Transformation of Litchi Pericarp-Derived Condensed Tannin with Aspergillus awamori
Abstract
:1. Introduction
2. Results and Discussion
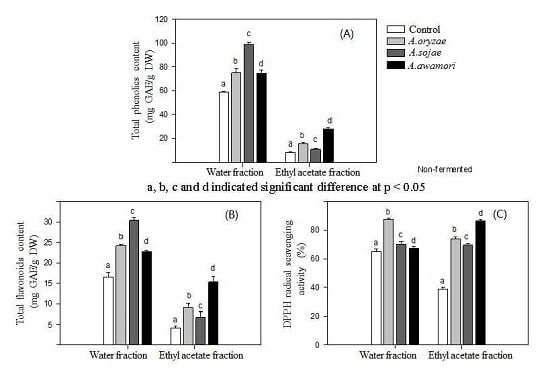
2.1. Effect of Aspergillus Treatments on Polyphenol Recovery and DPPH Radical Scavenging Activity
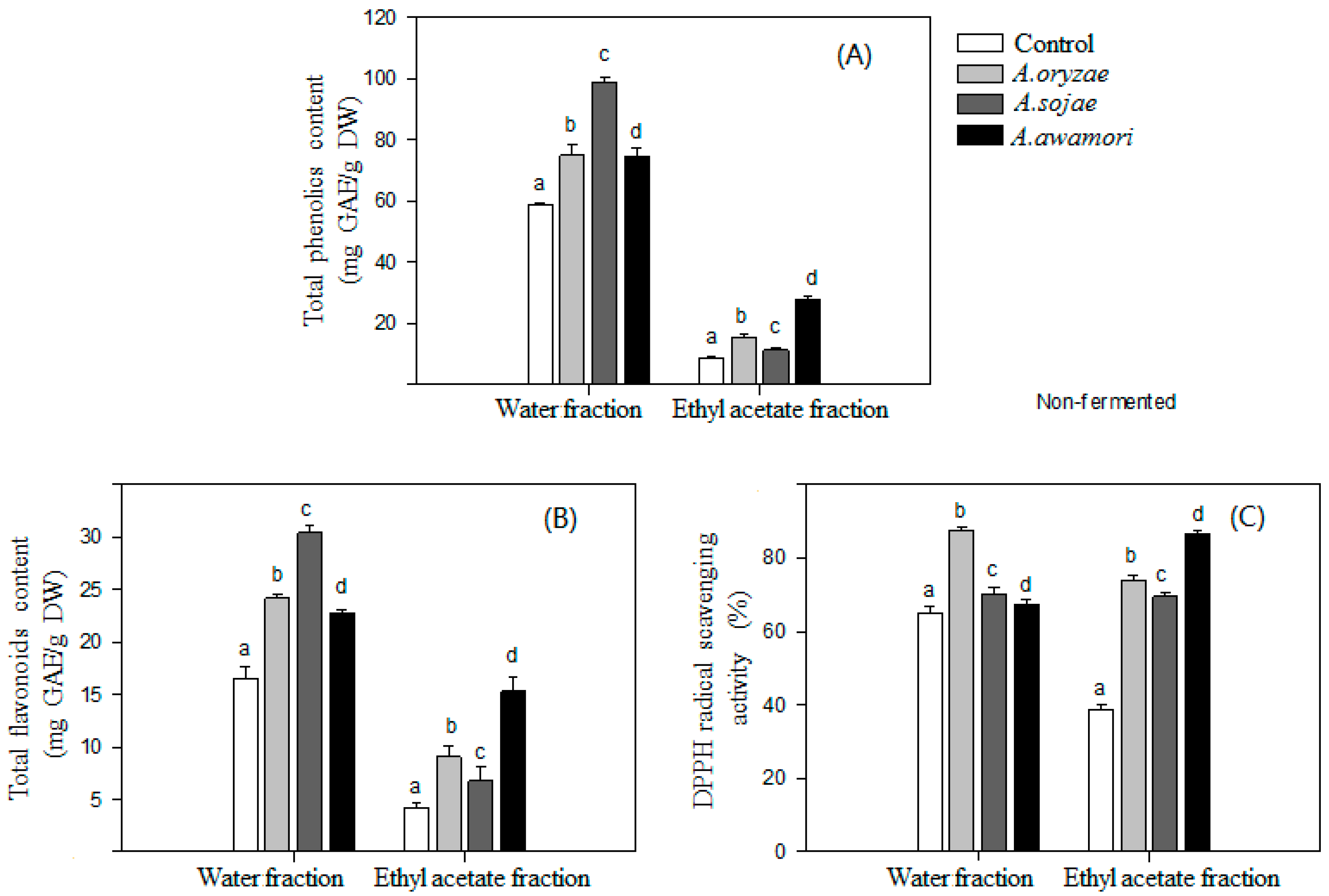
2.2. HPLC Analysis
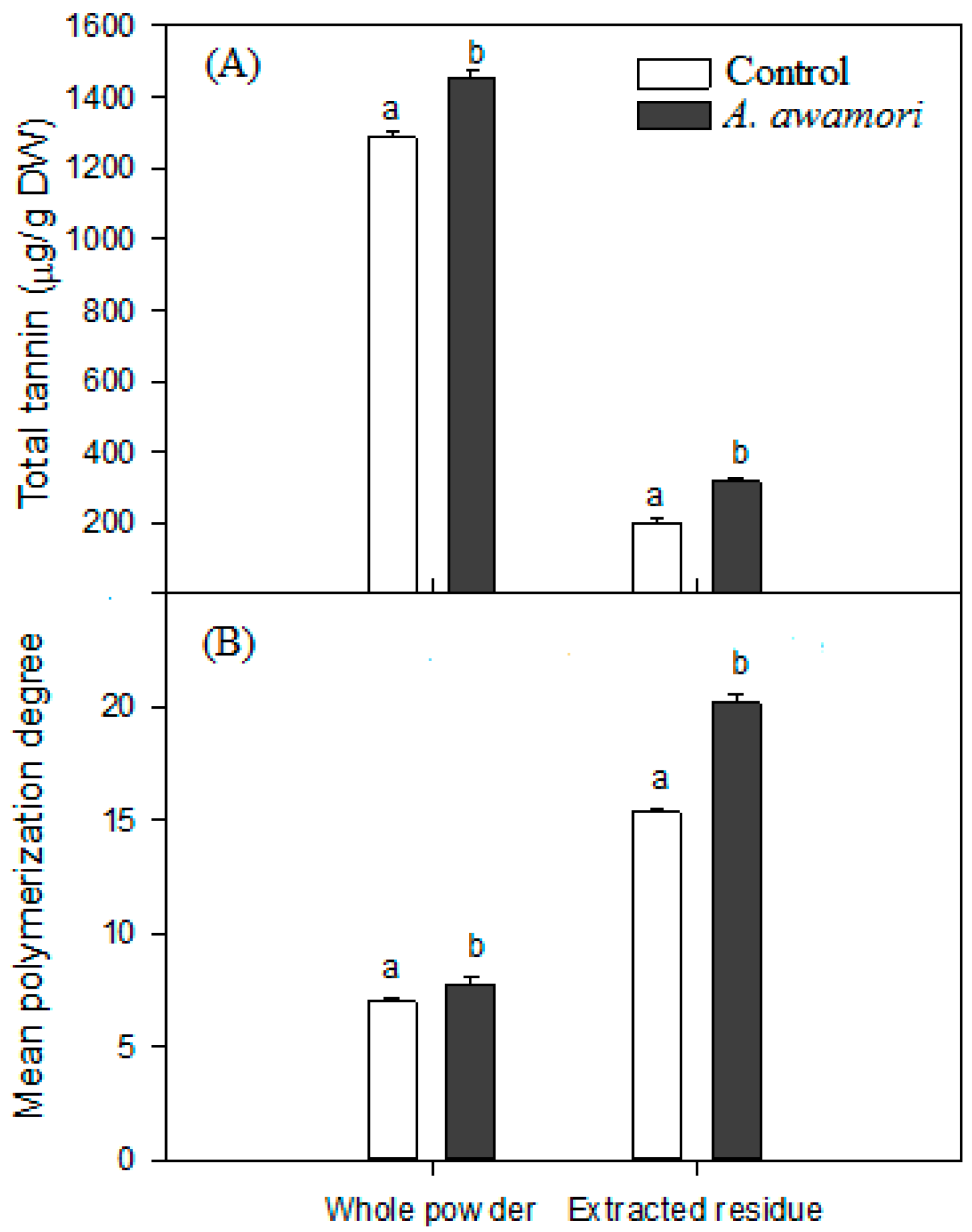
2.3. The Effect of A. awamori Treatment on Condensed Tannin
