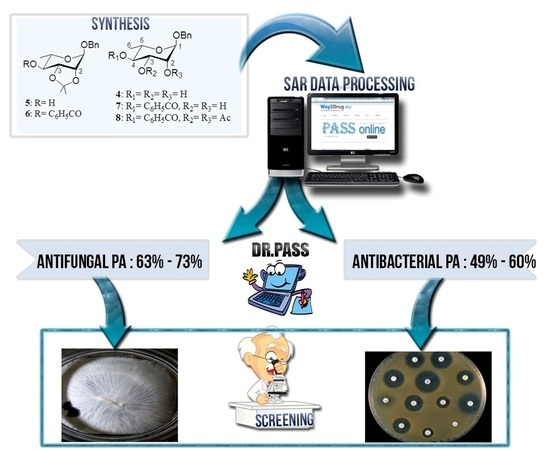
Synthesis, PASS-Predication and in Vitro Antimicrobial Activity of Benzyl 4-O-benzoyl-α-l-rhamnopyranoside Derivatives
Abstract
:1. Introduction
2. Results and Discussion
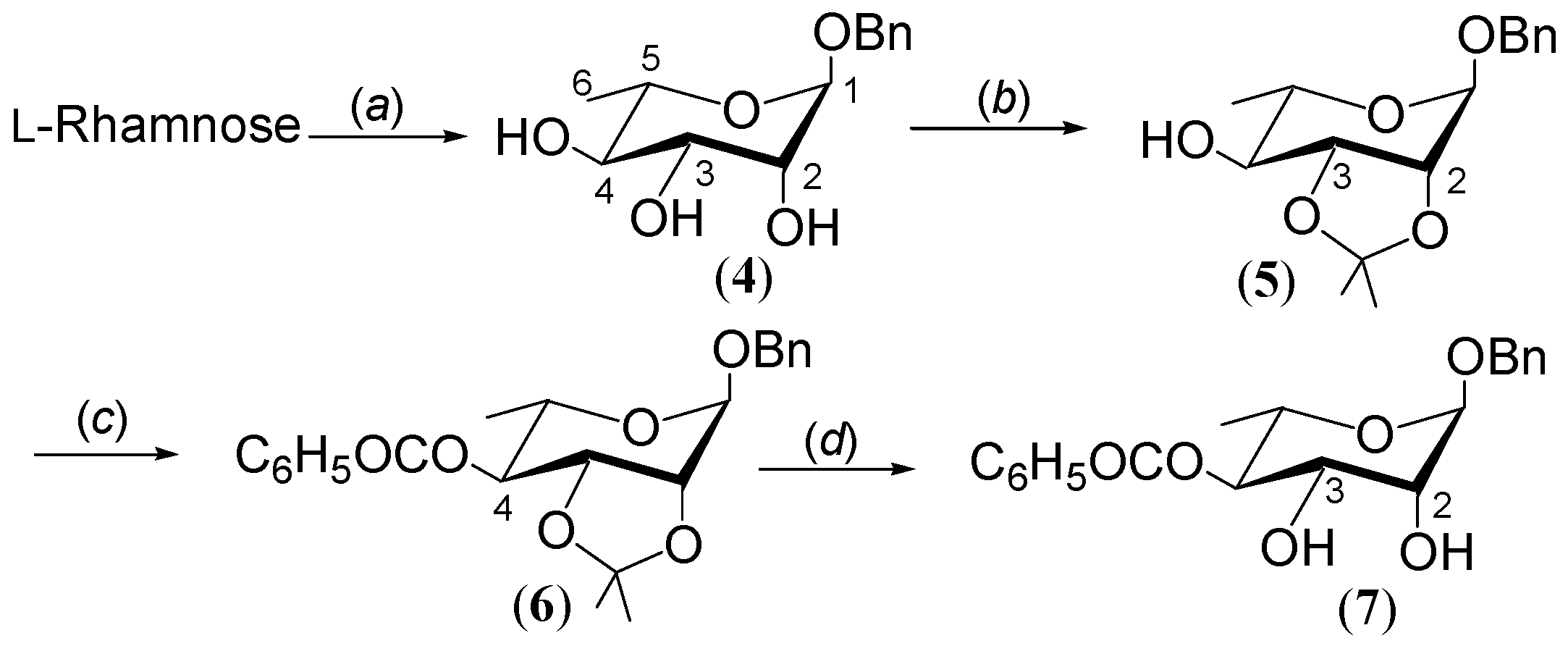
2.1. Synthesis of Benzyl 4-O-benzoyl-α-L-rhamnopyranoside 7
2.2. Synthesis of 2,3-di-O-Acetyl Derivative of 4-O-Benzoate 7
2.3. Conformational Study: Distortion of Rhamnopyranosides 5 and 6
2.4. Computational Evaluation of Antimicrobial Activities
2.5. Antimicrobial Studies
2.6. Structure Activity Relationship (SAR)
3. Materials and Methods
3.1. General Procedure: Synthesis
3.2. General Procedure for Acylation
3.3. Test Human and Phytopathogens
3.4. Antimicrobial Screening Procedure
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lüderitz, O.; Jann, K.; Wheat, R. Somatic and capsular antigens of Gram-negative bacteria. In Comprehensive Biochemistry: Extracellular and Supporting Structures; Florkin, M., Stotz, E.H., Eds.; Elsevier Publishing Company: Amsterdam, The Netherlands, 1968; Volume 26A. [Google Scholar]
- McKinnell, J.; Percival, E. Structural investigations on the water-soluble polysaccharide of the green seaweed Enteromorpha compressa. J. Chem. Soc. 1962. [Google Scholar] [CrossRef]
- Tiwari, K.; Choudhary, R. Two new steryl glycosides from Lindenbergia indica. Phytochemistry 1979, 18, 2044–2045. [Google Scholar] [CrossRef]
- Hilinski, M.K.; Mrozowski, R.M.; Clark, D.E.; Lannigan, D.A. Analogs of the RSK inhibitor SL0101: Optimization of in vitro biological stability. Bioorg. Med. Chem. Lett. 2012, 22, 3244–3247. [Google Scholar] [CrossRef] [PubMed]
- Perez-Tomas, R. Multidrug resistance: Retrospect and prospects in anti-cancer drug treatment. Curr. Med. Chem. 2006, 13, 1859–1876. [Google Scholar] [CrossRef] [PubMed]
- Pouillart, P.; Douillet, O.; Scappini, B.; Gozzini, A.; Santini, V.; Grossi, A.; Pagliai, G.; Strippoli, P.; Rigacci, L.; Ronco, G.; et al. Regioselective synthesis and biological profiling of butyric and phenylalkylcarboxylic esters derivated from d-mannose and xylitol: Influence of alkyl chain length on acute toxicity. Eur. J. Pharm. Sci. 1999, 7, 93–106. [Google Scholar] [CrossRef]
- Chortyk, O.T.; Pomonis, J.G.; Johnson, A.W. Syntheses and characterizations of insecticidal sucrose esters. J. Agric. Food Chem. 1996, 44, 1551–1557. [Google Scholar] [CrossRef]
- Kabir, A.K.M.S.; Matin, M.M.; Sanaullah, A.F.M.; Sattar, M.A.; Rahman, M.S.; Anwar, M.N. Antimicrobial activities of some lyxoside derivatives. Bangladesh J. Microbiol. 2001, 18, 89–95. [Google Scholar]
