Synthesis and Evaluation of Tricarbonyl 99mTc-Labeled 2-(4-Chloro)phenyl-imidazo[1,2-a]pyridine Analogs as Novel SPECT Imaging Radiotracer for TSPO-Rich Cancer
Abstract
:1. Introduction
2. Results and Discussion
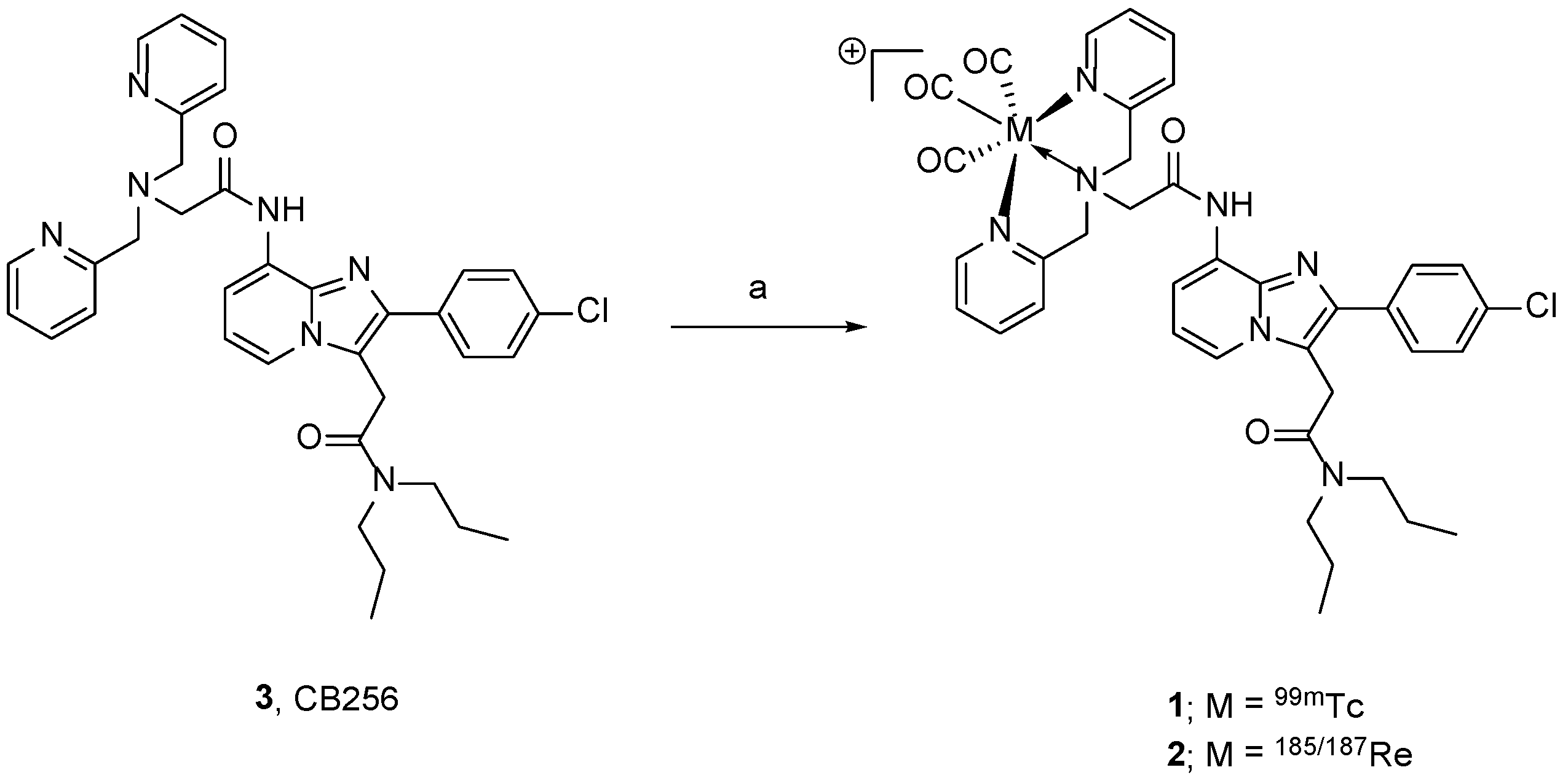
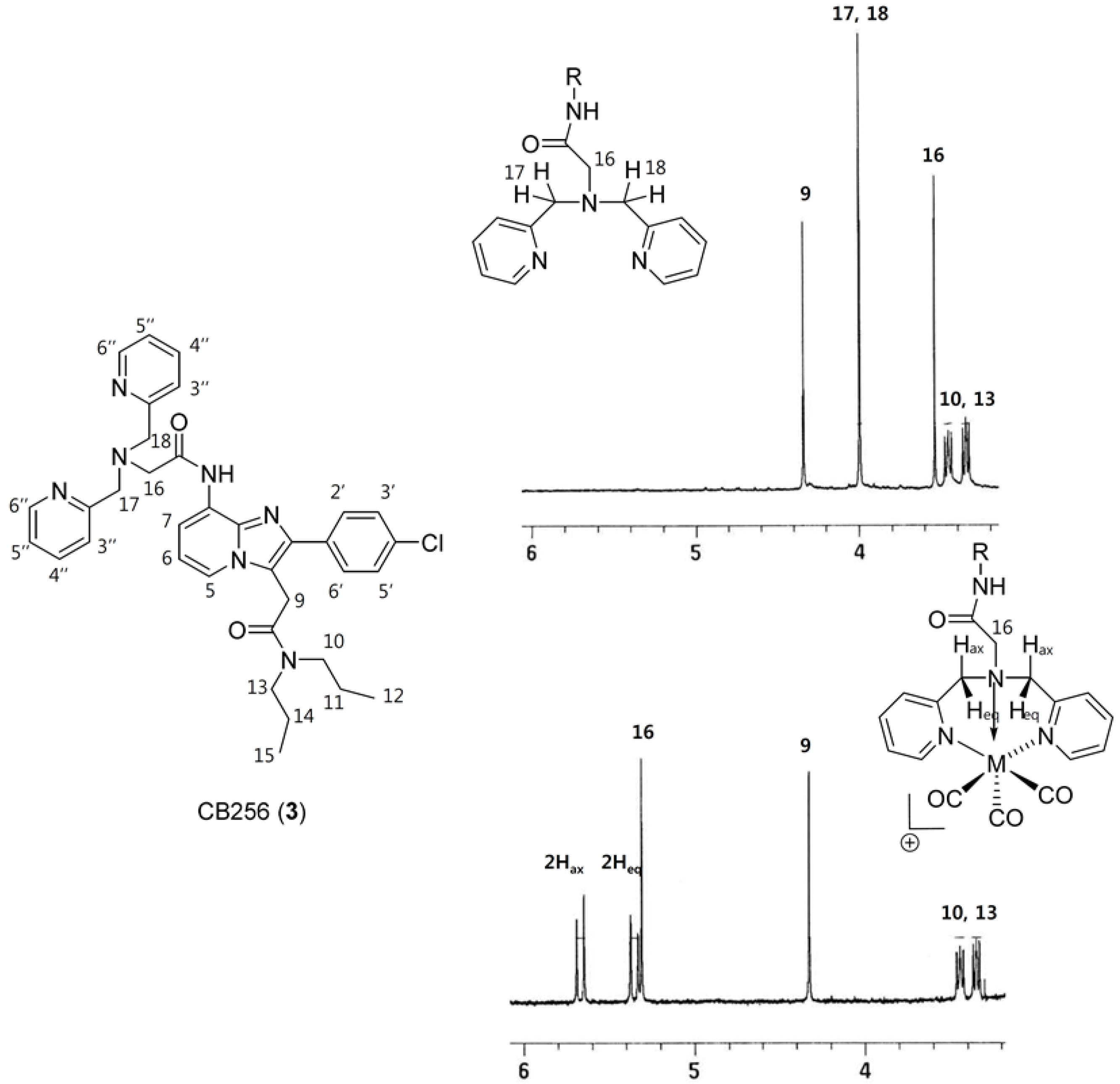
2.1. Synthesis of Re-CB256 and 99mTc-CB256
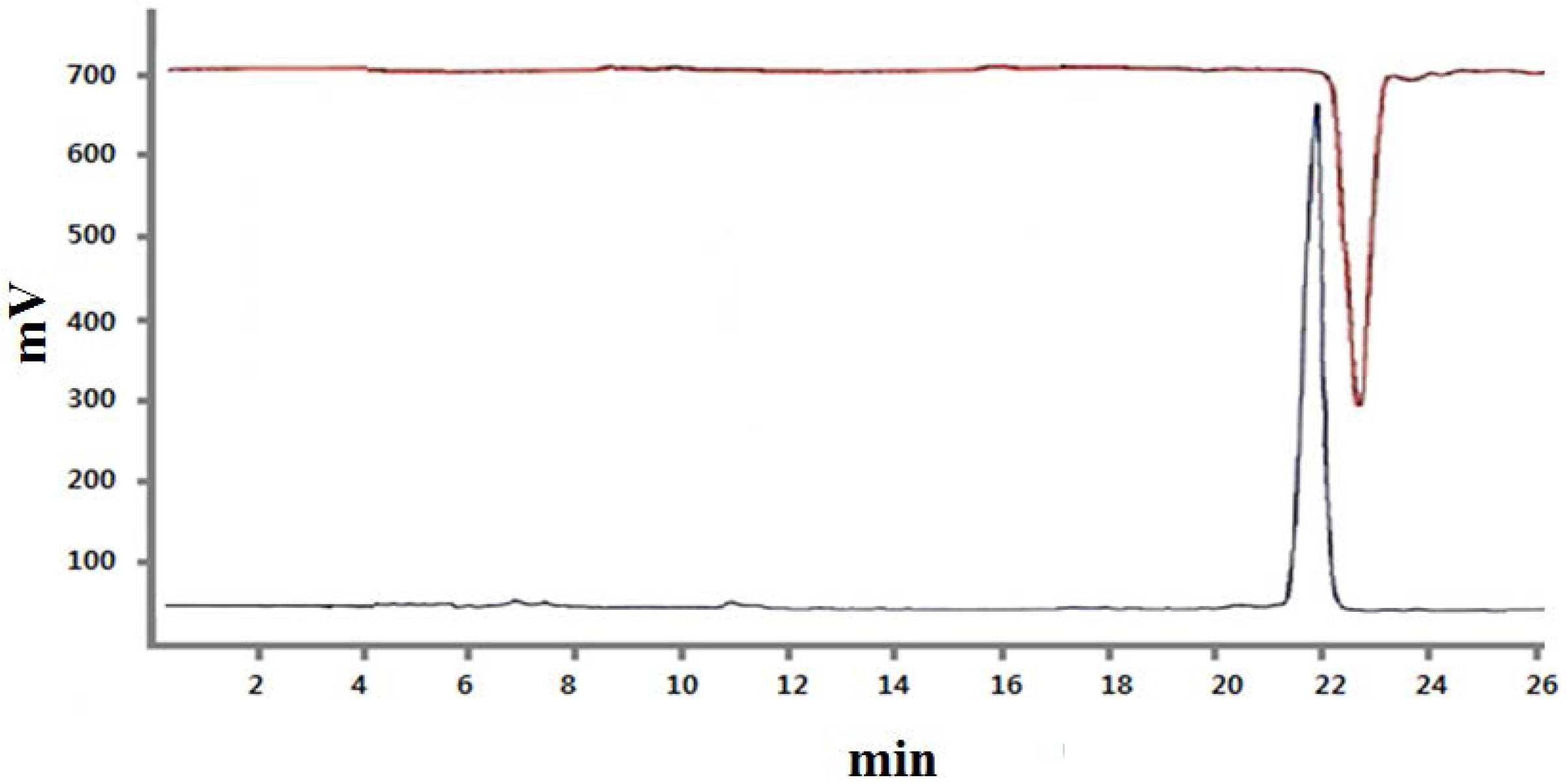
2.2. In Vitro Stability Studies and Partition Coefficient of 99mTc-CB256 (1)
2.3. In Vitro Cell Uptake Assay of 99mTc-CB256 (1)
2.4. In Vitro Cell Binding Affinity of CB256 (3) and Re-CB256 (2)
2.5. In Vitro Cytotoxicity Assays of CB256 (3) and Re-CB256 (2)
3. Experimental Section
3.1. Materials and Methods
3.2. Synthesis of Re-CB256 (2)
3.3. Synthesis of 99mTc-CB256 (1)
3.4. In Vitro Stability Study
3.5. LogD Determination
3.6. In Vitro Cell Uptake Assay of 99mTc-CB256 (1)
3.7. In Vitro Cell-Binding Assays
3.8. Cytotoxicity Assays
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| TSPO | 18-kDa mitochondrial translocator protein |
| PET | positron emission computed tomography |
| SPECT | single photon emission computed tomography |
| HPLC | high performance liquid chromatography |
| ESI-MS | electrospray mass spectrometry |
| HRMS | high resolution mass spectrometry |
| CBR | central benzodiazephine receptor |
| U87-MG | U87-malignant glioma |
| % ID | % injected dose |
| NMR | nuclear magnetic resonance |
| TLC | thin layer chromatography |
| S.D. | standard deviation |
References
- Papadopoulos, V.; Baraldi, M.; Guilarte, T.R.; Knudsen, T.B.; Lacapère, J.J.; Lindemann, P.; Norenberg, M.D.; Nutt, D.; Weizman, A.; Zhang, M.R.; et al. Translocator protein (18 kDa): New nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol. Sci. 2006, 27, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Rupprecht, R.; Papadopoulos, V.; Rammes, G.; Baghai, T.C.; Fan, J.; Akula, N.; Groyer, G.; Adams, D.; Schumacher, M. Translocator protein (18 kDa) (TSPO) as a therapeutic target for neurological and psychiatric disorders. Nat. Rev. Drug Discov. 2010, 9, 971–988. [Google Scholar] [CrossRef] [PubMed]
