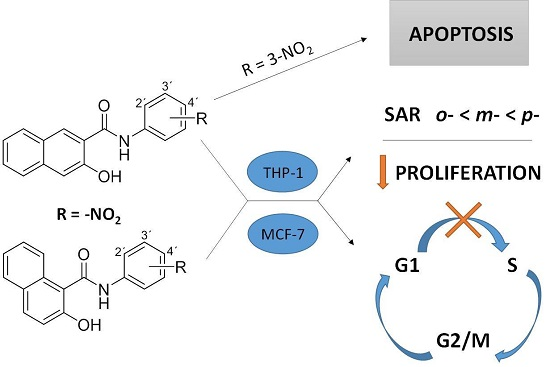
Antiproliferative and Pro-Apoptotic Effect of Novel Nitro-Substituted Hydroxynaphthanilides on Human Cancer Cell Lines
Abstract
:1. Introduction
2. Results
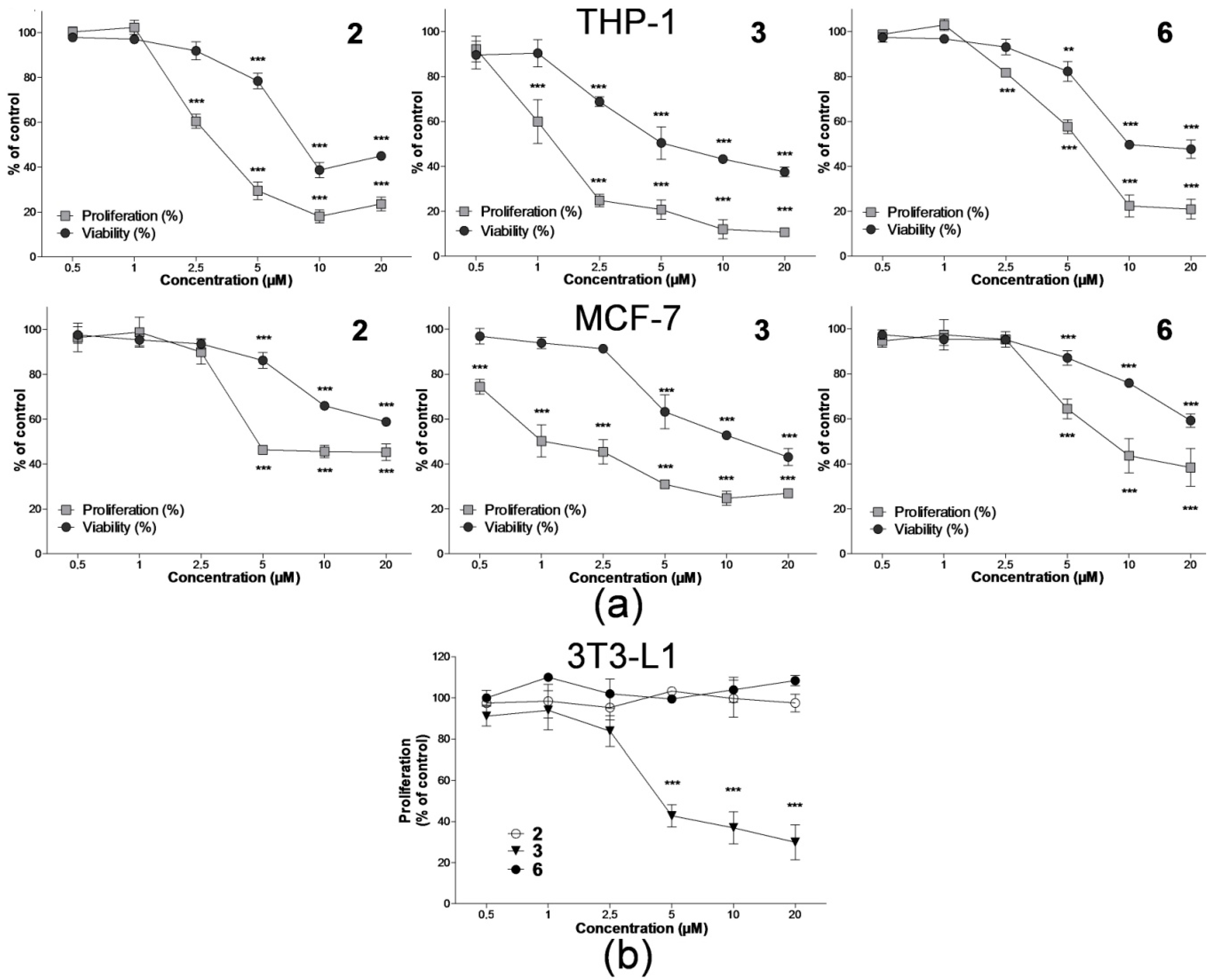
2.1. Effect on Cell Proliferation and Viability
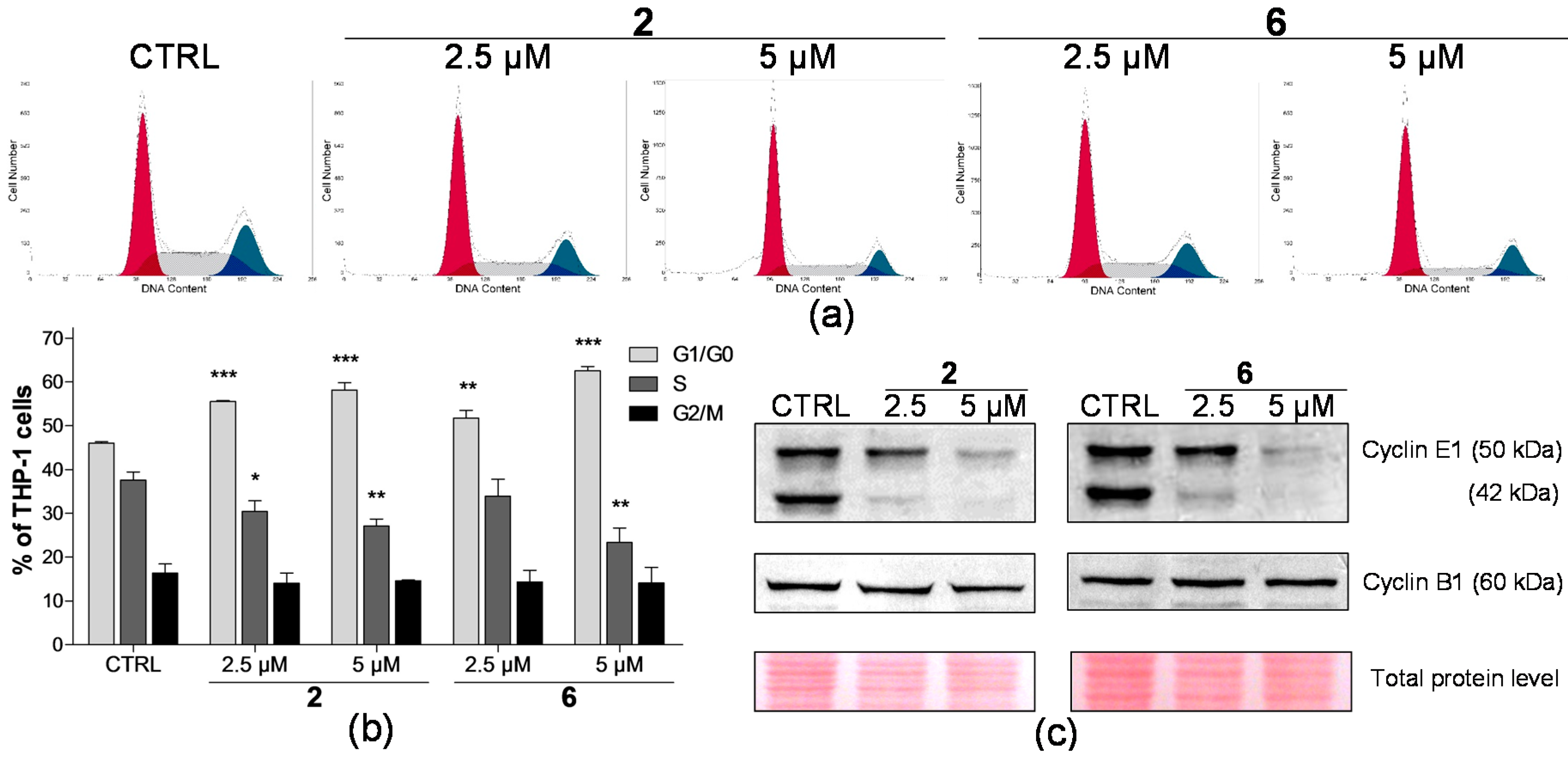
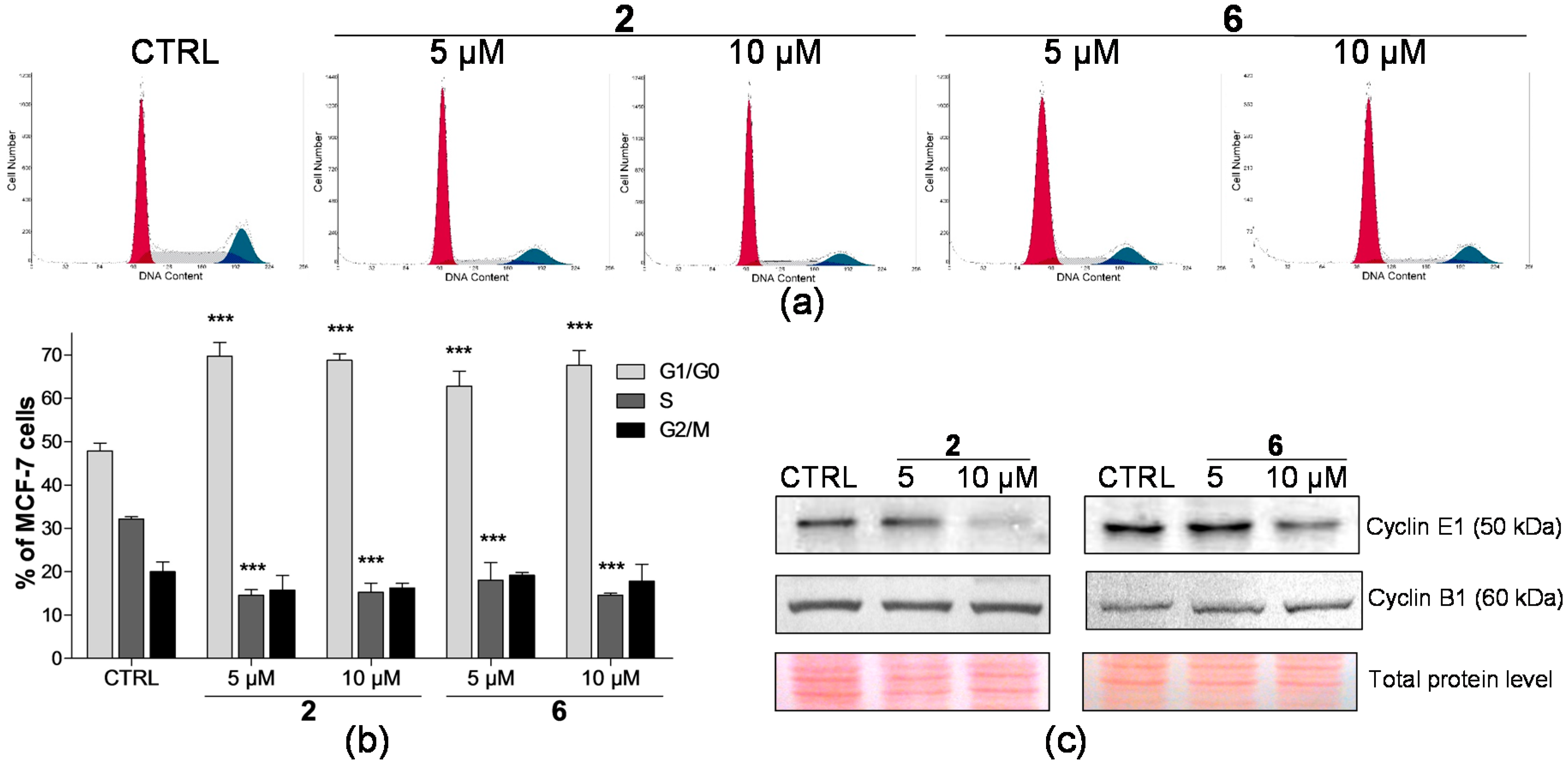
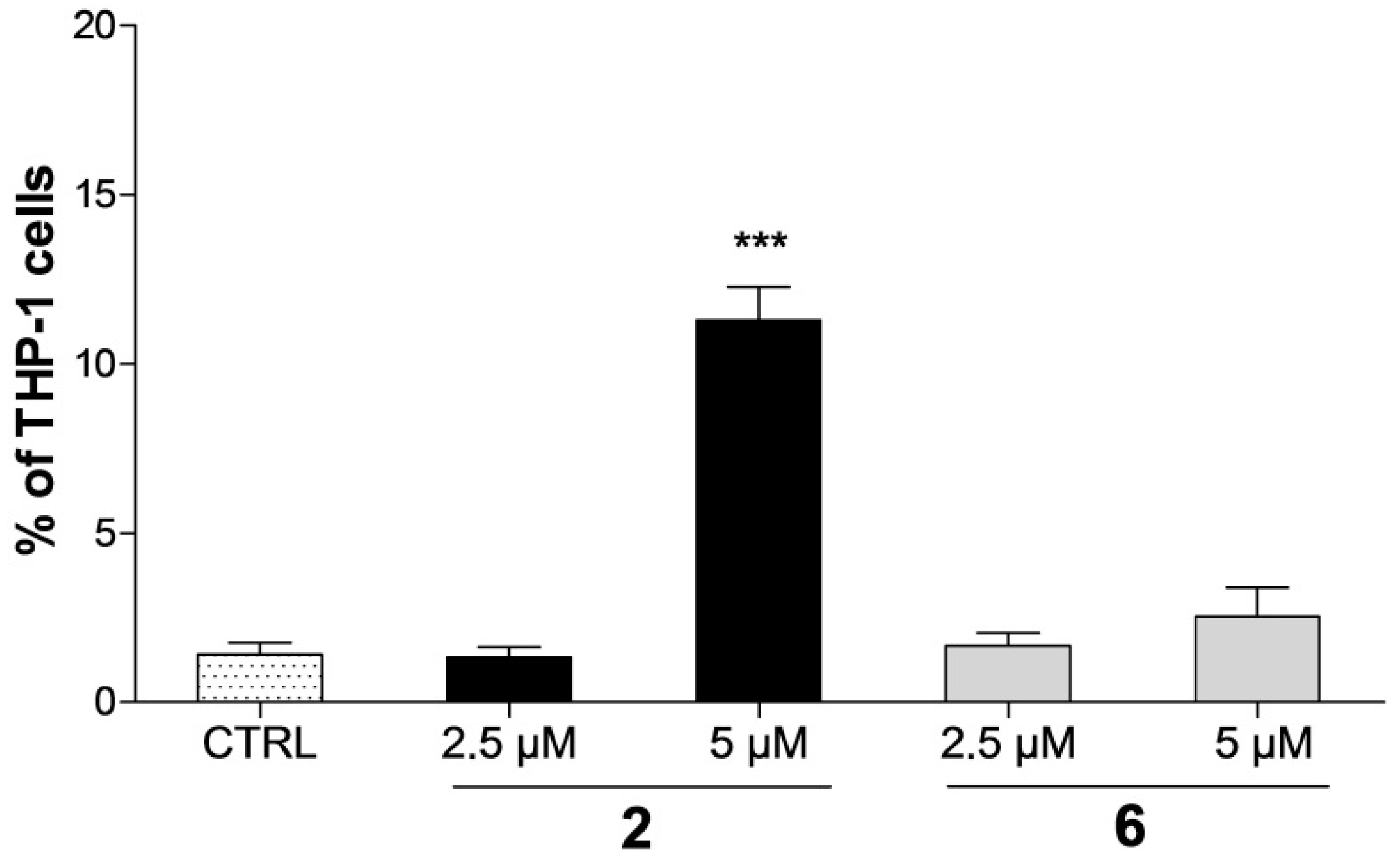
2.2. Effect on Distribution of Cells in Cell Cycle Phases
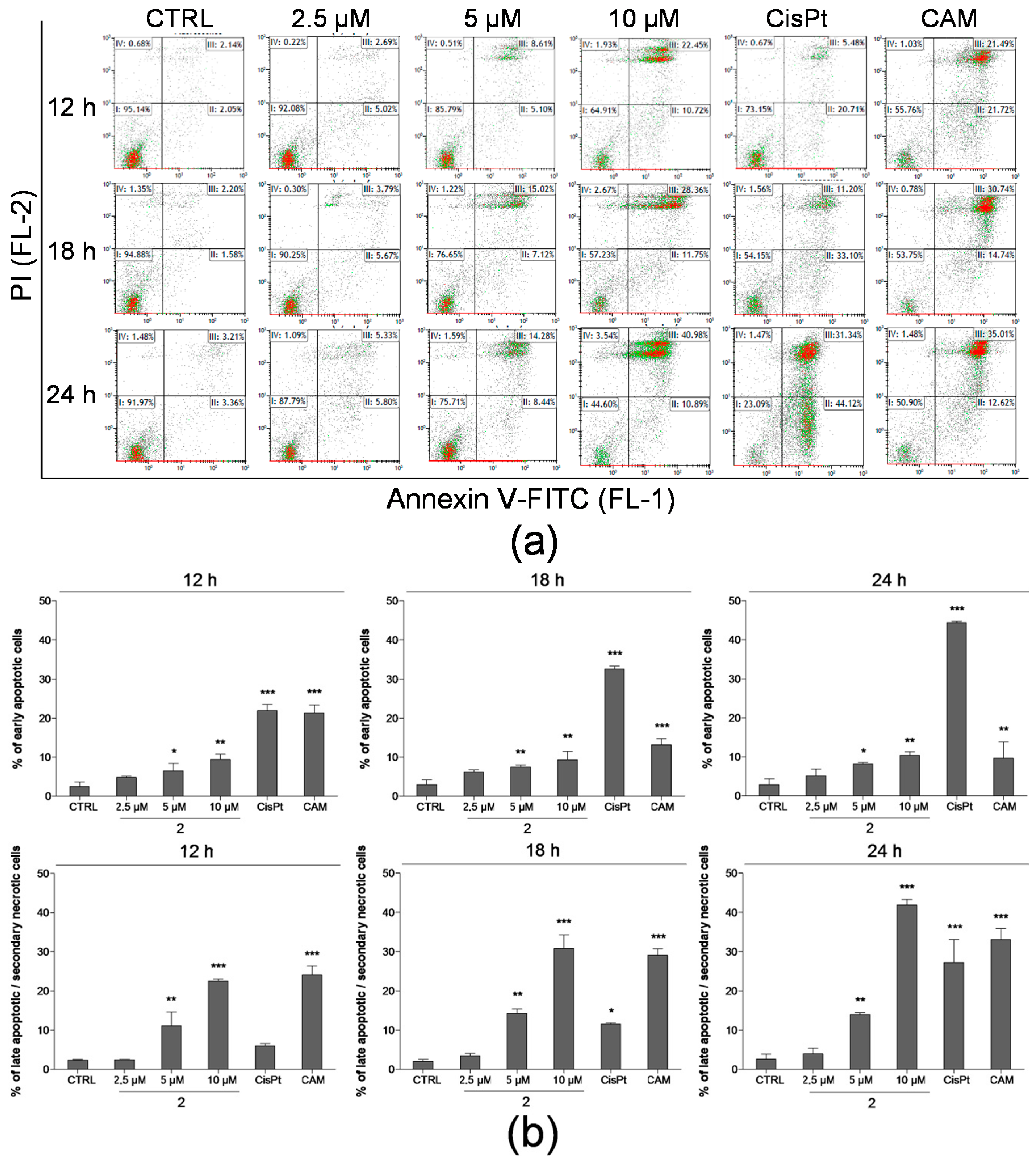
2.3. Detection of Apoptosis by Annexin V-FITC/PI Assay
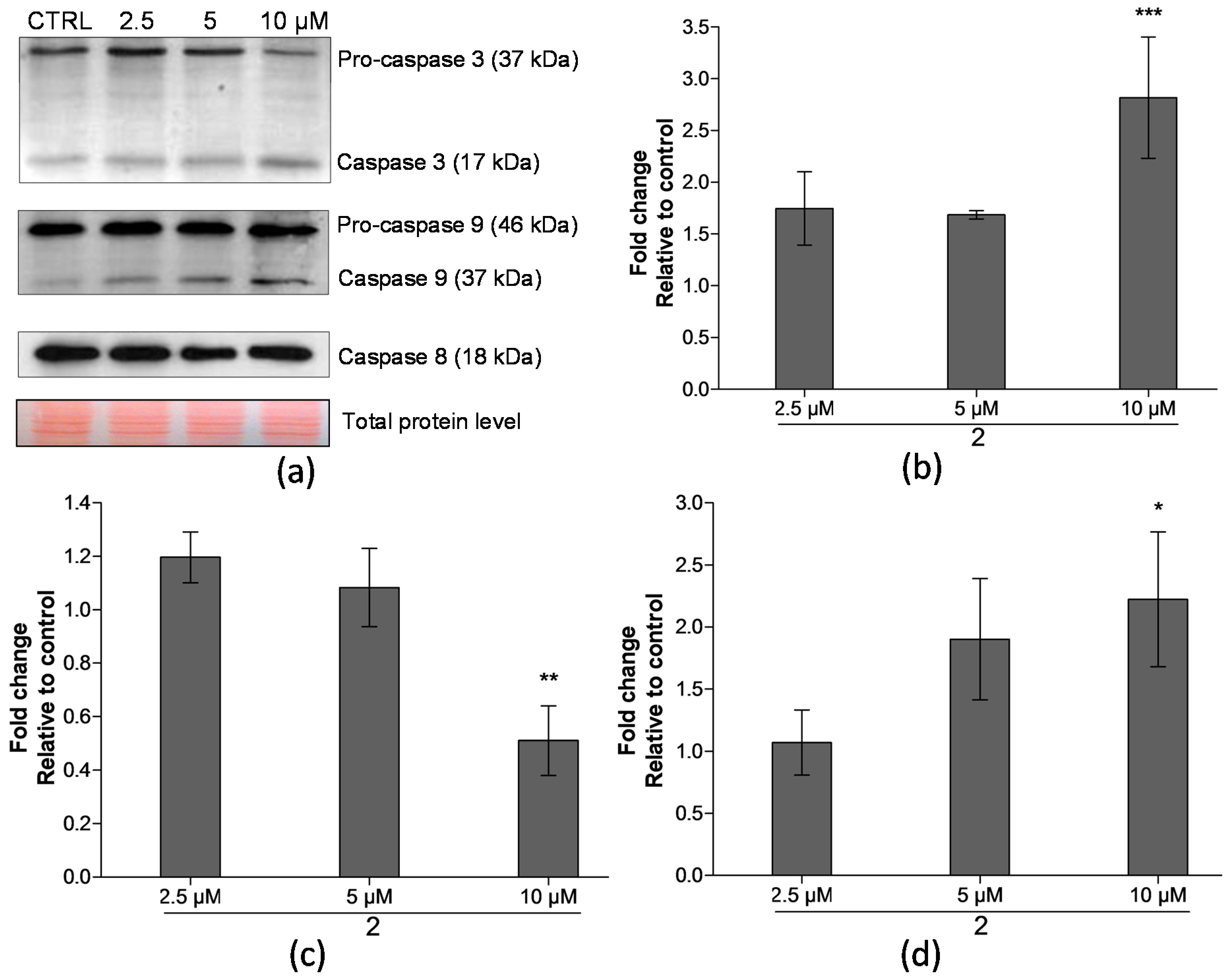
2.4. Analysis of Proteins Levels Involved in Apoptotic Pathways
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cell Culture
4.3. Analysis of Cell Proliferation and Viability
4.4. Cell Cycle Analysis
4.5. Detection of Apoptosis Using Annexin V-FITC/PI Assay
4.6. Western Blotting
4.7. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kratky, M.; Vinsova, J.; Novotna, E.; Mandikova, J.; Wsol, V.; Trejtnar, F.; Ulmann, V.; Stolarikova, J.; Fernandes, S.; Bhat, S.; et al. Salicylanilide derivatives block mycobacterium tuberculosis through inhibition of isocitrate lyase and methionine aminopeptidase. Tuberculosis (Edinburgh) 2012, 92, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Zadrazilova, I.; Pospisilova, S.; Masarikova, M.; Imramovsky, A.; Ferriz, J.M.; Vinsova, J.; Cizek, A.; Jampilek, J. Salicylanilide carbamates: Promising antibacterial agents with high in vitro