- Kabir, A.K.M.S.; Matin, M.M.; Bhuiyan, M.M.R.; Rahim, M.A.; Rahman, M.S. Biological evaluation of some monosaccharide derivatives. Int. J. Agric. Biol. 2005, 7, 218–221. [Google Scholar]
- Kabir, A.K.M.S.; Rahman, M.S.; Matin, M.M.; Bhuiyan, M.M.R.; Ali, M. Antimicrobial activities of some d-glucose derivatives. Chittagong Univ. J. Sci. 2001, 25, 123–128. [Google Scholar]
- Kabir, A.K.M.S.; Matin, M.M.; Mridha, M.A.U.; Shahed, S.M. Antifungal activities of some methyl 6-O-trityl-α-d-mannopyranosides. Chittagong Univ. J. Sci. 1998, 22, 41–46. [Google Scholar]
- Ahsan, F.; Arnold, J.J.; Meezan, E.; Pillion, D.J. Sucrose cocoate, a component of cosmetic preparations, enhances nasal and ocular peptide absorption. Int. J. Pharm. 2003, 251, 195–203. [Google Scholar] [CrossRef]
- Csóka, G.; Marton, S.; Zelko, R.; Otomo, N.; Antal, I. Application of sucrose fatty acid esters in transdermal therapeutic systems. Eur. J. Pharm. Biopharm. 2007, 65, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Kabir, A.K.M.S.; Matin, M.M.; Ali, M.; Anwar, M.N. Comparative studies on selective acylation and antimicrobial activities of some d-glucofuranose derivatives. J. Bangladesh Acad. Sci. 2003, 27, 43–50. [Google Scholar]
- Dhavale, D.D.; Matin, M.M. Piperidine homoazasugars: Natural occurrence, synthetic aspects and biological activity study. Arkivoc 2005, 3, 110–132. [Google Scholar] [CrossRef]
- Dhavale, D.D.; Matin, M.M. Selective sulfonylation of 4-C-hydroxymethyl-β-l-threo-pento-1, 4-furanose: Synthesis of bicyclic diazasugars. Tetrahedron 2004, 60, 4275–4281. [Google Scholar] [CrossRef]
- Matin, M.M. Synthesis of d-glucose derived oxetane: 1,2-O-ısopropylidene-4-(S)-3-O,4-C-methylene-5-O-methanesulfonyl-β-l-threo-pento-1,4-furanose. J. Appl. Sci. Res. 2008, 4, 1478–1482. [Google Scholar]
- Dong, H.; Zhou, Y.; Pan, X.; Cui, F.; Liu, W.; Liu, J.; Ramström, O. Stereoelectronic control in regioselective carbohydrate protection. J. Org. Chem. 2012, 77, 1457–1467. [Google Scholar] [CrossRef] [PubMed]
- Grindley, T.B. Applications of tin-containing intermediates to carbohydrate chemistry. Adv. Carbohydr. Chem. Biochem. 1998, 53, 17–142. [Google Scholar] [PubMed]
- Kabir, A.K.M.S.; Matin, M.M. Regioselective monoacylation of a derivative of l-rhamnose. J. Bangladesh Acad. Sci. 1997, 21, 83–88. [Google Scholar]
- Matin, M.M.; Bhuiyan, M.M.H.; Azad, A.K.M.S. Synthesis and antimicrobial evaluation of some n-butyl α- and β-d-glucopyranoside derivatives. RGUHS J. Pharm. Sci. 2013, 3, 53–59. [Google Scholar]