- Maaser, K.; Grabowski, P.; Sutter, A.P.; Höpfner, M.; Foss, H.D.; Stein, H.; Berger, G.; Gavish, M.; Zeitz, M.; Scherübl, H. Overexpression of the peripheral benzodiazepine receptor is a relevant prognostic factor in stage III colorectal cancer. Clin. Cancer Res. 2002, 8, 3205–3209. [Google Scholar] [PubMed]
- Corsi, L.; Geminiani, E.; Baraldi, M. Peripheral Benzodiazepine Receptor (PBR) new insight in cell proliferation and cell differentiation review. Curr. Clin. Pharmacol. 2008, 3, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Austin, C.J.D.; Kahlert, J.; Kassiou, M.; Rendina, L.M. The translocator protein (TSPO): A novel target for cancer chemotherapy. Int. J. Biochem. Cell Biol. 2013, 45, 1212–1216. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Boisgard, R.; Siquier-Pernet, K.; Decaudin, D.; Dollé, F.; Tavitian, B. Differential expression of the 18 kDa translocator protein (TSPO) by neoplastic and inflammatory cells in mouse tumors of breast cancer. Mol. Pharm. 2011, 8, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Slack, R.S.; Li, W.; Papadopoulos, V. Expression of peripheral benzodiazepine receptor (PBR) in human tumors: Relationship to breast, colorectal, and prostate tumor progression. J. Recept. Signal Transduct. 2003, 2, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Le Fur, G.; Perrier, M.L.; Vaucher, N.; Imbault, F.; Flamier, A.; Benavides, J.; Uzan, A.; Renault, C.; Dubroeucq, M.C.; Guérémy, C. Peripheral benzodiazepine binding sites: Effect of PK11195, 1-(2-chlorophenyl)-N-(1-methylpropyl)-3-isoquinolinecarboxamide I. In vitro studies. Life Sci. 1983, 32, 1839–1847. [Google Scholar] [CrossRef]
