Biomark. Prev. 2005, 14, 2898–2904. [Google Scholar] [CrossRef] [PubMed]
- Battle, M.; Gillespie, C.; Quarshie, A.; Lanier, V.; Harmon, T.; Wilson, K.; Torroella-Kouri, M.; Gonzalez-Perez, R.R. Obesity induced a leptin-Notch signaling axis in breast cancer. Int. J. Cancer 2014, 134, 1605–1616. [Google Scholar] [CrossRef] [PubMed]
- Cullen, P.J.; Lockyer, P.J. Integration of calcium and Ras signalling. Nat. Rev. Mol. Cell Biol. 2002, 3, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Cross, B.M.; Breitwieser, G.E.; Reinhardt, T.A.; Rao, R. Cellular calcium dynamics in lactation and breast cancer: From physiology to pathology. Am. J. Physiol. Cell Physiol. 2014, 306, C515–C526. [Google Scholar] [CrossRef] [PubMed]
- Thaw, S.S.H.; Sahmoun, A.; Schwartz, G.G. Serum calcium, tumor size, and hormone receptor status in women with untreated breast cancer. Cancer Biol. Ther. 2012, 13, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Huss, L.; Butt, S.; Borgquist, S.; Almquist, M.; Malm, J.; Manjer, J. Serum levels of vitamin D, parathyroid hormone and calcium in relation to survival following breast cancer. Cancer Causes Control 2014, 25, 1131–1140. [Google Scholar] [CrossRef] [PubMed]
- Bringhurst, F.; Demay, M.; Krane, S.; Kronenberg, H. Bone and mineral metabolism in health and disease. In Harrrison’s Principles of Internal Medicine; Fauci, A., Longo, D., Kaspe, D., Braunwald, E., Jameson, J., Loscalzo, J., Eds.; McGraw-Hill: New York, NY, USA, 2008. [Google Scholar]
- Holme, I.; Aastveit, A.H.; Hammar, N.; Jungner, I.; Walldius, G. Inflammatory markers, lipoprotein components and risk of major cardiovascular events in 65,005 men and women in the Apolipoprotein MOrtality RISk study (AMORIS). Atherosclerosis 2010, 213, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Wulaningsih, W.; Holmberg, L.; Garmo, H.; Malmstrom, H.; Lambe, M.; Hammar, N.; Walldius, G.; Jungner, I.; Van Hemelrijck, M. Prediagnostic serum inflammatory markers in relation to breast cancer risk, severity at diagnosis and survival in breast cancer patients. Carcinogenesis 2015, 36, 1121–1128. [Google Scholar] [CrossRef] [PubMed]
- Jorde, R.; Sundsfjord, J.; Bønaa, K.H. Determinants of serum calcium in men and women. The Tromsø Study. Eur. J. Epidemiol. 2001, 17, 1117–1123. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [Updated March 2011]. Available online: www.cochrane-handbook.org (accessed on 12 March 2011).
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Grp, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement (reprinted from annals of internal medicine). Phys. Ther. 2009, 89, 873–880. [Google Scholar] [PubMed]
- Greenland, S.; Longnecker, M.P. Methods for estimation from summarised dose-response data, with applications to meta-analysis. Am. J. Epidemiol. 1992, 135, 1301–1309. [Google Scholar] [PubMed]
- Il’yasova, D.; Hertz-Picciotto, I.; Peters, U.; Berlin, J.A.; Poole, C. Choice of exposure scores for categorical regression in meta-analysis: A case study of a common problem. Cancer Causes Control 2005, 16, 383–388. [Google Scholar] [CrossRef] [PubMed]
| Serum Calcium (mmol/L) | |||
|---|---|---|---|
| All (n = 229,674) | Q1 < 2.31 (n = 55,357) | Q4 ≥ 2.44 (n = 59,462) | |
| Age (years) Mean (SD) | 46.16 (15.19) | 44.49 (13.72) | 48.82 (16.25) |
| SES | |||
| High | 82,555 (35.94) | 21,707 (39.21) | 18,882 (31.75) |
| Low | 116,202 (50.59) | 27,295 (49.31) | 31,084 (52.28) |
| Unclassified | 30,917 (13.46) | 6355 (11.48) | 9496 (15.97) |
| Education Status | |||
| High | 56,856 (24.76) | 15,649 (28.27) | 12,233 (20.57) |
| Middle | 98,268 (42.79) | 23,918 (43.21) | 24,631 (41.42) |
| Low | 62,456 (27.19) | 13,182 (21.81) | 18,775 (31.57) |
| Missing | 12,094 (5.27)) | 2608 (4.71) | 3823 (6.43) |
| Parity | |||
| Yes | 155,706 (67.79) | 38,594 (69.72) | 40,094 (67.43) |
| No | 73,968 (32.21) | 16,763 (30.28) | 19,368 (32.57) |
| Breast Cancer | |||
| Yes | 10,863 (4.73) | 2656 (4.80) | 2829 (4.76) |
| No | 218,811 (95.27) | 52,701 (95.20) | 56,633 (95.24) |
| Calcium (mmol/L) Mean (SD) | 2.38 (0.10) | 2.26 (0.04) | 2.50 (0.06) |
| Corrected Calcium (mmol/L) Mean (SD) | 2.32 (0.09) | 2.23 (0.06) | 2.43 (0.08) |
| Albumin (g/L) Mean (SD) | 42.50 (2.74) | 41.15 (2.65) | 43.76 (2.64) |