- Matin, M.M.; Bhuiyan, M.M.H.; Debnath, D.C.; Manchur, M.A. Synthesis and comparative antimicrobial studies of some acylated d-glucofuranose and d-glucopyranose derivatives. Int. J. Biosci. 2013, 3, 279–287. [Google Scholar]
- Kabir, A.K.M.S.; Matin, M.M.; Hossain, A.; Rahman, M.S. Synthesis and antimicrobial activities of some acylated derivatives of l-rhamnose. Chittagong Univ. J. Sci. 2002, 26, 35–44. [Google Scholar]
- Kabir, A.K.M.S.; Matin, M.M.; Hossain, A.; Sattar, M.A. Synthesis and antimicrobial activities of some rhamno-pyranoside derivatives. J. Bangladesh Chem. Soc. 2003, 16, 85–93. [Google Scholar]
- Kabir, A.K.M.S.; Alauddin, M.; Matin, M.M.; Bhattacharjee, S.C. Regioselective monobenzoylation of methyl α-l-rhamnopyranoside. Chittagong Univ. Stud. 1997, 21, 59–63. [Google Scholar]
- Kadir, F.A.; Kassim, N.M.; Abdulla, M.A.; Yehye, W.A. PASS-predicted Vitex negundo activity: Antioxidant and antiproliferative properties on human hepatoma cells-an in vitro study. BMC Complement. Altern. Med. 2013, 13, 343. [Google Scholar] [CrossRef] [PubMed]
- Yehye, W.A.; Abdul Rahman, N.; A. Alhadi, A.; Khaledi, H.; Ng, S.W.; Ariffin, A. Butylated hydroxytoluene analogs: Synthesis and evaluation of their multipotent antioxidant activities. Molecules 2012, 17, 7645. [Google Scholar] [CrossRef] [PubMed]
- Ariffin, A.; Rahman, N.A.; Yehye, W.A.; Alhadi, A.A.; Kadir, F.A. PASS-assisted design, synthesis and antioxidant evaluation of new butylated hydroxytoluene derivatives. Eur. J. Med. Chem. 2014, 87, 564–577. [Google Scholar] [CrossRef] [PubMed]
- Kabir, A.K.M.S.; Matin, M.M.; Islam, K.R.; Anwar, M.N. Synthesis and antimicrobial activities of some acylated uridine derivatives. J. Bangladesh Chem. Soc. 2002, 15, 13–22. [Google Scholar]
- Lázár, L.; Csávás, M.; Borbás, A.; Gyémánt, G.; Lipták, A. Synthesis of methyl 6-deoxy-4-O-(sodium sulfonato)-α-l-talopyranoside, its C-4 epimer and both isosteric [4-C-(potassium sulfonatomethyl)] derivatives. Arkivoc 2004, 7, 196–207. [Google Scholar]
- Lipták, A.; Fügedi, P.; Nánási, P. Synthesis of mono- and di-benzyl ethers of benzyl α-l-rhamnopyranoside. Carbohydr. Res. 1978, 65, 209–217. [Google Scholar] [CrossRef]
- Matin, M.M. Synthesis and antimicrobial study of some methyl 4-O-palmitoyl-α-l-rhamnopyranoside derivatives. Orbital: Electron. J. Chem. 2014, 6, 20–28. [Google Scholar]