activity against methicillin-resistant staphylococcus aureus (MRSA). Eur. J. Pharm. Sci. 2015, 77, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Gonec, T.; Kos, J.; Zadrazilova, I.; Pesko, M.; Govender, R.; Keltosova, S.; Chambel, B.; Pereira, D.; Kollar, P.; Imramovsky, A.; et al. Antibacterial and herbicidal activity of ring-substituted 2-hydroxynaphthalene-1-carboxanilides. Molecules 2013, 18, 9397–9419. [Google Scholar] [CrossRef] [PubMed]
- Gonec, T.; Kos, J.; Zadrazilova, I.; Pesko, M.; Keltosova, S.; Tengler, J.; Bobal, P.; Kollar, P.; Cizek, A.; Kralova, K.; et al. Antimycobacterial and herbicidal activity of ring-substituted 1-hydroxynaphthalene-2-carboxanilides. Bioorg. Med. Chem. 2013, 21, 6531–6541. [Google Scholar] [CrossRef] [PubMed]
- Kos, J.; Zadrazilova, I.; Pesko, M.; Keltosova, S.; Tengler, J.; Gonec, T.; Bobal, P.; Kauerova, T.; Oravec, M.; Kollar, P.; et al. Antibacterial and herbicidal activity of ring-substituted 3-hydroxynaphthalene-2-carboxanilides. Molecules 2013, 18, 7977–7997. [Google Scholar] [CrossRef] [PubMed]
- Olliaro, P.; Seiler, J.; Kuesel, A.; Horton, J.; Clark, J.N.; Don, R.; Keiser, J. Potential drug development candidates for human soil-transmitted helminthiases. PLoS Negl. Trop. Dis. 2011, 5, e1138. [Google Scholar] [CrossRef] [PubMed]
- Mudduluru, G.; Walther, W.; Kobelt, D.; Dahlmann, M.; Treese, C.; Assaraf, Y.G.; Stein, U. Repositioning of drugs for intervention in tumor progression and metastasis: Old drugs for new targets. Drug Resist. Updat. 2016, 26, 10–27. [Google Scholar] [CrossRef] [PubMed]
