La Autoantigen Induces Ribosome Binding Protein 1 (RRBP1) Expression through Internal Ribosome Entry Site (IRES)-Mediated Translation during Cellular Stress Condition
Abstract
:1. Introduction
2. Results
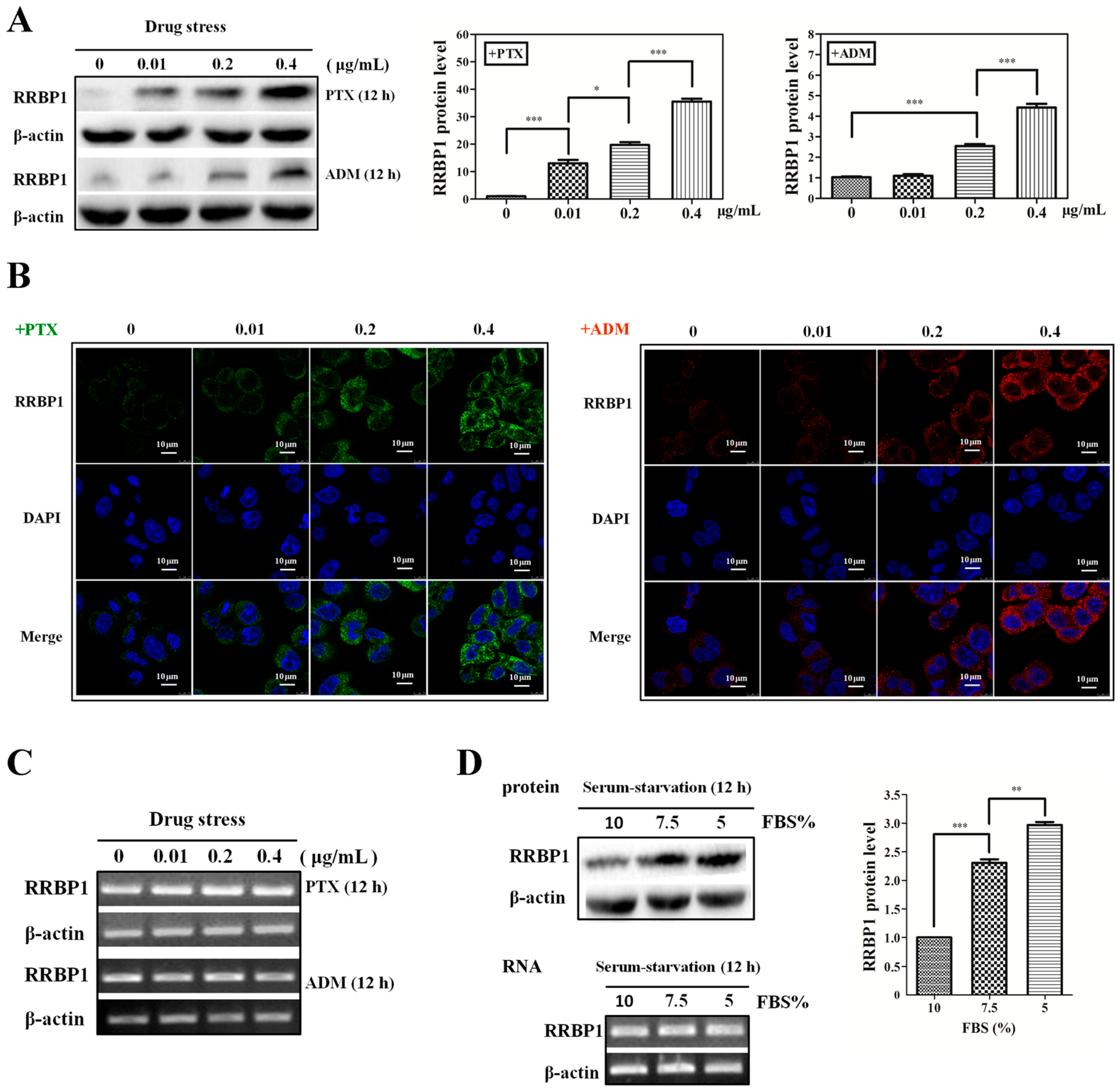
2.1. Enhanced Protein Synthesis Contributes to Overexpression of RRBP1 during Stress Conditions
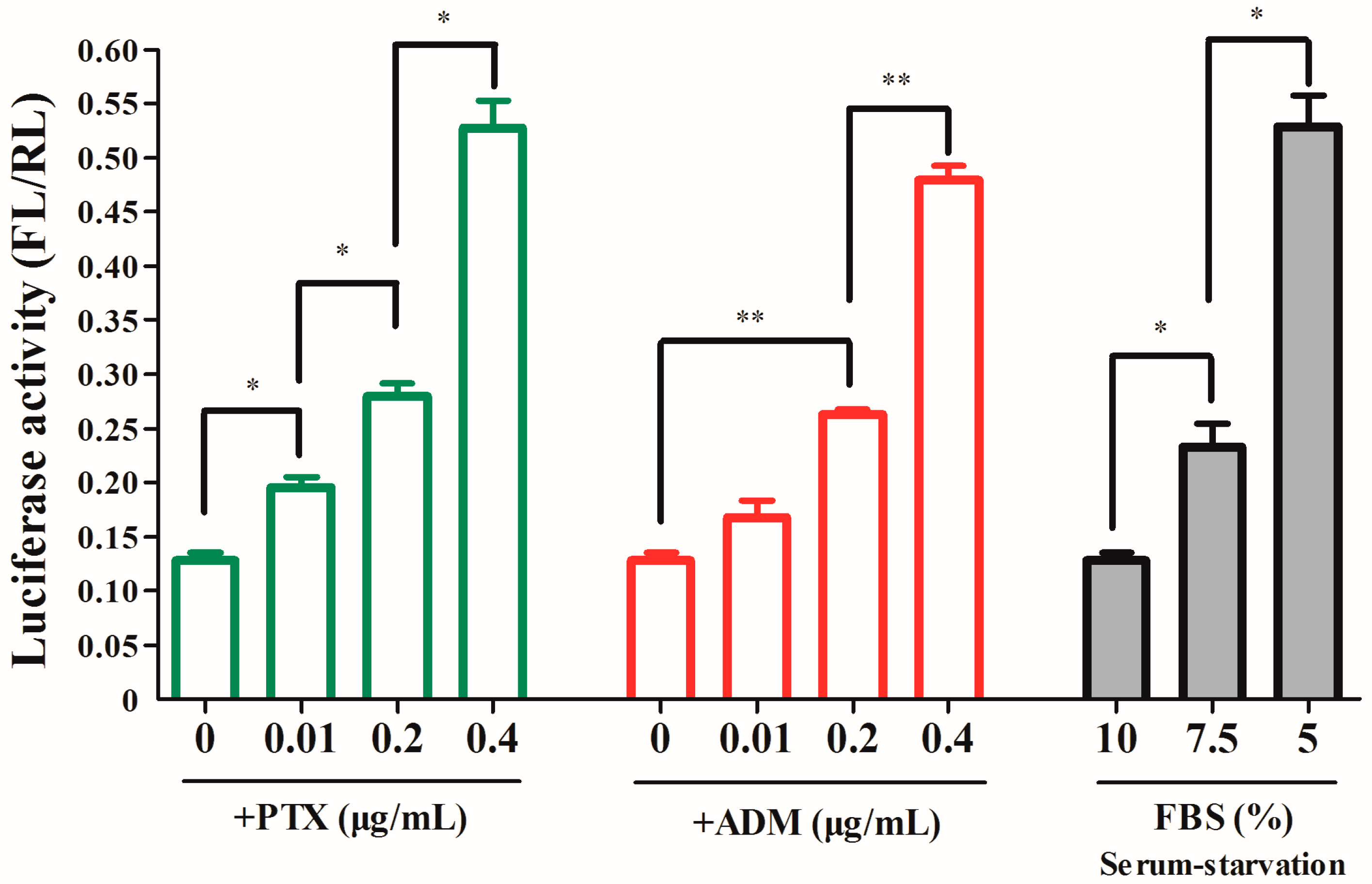
2.2. Analysis of IRES Activity within the 5′ UTR of RRBP1 mRNA
2.3. The RRBP1 IRES Upregulates Translation during Stress Conditions
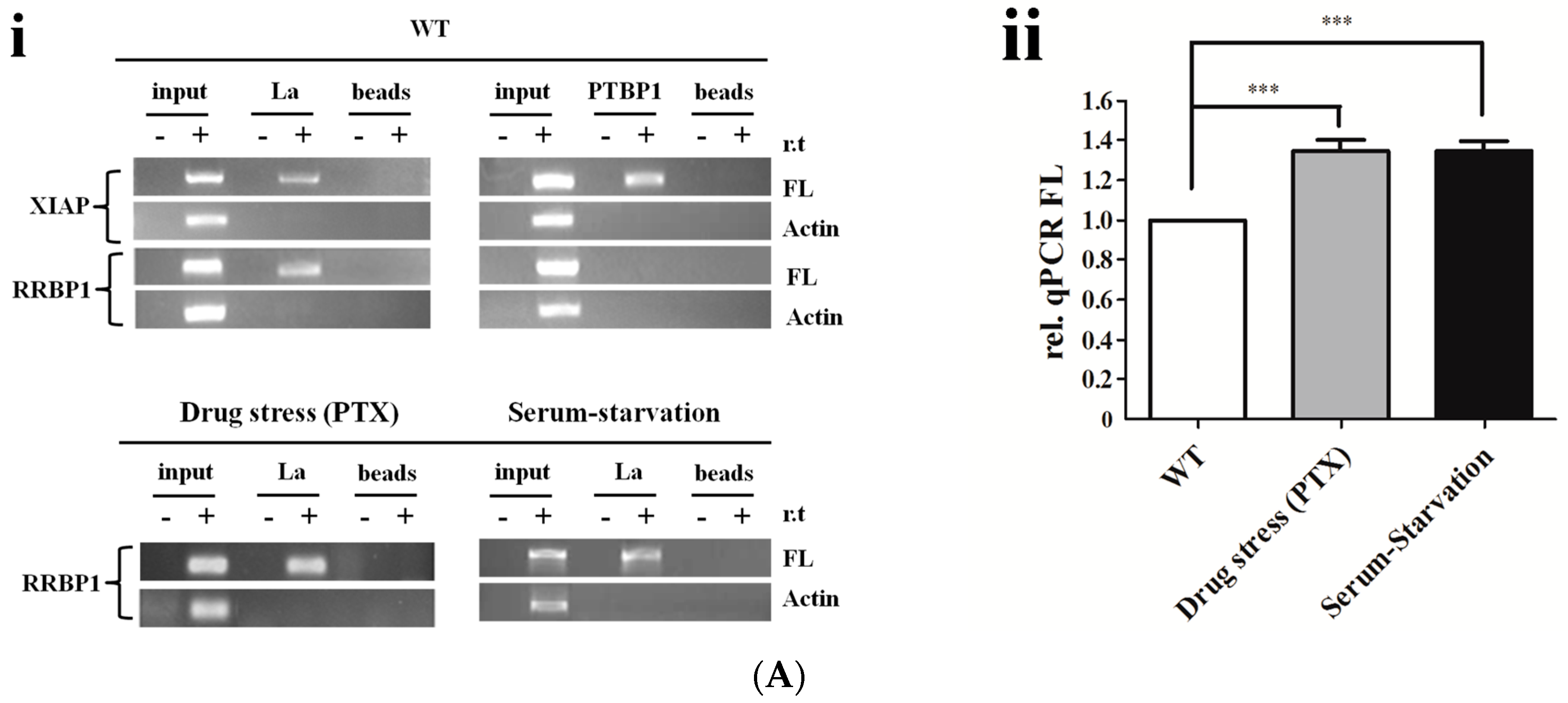
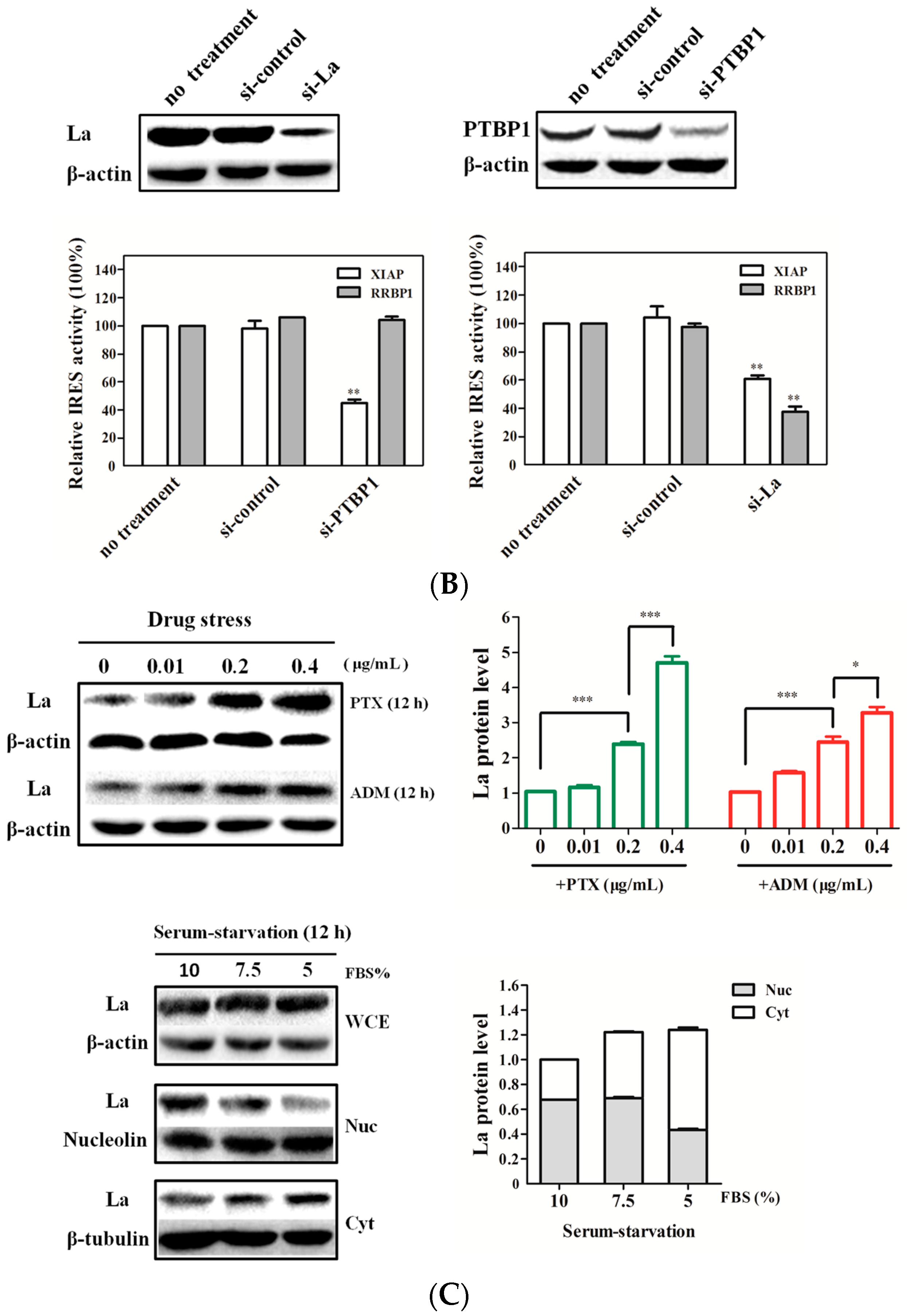
2.4. La Interacts with and Enhances the RRBP1 IRES under Drug Pressures
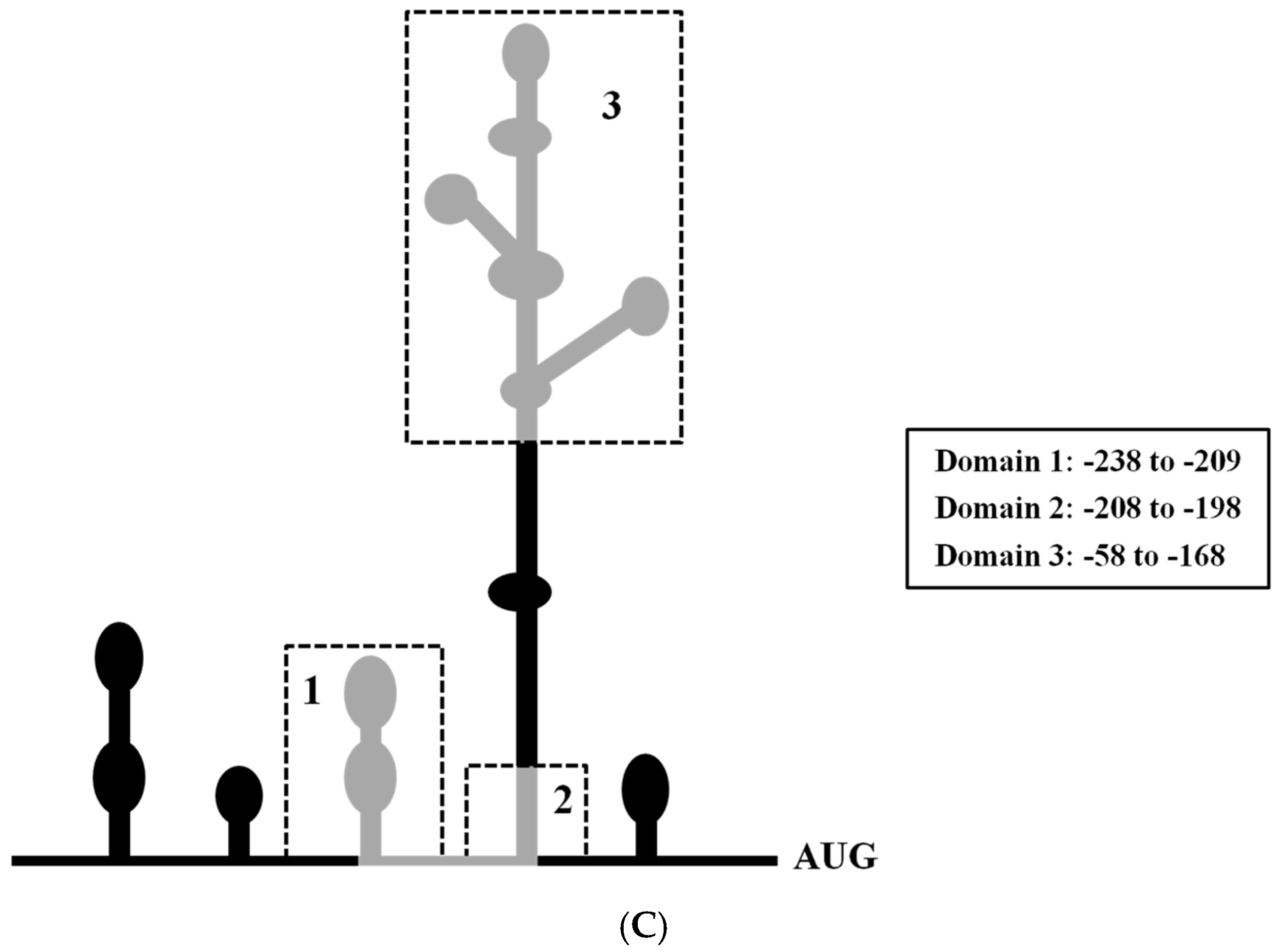
2.5. Mapping the RRBP1 IRES
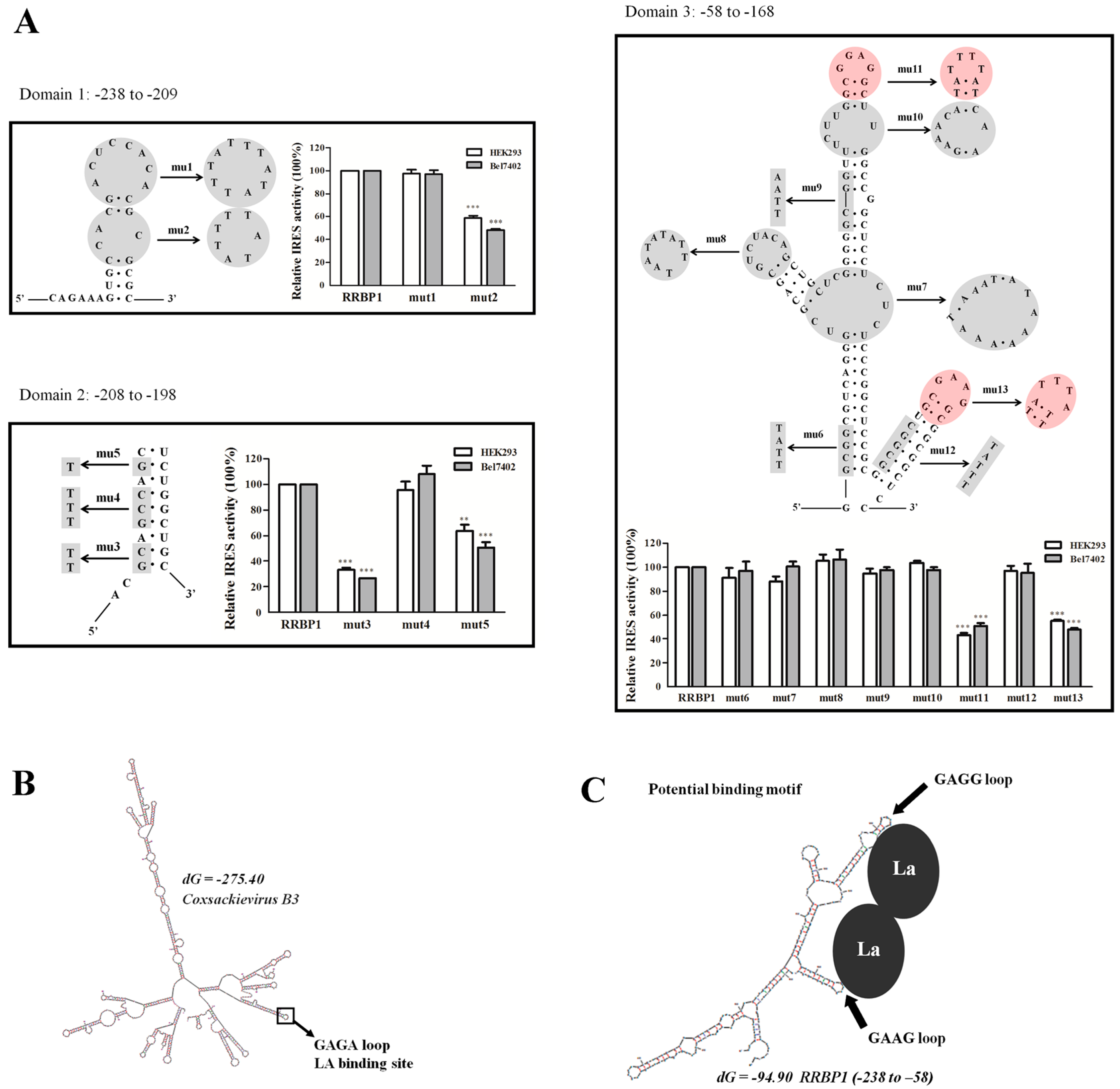
2.6. Mutational Analysis of RRBP1 IRES
3. Discussion
4. Experimental Section
4.1. Cell Lines, Cell Culture, Reagents, and Treatment
4.2. Plasmid Construction
4.3. Transient Transfection and Reporter Assay
4.4. Immunofluorescence
4.5. Reverse Transcriptase (RT)-PCR and Quantitative (q) PCR
4.6. Protein Extraction and Western Blot Analysis
4.7. RNA-Protein Immunoprecipitation
4.8. siRNA Transfection
4.9. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shatsky, I.N.; Dmitriev, S.E.; Terenin, I.M.; Andreev, D.E. Cap- and ires-independent scanning mechanism of translation initiation as an alternative to the concept of cellular iress. Mol. Cells 2010, 30, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Komar, A.A.; Hatzoglou, M. Cellular ires-mediated translation: The