- Stepanchikova, A.V.; Lagunin, A.A.; Filimonov, D.A.; Poroikov, V.V. Prediction of biological activity spectra for substances: Evaluation on the diverse sets of drug-like structures. Curr. Med. Chem. 2003, 10, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Anzali, S.; Barnickel, G.; Cezanne, B.; Krug, M.; Filimonov, D.; Poroikov, V. Discriminating between drugs and nondrugs by prediction of activity spectra for substances (PASS). J. Med. Chem. 2001, 44, 2432–2437. [Google Scholar] [CrossRef] [PubMed]
- Kadir, F.; Kassim, N.B.M.; Abdulla, M.A.; Kamalidehghan, B.; Ahmadipour, F.; Yehye, W.A. PASS-predicted hepatoprotective activity of Caesalpinia sappan in thioacetamide-induced liver fibrosis in rats. Sci. World J. 2014. [Google Scholar] [CrossRef] [PubMed]
- Poroikov, V.; Filimonov, D.; Lagunin, A.; Gloriozova, T.; Zakharov, A. PASS: Identification of probable targets and mechanisms of toxicity. SAR QSAR Environ. Res. 2007, 18, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Delmas, F.; di Giorgio, C.; Robin, M.; Azas, N.; Gasquet, M.; Detang, C.; Costa, M.; Timon-David, P.; Galy, J.-P. In vitro activities of position 2 substitution-bearing 6-nitro-and 6-amino-benzothiazoles and their corresponding anthranilic acid derivatives against Leishmania infantum and Trichomonas vaginalis. Antimicrob. Agents Chemother. 2002, 46, 2588–2594. [Google Scholar] [CrossRef] [PubMed]
- Di Giorgio, C.; Delmas, F.; Filloux, N.; Robin, M.; Seferian, L.; Azas, N.; Gasquet, M.; Costa, M.; Timon-David, P.; Galy, J.-P. In vitro activities of 7-substituted 9-chloro and 9-amino-2-methoxyacridines and their bis-and tetra-acridine complexes against Leishmania infantum. Antimicrob. Agents Chemother. 2003, 47, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Di Giorgio, C.; Delmas, F.; Ollivier, E.; Elias, R.; Balansard, G.; Timon-David, P. In vitro activity of the β-carboline alkaloids harmane, harmine, and harmaline toward parasites of the species Leishmania infantum. Exp. Parasitol. 2004, 106, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Hunt, W.A. The effects of aliphatic alcohols on the biophysical and biochemical correlates of membrane function. In Biochemical Pharmacology of Ethanol; Majchrowicz, E., Ed.; Plenum Press: New York, USA, 1975; pp. 195–210. [Google Scholar]
- Judge, V.; Narasimhan, B.; Ahuja, M.; Sriram, D.; Yogeeswari, P.; de Clercq, E.; Pannecouque, C.; Balzarini, J. Synthesis, antimycobacterial, antiviral, antimicrobial activity and QSAR studies of N2-acyl isonicotinic acid hydrazide derivatives. Med. Chem. 2013, 9, 53–76. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are not available.