- Osada, T.; Chen, M.; Yang, X.Y.; Spasojevic, I.; Vandeusen, J.B.; Hsu, D.; Clary, B.M.; Clay, T.M.; Chen, W.; Morse, M.A.; et al. Antihelminth compound niclosamide downregulates Wnt signaling and elicits antitumor responses in tumors with activating APC mutations. Cancer Res. 2011, 71, 4172–4182. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, P.K.; Roberts, M.J.; Arend, R.C.; Samant, R.S.; Buchsbaum, D.J. Multi-targeted therapy of cancer by niclosamide: A new application for an old drug. Cancer Lett. 2014, 349, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, B.D.; Diering, G.H.; Bidinosti, M.A.; Dalal, K.; Alain, T.; Balgi, A.D.; Forestieri, R.; Nodwell, M.; Rajadurai, C.V.; Gunaratnam, C.; et al. Structure-activity analysis of niclosamide reveals potential role for cytoplasmic pH in control of mammalian target of rapamycin complex 1 (mTORC1) signaling. J. Biol. Chem. 2012, 287, 17530–17545. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Lin, C.; Roberts, M.J.; Waud, W.R.; Piazza, G.A.; Li, Y. Niclosamide suppresses cancer cell growth by inducing Wnt co-receptor LRP6 degradation and inhibiting the Wnt/β-catenin pathway. PLoS ONE 2011, 6, e29290. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Lu, Z.; Ding, K.; Li, J.; Du, X.; Chen, C.; Sun, X.; Wu, Y.; Zhou, J.; Pan, J. Antineoplastic mechanisms of niclosamide in acute myelogenous leukemia stem cells: Inactivation of the NF-kappaB pathway and generation of reactive oxygen species. Cancer Res. 2010, 70, 2516–2527. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.-R.; Shan, A.-L.; Li, Y.-P.; Xu, J.; Lu, S.-M.; Zhang, S.-Q. Discovery of 2-aryl-8-hydroxy (or methoxy)-isoquinolin-1(2H)-ones as novel EGFR inhibitor by scaffold hopping. Bioorg. Med. Chem. 2013, 21, 6956–6964. [Google Scholar] [CrossRef] [PubMed]
- Liechti, C.; Sequin, U.; Bold, G.; Furet, P.; Meyer, T.; Traxler, P. Salicylanilides as inhibitors of the protein tyrosine kinase epidermal growth factor receptor. Eur. J. Med. Chem. 2004, 39, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Guo, Z.; Guo, Y.; Feng, Z.; Jiang, Y.; Chu, F. Acryloylamino-salicylanilides as EGFR PTK inhibitors. Bioorg. Med. Chem. Lett. 2016, 16, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.F.; Wang, J.S.; Cai, L.L.; Zeng, Y.X.; Yang, D. SUCI02 inhibits the erbb-2 tyrosine kinase receptor signaling pathway and arrests the cell cycle in G1 phase in breast cancer cells. Cancer Sci. 2006, 97, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Imramovsky, A.; Jorda, R.; Pauk, K.; Reznickova, E.; Dusek, J.; Hanusek, J.; Krystof, V. Substituted 2-hydroxy-N-(arylalkyl)benzamides induce apoptosis in cancer cell lines. Eur. J. Med. Chem. 2016, 68, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Zuo, M.; Zheng, Y.W.; Lu, S.M.; Li, Y.; Zhang, S.Q. Synthesis and biological evaluation of N-aryl salicylamides with a hydroxamic acid moiety at 5-position as novel HDAC-EGFR dual inhibitors. Bioorg. Med. Chem. 2012, 20, 4405–4412. [Google Scholar] [CrossRef] [PubMed]