war of itafs in pathophysiological states. Cell Cycle 2011, 10, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Oumard, A.; Hennecke, M.; Hauser, H.; Nourbakhsh, M. Translation of NRF mRNA is mediated by highly efficient internal ribosome entry. Mol. Cell. Biol. 2000, 20, 2755–2759. [Google Scholar] [CrossRef] [PubMed]
- Arguelles, S.; Camandola, S.; Cutler, R.G.; Ayala, A.; Mattson, M.P. Elongation factor 2 diphthamide is critical for translation of two IRES-dependent protein targets, XIAP and FGF2, under oxidative stress conditions. Free Radic. Biol. Med. 2014, 67, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Subkhankulova, T.; Mitchell, S.A.; Willis, A.E. Internal ribosome entry segment-mediated initiation of c-myc protein synthesis following genotoxic stress. Biochem. J. 2001, 359, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Stoneley, M.; Chappell, S.A.; Jopling, C.L.; Dickens, M.; MacFarlane, M.; Willis, A.E. C-Myc protein synthesis is initiated from the internal ribosome entry segment during apoptosis. Mol. Cell. Biol. 2000, 20, 1162–1169. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.L.; Dibbens, J.A.; Damert, A.; Risau, W.; Vadas, M.A.; Goodall, G.J. The vascular endothelial growth factor mRNA contains an internal ribosome entry site. FEBS Lett. 1998, 434, 417–420. [Google Scholar] [CrossRef]
- Bastide, A.; Karaa, Z.; Bornes, S.; Hieblot, C.; Lacazette, E.; Prats, H.; Touriol, C. An upstream open reading frame within an IRES controls expression of a specific vegf-a isoform. Nucleic Acids Res. 2008, 36, 2434–2445. [Google Scholar] [CrossRef] [PubMed]