| Charlson Comorbidity Index | |||
| 0 | 213,122 (92.79) | 51,713 (93.42) | 54,205 (91.16) |
| 1 | 8819 (3.84) | 2023 (3.65) | 2712 (4.56) |
| 2 | 5692 (2.48) | 1155 (2.09) | 1870 (3.14) |
| 3+ | 2041 (0.89) | 466 (0.84) | 675 (1.14) |
| History of Fractures | |||
| Yes | 173 (0.08) | 45 (0.08) | 43 (0.07) |
| No | 229,501 (99.92) | 55,313 (99.92) | 59,419 (99.93) |
| Seasonality | |||
| Spring | 66,166 (28.81) | 16,150 (29.17) | 17,455 (29.35) |
| Summer | 37,733 (16.43) | 8996 (16.25) | 10,150 (17.07) |
| Autumn | 68,503 (29.83) | 16,620 (30.02 | 16,924 (28.46) |
| Winter | 57,272 (24.94) | 13,591 (24.55) | 14,933 (25.11) |
| Follow-up (years) Mean (SD) | 19.40 (5.91) | 19.58 (5.75) | 18.99 (6.31) |
| N (%) | Hazard Ratio (95% CI) | ||
|---|---|---|---|
| Breast Cancer | No Breast Cancer | ||
| Calcium, mmol/L | 0.78 (0.63–0.97) | ||
| Quartiles of calcium | |||
| <2.31 | 2656 (24.45) | 52,701 (24.09) | 1.00 (Reference) |
| 2.31–2.36 | 2416 (22.24) | 49,109 (22.44) | 0.95 (0.90–1.00) |
| 2.36–2.44 | 2962 (27.27) | 60,368 (27.59) | 0.96 (0.91–1.01) |
| ≥2.44 | 2829 (26.04) | 56,633 (25.88) | 0.94 (0.88–0.99) |
| p-value for trend | 0.04 | ||
| Calcium according to age-specific cut-offs | |||
| Low | 128 (1.18) | 2337 (1.07) | 1.12 (0.94–1.34) |
| Normal | 10,557 (97.18) | 212,101 (96.93) | 1.00 (Reference) |
| High | 178 (1.64) | 4373 (2.00) | 0.94 (0.81-1.09) |
| p-value for trend | 0.78 | ||
| Albumin-corrected calcium, mmol/L | 0.79 (0.63–0.99) | ||
| Quartiles of albumin-corrected calcium * | |||
| <2.26 | 2320 (21.36) | 49,316 (22.54) | 1.00 (Reference) |
| 2.26–2.32 | 2729 (25.12) | 56,756 (25.94) | 0.94 (0.89–1.00) |
| 2.32–2.38 | 2697 (24.83) | 53,093 (24.26) | 0.96 (0.91–1.01) |
| ≥2.38 | 3117 (28.69) | 59,645 (27.26) | 0.93 (0.88–0.99) |
| p-value for trend | 0.05 | ||
| Study | Country | Study Design | Sample Size | Participant Characteristics | Follow-up Duration | Assessment of Serum Calcium | Findings | Adjustment | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Estimates | Total Calcium (mmol/L) | Breast Cancer Risk | ||||||||
| Almquist et al. 2007 [9] | Sweden | Cohort | 7847 | Mean age 52.3 years | Mean: 17.8 years | Serum total calcium by photometry | Relative risk (RR) | <2.29 | Reference | Age, educational level, BMI, age at menarche, use of oral contraception, number of children, use of hormone-replacement therapy (HRT), smoking status, marital status, and alcohol consumption |
| 2.29–2.34 | 0.99 (0.76–1.28) | |||||||||
| 2.35–2.40 | 1.05 (0.81–1.36) | |||||||||
| ≥2.40 | 0.89 (0.67–1.19) | |||||||||
| Sprague et al. 2010 [6] | USA | Cohort | 2338 | Mean age 62.0 years | Mean: 14.3 years | Serum total calcium by ion-selective electrode analyzer | Hazard ratio (HR) | 1.40–2.28 | Reference | Age, education, menopausal status, age at menarche, age at menopause, parity, age at first birth, alcohol consumption, body mass index, and postmenopausal hormone use |
| 2.39–2.44 | 0.70 (0.42–1.17) | |||||||||
| 2.45–2.52 | 0.84 (0.51–1.40) | |||||||||
| 2.53–3.16 | 0.98 (0.60–1.60) | |||||||||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wulaningsih, W.; Sagoo, H.K.; Hamza, M.; Melvin, J.; Holmberg, L.; Garmo, H.; Malmström, H.; Lambe, M.; Hammar, N.; Walldius, G.; et al. Serum Calcium and the Risk of Breast Cancer: Findings from the Swedish AMORIS Study and a Meta-Analysis of Prospective Studies. Int. J. Mol. Sci. 2016, 17, 1487. https://doi.org/10.3390/ijms17091487
Wulaningsih W, Sagoo HK, Hamza M, Melvin J, Holmberg L, Garmo H, Malmström H, Lambe M, Hammar N, Walldius G, et al. Serum Calcium and the Risk of Breast Cancer: Findings from the Swedish AMORIS Study and a Meta-Analysis of Prospective Studies. International Journal of Molecular Sciences. 2016; 17(9):1487. https://doi.org/10.3390/ijms17091487
Chicago/Turabian StyleWulaningsih, Wahyu, Harkiran K. Sagoo, Mustafa Hamza, Jennifer Melvin, Lars Holmberg, Hans Garmo, Håkan Malmström, Mats Lambe, Niklas Hammar, Göran Walldius, and et al. 2016. "Serum Calcium and the Risk of Breast Cancer: Findings from the Swedish AMORIS Study and a Meta-Analysis of Prospective Studies" International Journal of Molecular Sciences 17, no. 9: 1487. https://doi.org/10.3390/ijms17091487