- Solomon, V.R.; Lee, H. Quinoline as a privileged scaffold in cancer drug discovery. Curr. Med. Chem. 2011, 18, 1488–1508. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Wang, Q.L.; Jiang, Q.Q.; Jiang, Q.J.; Jiang, Y.B. Anion-triggered substituent-dependent conformational switching of salicylanilides. New hints for understanding the inhibitory mechanism of salicylanilides. J. Org. Chem. 2007, 72, 9947–9953. [Google Scholar] [CrossRef] [PubMed]
- Walters Haygood, C.L.; Arend, R.C.; Gangrade, A.; Chettiar, S.; Regan, N.; Hassmann, C.J., 2nd; Li, P.K.; Hidalgo, B.; Straughn, J.M., Jr.; Buchsbaum, D.J. Niclosamide analogs for treatment of ovarian cancer. Int. J. Gynecol. Cancer 2015, 25, 1377–1385. [Google Scholar] [CrossRef] [PubMed]
- Brumatti, G.; Sheridan, C.; Martin, S.J. Expression and purification of recombinant annexin V for the detection of membrane alterations on apoptotic cells. Methods 2008, 44, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S. Caspase function in programmed cell death. Cell Death Differ. 2007, 14, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Malik, I.; Bukovsky, M.; Andriamainty, F.; Galisinova, J. Antimicrobial activity of meta-alkoxyphenylcarbamates containing substituted N-phenylpiperazine fragment. Braz. J. Microbiol. 2012, 3, 959–965. [Google Scholar]