- Holcik, M.; Yeh, C.; Korneluk, R.G.; Chow, T. Translational upregulation of X-linked inhibitor of apoptosis (XIAP) increases resistance to radiation induced cell death. Oncogene 2000, 19, 4174–4177. [Google Scholar] [CrossRef] [PubMed]
- Hellen, C.U.; Sarnow, P. Internal ribosome entry sites in eukaryotic mrna molecules. Genes Dev. 2001, 15, 1593–1612. [Google Scholar] [CrossRef] [PubMed]
- Komar, A.A.; Hatzoglou, M. Exploring internal ribosome entry sites as therapeutic targets. Front. Oncol. 2015, 5, 233. [Google Scholar] [CrossRef] [PubMed]
- Costa-Mattioli, M.; Svitkin, Y.; Sonenberg, N. La autoantigen is necessary for optimal function of the poliovirus and hepatitis C virus internal ribosome entry site in vivo and in vitro. Mol. Cell. Biol. 2004, 24, 6861–6870. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.N.; Lin, J.Y.; Locker, N.; Kung, Y.A.; Hung, C.T.; Lin, J.Y.; Huang, H.I.; Li, M.L.; Shih, S.R. Far upstream element binding protein 1 binds the internal ribosomal entry site of enterovirus 71 and enhances viral translation and viral growth. Nucleic Acids Res. 2011, 39, 9633–9648. [Google Scholar] [CrossRef] [PubMed]
- Agol, V.I. Translational control of the picornavirus phenotype. Mol. Biol. 2001, 35, 691–701. [Google Scholar] [CrossRef]
- Petz, M.; Them, N.C.; Huber, H.; Mikulits, W. PDGF enhances IRES-mediated translation of laminin b1 by cytoplasmic accumulation of La during epithelial to mesenchymal transition. Nucleic Acids Res. 2012, 40, 9738–9749. [Google Scholar] [CrossRef] [PubMed]