- Marangos, P.L.; Pate, J.; Boulenger, J.P.; Clark-Rosenberg, R. Characterization of peripheral-type benzodiazepine binding sites in brain using [3H]Ro 5-4864. Mol. Pharmacol. 1982, 22, 26–32. [Google Scholar] [PubMed]
- Romeo, E.; Auta, J.; Kozikowski, A.P.; Ma, D.; Papadopoulos, V.; Puia, G.; Costa, E.; Guidotti, A. 2-Aryl-3-indoleacetamides (FGIN-1): A new class of potent and specific ligands for the mitochondrial DBI receptor (MDR). J. Pharmacol. Exp. Ther. 1992, 262, 971–978. [Google Scholar] [PubMed]
- Imaizumi, M.; Briard, E.; Zoghbi, S.S.; Gourley, J.P.; Hong, J.; Fujimura, Y.; Pike, V.W.; Innis, R.B.; Fujita, M. Brain and whole-body imaging in nonhuman primates of [11C]PBR28, a promising PET radioligand for peripheral benzodiazepine receptors. NeuroImage 2008, 39, 1289–1298. [Google Scholar] [CrossRef] [PubMed]
- Denora, N.; Laquintana, V.; Pisu, M.G.; Dore, R.; Murru, L.; Latrofa, A.; Trapani, G.; Sanna, E. 2-Phenyl-imidazo[1,2-a]pyridine compounds containing hydrophilic groups as potent and selective ligands for peripheral benzodiazepine receptors: Synthesis, binding affinity and electrophysiological studies. J. Med. Chem. 2008, 51, 6876–6888. [Google Scholar] [CrossRef] [PubMed]
- Cappelli, A.; Mancini, A.; Sudati, F.; Valenti, S.; Anzini, M.; Belloli, S.; Moresco, R.M.; Matarrese, M.; Vaghi, M.; Fabro, A.; et al. Synthesis and biological characterization of novel 2-quinolinecarboxamide ligands of the peripheral benzodiazepine receptors bearing technetium-99m or rhenium. Bioconjug. Chem. 2008, 19, 1143–1153. [Google Scholar] [CrossRef] [PubMed]