- Williams, G.H.; Stoeber, K. The cell cycle and cancer. J. Pathol. 2012, 226, 352–364. [Google Scholar] [CrossRef] [PubMed]
- Malumbres, M.; Barbacid, M. Cell cycle, CDKs and cancer: A changing paradigm. Nat. Rev. Cancer 2009, 9, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Moralli, S.; Tarrado-Castellarnau, M.; Miranda, A.; Cascante, M. Targeting cell cycle regulation in cancer therapy. Pharmacol. Ther. 2013, 138, 255–271. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Hartley, R.S. Hur contributes to cyclin E1 deregulation in MCF-7 breast cancer cells. Cancer Res. 2006, 66, 7948–7956. [Google Scholar] [CrossRef] [PubMed]
- Wingate, H.; Bedrosian, I.; Akli, S.; Keyomarsi, K. The low molecular weight (LMW) isoforms of cyclin E deregulate the cell cycle of mammary epithelial cells. Cell Cycle 2003, 2, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Akli, S.; Keyomarsi, K. Cyclin E and its low molecular weight forms in human cancer and as targets for cancer therapy. Cancer Biol. Ther. 2003, 2, S38–S47. [Google Scholar] [CrossRef] [PubMed]
- Kollar, P.; Barta, T.; Zavalova, V.; Smejkal, K.; Hampl, A. Geranylated flavanone tomentodiplacone B inhibits proliferation of human monocytic leukaemia (THP-1) cells. Br. J. Pharmacol. 2011, 162, 1534–1541. [Google Scholar] [CrossRef] [PubMed]
- Kajstura, M.; Halicka, H.D.; Pryjma, J.; Darzynkiewicz, Z. Discontinuous fragmentation of nuclear DNA during apoptosis revealed by discrete “sub-G1” peaks on DNA content histograms. Cytom. A 2007, 71, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.F.; Desai, S.D.; Li, T.K.; Mao, Y.; Sun, M.; Sim, S.P. Mechanism of action of camptothecin. Ann. N. Y. Acad. Sci. 2000, 922, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, D.A.; Toltl, L.J.; Beaudin, S.; Liaw, P.C. Modulation of monocyte function by activated protein C, a natural anticoagulant. J. Immunol. 2006, 177, 2115–2122. [Google Scholar] [CrossRef] [PubMed]
- Amran, D.; Sancho, P.; Fernandez, C.; Esteban, D.; Ramos, A.M.; de Blas, E.; Gomez, M.; Palacios, M.A.; Aller, P. Pharmacological inhibitors of extracellular signal-regulated protein kinases attenuate the apoptotic action of cisplatin in human myeloid leukemia cells via glutathione-independent reduction in intracellular drug accumulation. Biochim. Biophys. Acta 2005, 1743, 269–279. [Google Scholar] [CrossRef] [PubMed]
- McIlwain, D.R.; Berger, T.; Mak, T.W. Caspase functions in cell death and disease. Cold Spring Harb. Perspect. Biol. 2013, 5, a008656. [Google Scholar] [CrossRef] [PubMed]
- Degterev, A.; Boyce, M.; Yuan, J. A decade of caspases. Oncogene 2003, 22, 8543–8567. [Google Scholar] [CrossRef] [PubMed]
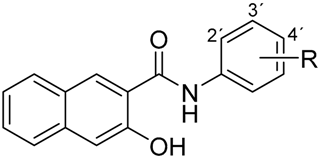 | 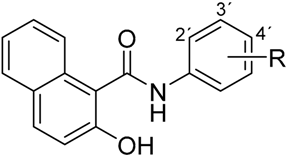 | ||
|---|---|---|---|
| (a) | (b) | ||
| Compound | R | Compound | R |
| 1 | 2-NO2 | 4 | 2-NO2 |
| 2 | 3-NO2 | 5 | 3-NO2 |
| 3 | 4-NO2 | 6 | 4-NO2 |
| Compound | THP-1 | MCF-7 | 3T3-L1 | ||
|---|---|---|---|---|---|
| IC50 (μM) | LC50 (μM) | IC50 (μM) | LC50 (μM) | IC50 (μM) | |