- Cobbold, L.C.; Wilson, L.A.; Sawicka, K.; King, H.A.; Kondrashov, A.V.; Spriggs, K.A.; Bushell, M.; Willis, A.E. Upregulated c-Myc expression in multiple myeloma by internal ribosome entry results from increased interactions with and expression of PTB-1 and YB-1. Oncogene 2010, 29, 2884–2891. [Google Scholar] [CrossRef] [PubMed]
- Knoch, K.P.; Nath-Sain, S.; Petzold, A.; Schneider, H.; Beck, M.; Wegbrod, C.; Sonmez, A.; Munster, C.; Friedrich, A.; Roivainen, M.; et al. PTBP1 is required for glucose-stimulated cap-independent translation of insulin granule proteins and coxsackieviruses in beta cells. Mol. Metab. 2014, 3, 518–530. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Hu, P.; Lin, X.; Han, W.; Zhu, L.; Tan, X.; Ye, F.; Wang, G.; Wu, F.; Yin, B.; et al. PTBP1 induces ADAR1 p110 isoform expression through IRES-like dependent translation control and influences cell proliferation in gliomas. Cell. Mol. Life Sci. 2015, 72, 4383–4397. [Google Scholar] [CrossRef] [PubMed]
- Telikicherla, D.; Marimuthu, A.; Kashyap, M.K.; Ramachandra, Y.L.; Mohan, S.; Roa, J.C.; Maharudraiah, J.; Pandey, A. Overexpression of ribosome binding protein 1 (RRBP1) in breast cancer. Clin. Proteom. 2012, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Cao, F.; Guo, A.; Chang, W.; Chen, X.; Ma, W.; Gao, X.; Guo, S.; Fu, C.; Zhu, J. Endoplasmic reticulum ribosome-binding protein 1, RRBP1, promotes progression of colorectal cancer and predicts an unfavourable prognosis. Br. J. Cancer 2015, 113, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.Y.; Yang, Y.F.; Wu, A.T.; Yang, C.J.; Liu, Y.P.; Jan, Y.H.; Lee, C.H.; Hsiao, Y.W.; Yeh, C.T.; Shen, C.N.; et al. Endoplasmic reticulum ribosome-binding protein 1 (RRBP1) overexpression is frequently found in lung cancer patients and alleviates intracellular stress-induced apoptosis through the enhancement of GRP78. Oncogene 2013, 32, 4921–4931. [Google Scholar] [CrossRef] [PubMed]
- Ogawa-Goto, K.; Tanaka, K.; Ueno, T.; Tanaka, K.; Kurata, T.; Sata, T.; Irie, S. P180 is involved in the interaction between the endoplasmic reticulum and microtubules through a novel microtubule-binding and bundling domain. Mol. Biol. Cell 2007, 18, 3741–3751. [Google Scholar] [CrossRef] [PubMed]
- Reboll, M.R.; Oumard, A.; Gazdag, A.C.; Renger, I.; Ritter, B.; Schwarzer, M.; Hauser, H.; Wood, M.; Yamada, M.; Resch, K.; et al. NRF IRES activity is mediated by rna binding protein JKTBP1 and a 14-nt RNA element. RNA 2007, 13, 1328–1340. [Google Scholar] [CrossRef] [PubMed]
- Hennecke, M.; Kwissa, M.; Metzger, K.; Oumard, A.; Kroger, A.; Schirmbeck, R.; Reimann, J.; Hauser, H. Composition and arrangement of genes define the strength of IRES-driven translation in bicistronic mrnas. Nucleic Acids Res. 2001, 29, 3327–3334. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Richie, C.; Legerski, R.J. Translation of hsnm1 is mediated by an internal ribosome entry site that upregulates expression during mitosis. DNA Repair 2002, 1, 379–390. [Google Scholar] [PubMed]