- Ching, A.S.C.; Kuhnast, B.; Damont, A.; Roeda, D.; Tavitian, B.; Dollé, F. Current paradigm of the 18-kDa translocator protein (TSPO) as a molecular target for PET imaging in neuroinflammation and neurodegenerative diseases. Insights Imaging 2012, 3, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Turkheimer, F.E.; Rizzo, G.; Bloomfield, P.S.; Howes, O.; Zanotti-Fregonara, P.; Bertoldo, A.; Veronese, M. The methodology of TSPO imaging with positron emission tomography. Biochem. Soc. Trans. 2015, 43, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Vivash, L.; O’Brien, T.J. Imaging microglial activation with TSPO PET: Lighting up neurologic diseases? J. Nucl. Med. 2016, 57, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Schwochau, K. Technetium radiopharmaceuticals. Fundamentals, synthesis, structure, and development. Angew. Chem. Int. Ed. 1994, 33, 2258–2267. [Google Scholar] [CrossRef]
- Jurisson, S.; Berning, D.; Jia, W.; Ma, D.S. Coordination compounds in nuclear medicine. Chem. Rev. 1993, 93, 1137–1156. [Google Scholar] [CrossRef]
- Schibli, R.; Netter, M.; Scapozza, L.; Birringer, M.; Schelling, P.; Dumas, C.; Schoch, J.; Schubiger, P.A. First organometallic inhibitors for human thymidine kinase: Synthesis and in vitro evaluation of rhenium(I)- and technetium(I)-tricarbonyl complexes of thymidine. J. Oranomet. Chem. 2003, 668, 67–74. [Google Scholar] [CrossRef]
- Lee, B.C.; Kim, D.H.; Lee, J.H.; Sung, H.J.; Choe, Y.S.; Chi, D.Y.; Lee, K.H.; Choi, Y.; Kim, B.T. 99mTc(CO)3-15-[N-(Acetyloxy)-2-picolylamino]pentadecanoic acid: A potential radiotracer for evaluation of fatty acid metabolism. Bioconjug. Chem. 2007, 18, 1332–1337. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.C.; Moon, B.S.; Kim, J.S.; Jung, J.H.; Park, H.S.; Katzenellenbogen, J.A.; Kim, S.E. Synthesis and biological evaluation of RGD peptides with the 99mTc/188Re chelated iminodiacetate core: Highly enhanced uptake and excretion kinetics of theranostics against tumor angiogenesis. RSC Adv. 2013, 3, 782–792. [Google Scholar] [CrossRef]