| Compounds | Coupling Constants (Hz) | ||
|---|---|---|---|
| J2,3 | J3,4 | J4,5 | |
| 5 | 5.8 | 6.9 | – |
| 6 | 2.9 | 10.1 | 6.9 |
| 7 | 3.4 | 9.6 | 10.0 |
| 8 | 3.2 | 10.0 | 10.0 |
| Compound No. | Biological Activity | |||
|---|---|---|---|---|
| Antibacterial | Antifungal | |||
| Pa | Pi | Pa | Pi | |
| 4 | 0.561 | 0.011 | 0.654 | 0.013 |
| 5 | 0.495 | 0.017 | 0.733 | 0.008 |
| 6 | 0.479 | 0.018 | 0.715 | 0.009 |
| 7 | 0.582 | 0.010 | 0.665 | 0.012 |
| 8 | 0.608 | 0.008 | 0.670 | 0.012 |
| Name of Bacteria | Diameter of Zone of Inhibition in mm, 50 μg/dw/disc | |||||
|---|---|---|---|---|---|---|
| 4 | 5 | 6 | 7 | 8 | Ampicillin ** | |
| Bacillus cereus | NI | 08 | NI | NI | 06 | 22 * |
| Bacillus megaterium | NI | NI | 10 | NI | NI | 19 |
| Bacillus subtilis | NI | NI | 11 | NI | 08 | 25 * |
| Staphylococcus aureus | NI | NI | NI | NI | NI | 21 * |
| Escherichia coli | NI | NI | NI | 08 | NI | 25 * |
| Klebsiella pneumoniae | NI | 06 | NI | 09 | NI | 22P |
| Pseudomonas aeruginosa | NI | NI | NI | NI | NI | 17 |
| Salmonella typhi | NI | NI | NI | NI | NI | 13 * |
| Name of Fungus | % Inhibition of Fungal Mycelial Growth, Sample 100 μg/dw/mL PDA | |||||
|---|---|---|---|---|---|---|
| 4 | 5 | 6 | 7 | 8 | Nystatin ** | |
| Aspergillus brasiliensis | 35.0 | 30.0 | 25.0 | 20.0 | NI | 66.4 * |
| Candida albicans | 60.0 * | 58.8 | 63.8 * | 65.0 * | NI | 63.1 * |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matin, M.M.; Nath, A.R.; Saad, O.; Bhuiyan, M.M.H.; Kadir, F.A.; Abd Hamid, S.B.; Alhadi, A.A.; Ali, M.E.; Yehye, W.A. Synthesis, PASS-Predication and in Vitro Antimicrobial Activity of Benzyl 4-O-benzoyl-α-l-rhamnopyranoside Derivatives. Int. J. Mol. Sci. 2016, 17, 1412. https://doi.org/10.3390/ijms17091412
Matin MM, Nath AR, Saad O, Bhuiyan MMH, Kadir FA, Abd Hamid SB, Alhadi AA, Ali ME, Yehye WA. Synthesis, PASS-Predication and in Vitro Antimicrobial Activity of Benzyl 4-O-benzoyl-α-l-rhamnopyranoside Derivatives. International Journal of Molecular Sciences. 2016; 17(9):1412. https://doi.org/10.3390/ijms17091412
Chicago/Turabian StyleMatin, Mohammed Mahbubul, Amit R. Nath, Omar Saad, Mohammad M. H. Bhuiyan, Farkaad A. Kadir, Sharifah Bee Abd Hamid, Abeer A. Alhadi, Md. Eaqub Ali, and Wageeh A. Yehye. 2016. "Synthesis, PASS-Predication and in Vitro Antimicrobial Activity of Benzyl 4-O-benzoyl-α-l-rhamnopyranoside Derivatives" International Journal of Molecular Sciences 17, no. 9: 1412. https://doi.org/10.3390/ijms17091412