| 1 | >20 | >20 | >20 | >20 | >20 |
| 2 | 3.06 ± 0.206 | 7.91 ± 0.240 | 4.61 ± 0.068 | >20 | >20 |
| 3 | 1.05 ± 0.199 | 3.44 ± 1.209 | 1.65 ± 0.938 | 12.91 ± 1.984 | 4.41 ± 0.293 |
| 4 | >20 | >20 | >20 | >20 | >20 |
| 5 | >20 | >20 | >20 | >20 | >20 |
| 6 | 5.80 ± 0.370 | 9.98 ± 0.349 | 5.23 ± 0.802 | >20 | >20 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kauerova, T.; Kos, J.; Gonec, T.; Jampilek, J.; Kollar, P. Antiproliferative and Pro-Apoptotic Effect of Novel Nitro-Substituted Hydroxynaphthanilides on Human Cancer Cell Lines. Int. J. Mol. Sci. 2016, 17, 1219. https://doi.org/10.3390/ijms17081219
Kauerova T, Kos J, Gonec T, Jampilek J, Kollar P. Antiproliferative and Pro-Apoptotic Effect of Novel Nitro-Substituted Hydroxynaphthanilides on Human Cancer Cell Lines. International Journal of Molecular Sciences. 2016; 17(8):1219. https://doi.org/10.3390/ijms17081219
Chicago/Turabian StyleKauerova, Tereza, Jiri Kos, Tomas Gonec, Josef Jampilek, and Peter Kollar. 2016. "Antiproliferative and Pro-Apoptotic Effect of Novel Nitro-Substituted Hydroxynaphthanilides on Human Cancer Cell Lines" International Journal of Molecular Sciences 17, no. 8: 1219. https://doi.org/10.3390/ijms17081219
APA StyleKauerova, T., Kos, J., Gonec, T., Jampilek, J., & Kollar, P. (2016). Antiproliferative and Pro-Apoptotic Effect of Novel Nitro-Substituted Hydroxynaphthanilides on Human Cancer Cell Lines. International Journal of Molecular Sciences, 17(8), 1219. https://doi.org/10.3390/ijms17081219