- Alves, S.; Correia, J.D.; Gano, L.; Rold, T.L.; Prasanphanich, A.; Haubner, R.; Rupprich, M.; Alberto, R.; Decristoforo, C.; Santos, I.; et al. In vitro and in vivo evaluation of a novel 99mTc(CO)3-pyrazolyl conjugate of cyclo-(Arg-Gly-Asp-d-Tyr-Lys). Bioconjug. Chem. 2007, 18, 530–537. [Google Scholar] [CrossRef] [PubMed]
- North, A.J.; Hayne, D.J.; Schieber, C.; Price, K.; White, A.R.; Crouch, P.J.; Rigopoulos, A.; O’Keefe, G.J.; Tochon-Danguy, H.; Scott, A.M.; et al. Toward hypoxia-selective rhenium and technetium tricarbonyl complexes. Inorg. Chem. 2015, 54, 9594–9610. [Google Scholar] [CrossRef] [PubMed]
- Nayak, D.K.; Halder, K.K.; Baishya, R.; Sen, T.; Mitra, P.; Debnath, M.C. Tricarbonyltechnetium(I) and tricarbonylrhenium(I) complexes of amino acids: Crystal and molecular structure of a novel cyclic dimeric Re(CO)3-amino acid complex comprised of the OON donor atom set of the tridentate ligand. Dalton Trans. 2013, 42, 13565–13575. [Google Scholar] [CrossRef] [PubMed]
- Margiotta, N.; Ostuni, R.; Ranaldo, R.; Denora, N.; Laquintana, V.; Trapani, G.; Liso, G.; Natile, G. Synthesis and characterization of a platinum(II) complex tethered to a ligand of the peripheral benzodiazepine receptor. J. Med. Chem. 2007, 50, 1019–1027. [Google Scholar] [CrossRef] [PubMed]
- Margiotta, N.; Denora, N.; Ostuni, R.; Laquintana, V.; Anderson, A.; Johnson, S.W.; Trapani, G.; Natile, G. Platinum(II) complexes with bioactive carrier ligands having high affinity for the translocator protein. J. Med. Chem. 2010, 53, 5144–5154. [Google Scholar] [CrossRef] [PubMed]
- Piccinonna, S.; Margiotta, N.; Denora, N.; Iacobazzi, R.M.; Pacifico, C.; Trapani, G.; Natile, G. A model radiopharmaceutical agent targeted to translocator protein 18 kDa (TSPO). Dalton Trans. 2013, 42, 10112–10115. [Google Scholar] [CrossRef] [PubMed]