- Holcik, M.; Korneluk, R.G. Functional characterization of the x-linked inhibitor of apoptosis (XIAP) internal ribosome entry site element: Role of La autoantigen in xiap translation. Mol. Cell. Biol. 2000, 20, 4648–4657. [Google Scholar] [CrossRef] [PubMed]
- Baird, S.D.; Turcotte, M.; Korneluk, R.G.; Holcik, M. Searching for ires. RNA 2006, 12, 1755–1785. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Das, S. An apical GAGA loop within 5′ UTR of the coxsackievirus B3 RNA maintains structural organization of the IRES element required for efficient ribosome entry. RNA Biol. 2006, 3, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Das, S. Mapping of secondary structure of the spacer region within the 5′-untranslated region of the coxsackievirus B3 RNA: Possible role of an apical GAGA loop in binding la protein and influencing internal initiation of translation. Virus Res. 2005, 108, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.H.; Wei, W.Y.; Cao, W.L.; Zhang, X.S.; Xie, Y.B.; Xiao, Q. Overexpression of CDX2 in gastric cancer cells promotes the development of multidrug resistance. Am. J. Cancer Res. 2015, 5, 321–332. [Google Scholar] [PubMed]
- Yan, L.H.; Wei, W.Y.; Xie, Y.B.; Xiao, Q. New insights into the functions and localization of the homeotic gene CDX2 in gastric cancer. World J. Gastroenterol. 2014, 20, 3960–3966. [Google Scholar] [CrossRef] [PubMed]
- Takakura, Y.; Hinoi, T.; Oue, N.; Sasada, T.; Kawaguchi, Y.; Okajima, M.; Akyol, A.; Fearon, E.R.; Yasui, W.; Ohdan, H. CDX2 regulates multidrug resistance 1 gene expression in malignant intestinal epithelium. Cancer Res. 2010, 70, 6767–6778. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; Evans, B.G.; Yao, J.; Cooper, S.; Cornetta, K.; Ballas, C.B.; Hangoc, G.; Broxmeyer, H.E. Enhanced green fluorescent protein is a nearly ideal long-term expression tracer for hematopoietic stem cells, whereas dsred-express fluorescent protein is not. Stem Cells 2007, 25, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Kenan, D.J.; Query, C.C.; Keene, J.D. RNA recognition: Towards identifying determinants of specificity. Trends Biochem. Sci. 1991, 16, 214–220. [Google Scholar] [CrossRef]
- Martinez-Salas, E.; Lozano, G.; Fernandez-Chamorro, J.; Francisco-Velilla, R.; Galan, A.; Diaz, R. RNA-binding proteins impacting on internal initiation of translation. Int. J. Mol. Sci. 2013, 14, 21705–21726. [Google Scholar] [CrossRef] [PubMed]
- Maraia, R.J. Transcription termination factor La is also an initiation factor for RNA polymerase iii. Proc. Natl. Acad. Sci. USA 1996, 93, 3383–3387. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Ray, U.; Das, S. Human La protein interaction with GCAC near the initiator AUG enhances hepatitis C virus RNA replication by promoting linkage between 5′ and 3′ untranslated regions. J. Virol 2013, 87, 6713–6726. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Dinh, T.N.; Kappeler, K.; Tsaprailis, G.; Chen, Q.M. La autoantigen mediates oxidant induced de novo Nrf2 protein translation. Mol. Cell. Proteom. 2012, 11, M111.015032. [Google Scholar] [CrossRef] [PubMed]