- Piccinonna, S.; Denora, N.; Margiotta, N.; Laquintana, V.; Trapani, G.; Natile, G. Synthesis, characterization, and binding to the translocator protein (18 kDa, TSPO) of a new rhenium complex as a model of radiopharmaceutical agents. Z. Anorg. Allg. Chem. 2013, 639, 1606–1612. [Google Scholar] [CrossRef]
- Denora, N.; Margiotta, N.; Laquintana, V.; Lopedota, A.; Cutrignelli, A.; Losacco, M.; Franco, M.; Natile, G. Synthesis, characterization, and in vitro evaluation of a new TSPO selective bifunctional chelate ligand. ACS Med. Chem. Lett. 2014, 5, 685–689. [Google Scholar] [CrossRef] [PubMed]
- Margiotta, N.; Denora, N.; Piccinonna, S.; Laquintana, V.; Lasorsa, F.M.; Franco, M.; Natile, G. Synthesis, characterization, and in vitro evaluation of new coordination complexes of platinum(II) and rhenium(I) with a ligand targeting the translocator protein (TSPO). Dalton Trans. 2014, 43, 16252–16264. [Google Scholar] [CrossRef] [PubMed]
- Alberto, R.; Schibli, R.; Egli, A.; Schubiger, A.P. A Novel Organometallic Aqua Complex of Technetium for the Labeling of Biomolecules: Synthesis of [99mTc(OH2)3(CO)3]+ from [99mTcO4]− in Aqueous Solution and Its Reaction with a Bifunctional Ligand. J. Am. Chem. Soc. 1998, 120, 7987–7988. [Google Scholar] [CrossRef]
- Perrone, M.; Moon, B.S.; Park, H.S.; Laquintana, V.; Jung, J.H.; Cutrignelli, A.; Lopedota, A.; Franco, M.; Kim, S.E.; Lee, B.C.; et al. A novel pet imaging probe for the detection and monitoring of translocator protein 18 kDa expression in pathological disorders. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Schibli, R.; Katti, K.V.; Volkert, W.A.; Barnes, C.L. Novel coordination behavior of fac-[ReBr3(CO)3]2- with 1,3,5-triaza-7-phosphaadamantane (PTA). Systematic investigation on stepwise replacement of the halides by PTA ligand. Phase transfer studies and X-ray crystal structure of [NEt4][ReBr2((PTA)(CO)3], [ReBr(PTA)2(CO)3], and [Re(PTA)3(CO)3]PF6. Inorg. Chem. 1998, 37, 5306–5312. [Google Scholar]

| Compound | Ki (nM) for TSPO |
|---|---|
| PK 11195 | 9.10 ± 1.2 |
| CB256 (3) | 148.2 ± 11.3 |
| Re-CB256 (2) | 159.3 ± 8.70 |
| Compound | IC50 (µM) a | ||
|---|---|---|---|
| HepG2 | MCF7 | U87 | |
| CB256 (3) | 30 ± 5 | 38 ± 3 | 35 ± 2 |
| Re-CB256 (2) | >50 (67%) b | >50 (56%) b | >50 (59%) b |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.Y.; Iacobazzi, R.M.; Perrone, M.; Margiotta, N.; Cutrignelli, A.; Jung, J.H.; Park, D.D.; Moon, B.S.; Denora, N.; Kim, S.E.; et al. Synthesis and Evaluation of Tricarbonyl 99mTc-Labeled 2-(4-Chloro)phenyl-imidazo[1,2-a]pyridine Analogs as Novel SPECT Imaging Radiotracer for TSPO-Rich Cancer. Int. J. Mol. Sci. 2016, 17, 1085. https://doi.org/10.3390/ijms17071085
Choi JY, Iacobazzi RM, Perrone M, Margiotta N, Cutrignelli A, Jung JH, Park DD, Moon BS, Denora N, Kim SE, et al. Synthesis and Evaluation of Tricarbonyl 99mTc-Labeled 2-(4-Chloro)phenyl-imidazo[1,2-a]pyridine Analogs as Novel SPECT Imaging Radiotracer for TSPO-Rich Cancer. International Journal of Molecular Sciences. 2016; 17(7):1085. https://doi.org/10.3390/ijms17071085
Chicago/Turabian StyleChoi, Ji Young, Rosa Maria Iacobazzi, Mara Perrone, Nicola Margiotta, Annalisa Cutrignelli, Jae Ho Jung, Do Dam Park, Byung Seok Moon, Nunzio Denora, Sang Eun Kim, and et al. 2016. "Synthesis and Evaluation of Tricarbonyl 99mTc-Labeled 2-(4-Chloro)phenyl-imidazo[1,2-a]pyridine Analogs as Novel SPECT Imaging Radiotracer for TSPO-Rich Cancer" International Journal of Molecular Sciences 17, no. 7: 1085. https://doi.org/10.3390/ijms17071085