- Rutjes, S.A.; Utz, P.J.; van der Heijden, A.; Broekhuis, C.; van Venrooij, W.J.; Pruijn, G.J. The La (ss-b) autoantigen, a key protein in RNA biogenesis, is dephosphorylated and cleaved early during apoptosis. Cell Death Differ. 1999, 6, 976–986. [Google Scholar] [CrossRef] [PubMed]
- Filbin, M.E.; Kieft, J.S. Toward a structural understanding of IRES RNA function. Curr. Opin. Struct. Biol. 2009, 19, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Ma, W.; Li, Q.; Tao, Y.; Ding, P.; Zhu, R.; Jin, J. The 5′-UTR of ddb2 harbors an IRES element and upregulates translation during stress conditions. Gene 2015, 573, 57–63. [Google Scholar] [CrossRef] [PubMed]


© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, W.; Li, Q.; Zhu, R.; Jin, J. La Autoantigen Induces Ribosome Binding Protein 1 (RRBP1) Expression through Internal Ribosome Entry Site (IRES)-Mediated Translation during Cellular Stress Condition. Int. J. Mol. Sci. 2016, 17, 1174. https://doi.org/10.3390/ijms17071174
Gao W, Li Q, Zhu R, Jin J. La Autoantigen Induces Ribosome Binding Protein 1 (RRBP1) Expression through Internal Ribosome Entry Site (IRES)-Mediated Translation during Cellular Stress Condition. International Journal of Molecular Sciences. 2016; 17(7):1174. https://doi.org/10.3390/ijms17071174
Chicago/Turabian StyleGao, Wenqing, Qi Li, Ruiyu Zhu, and Jian Jin. 2016. "La Autoantigen Induces Ribosome Binding Protein 1 (RRBP1) Expression through Internal Ribosome Entry Site (IRES)-Mediated Translation during Cellular Stress Condition" International Journal of Molecular Sciences 17, no. 7: 1174. https://doi.org/10.3390/ijms17071174