2.4. ESI-TOF-MS
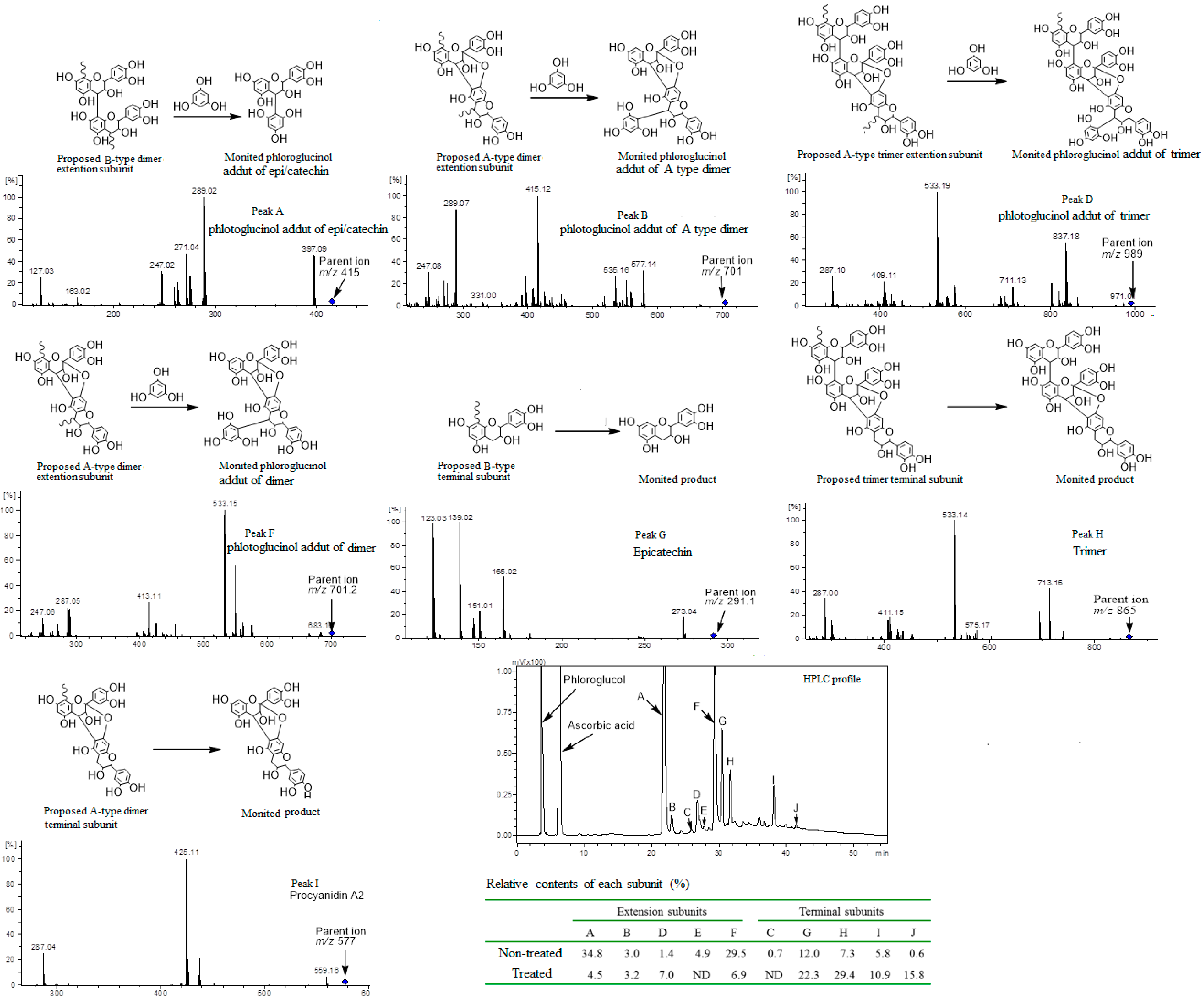
2.5. Phloroglucinolysis Analysis of Condensed Tannin
3. Materials and Methods
3.1. Plant Materials and the Starter Microorganisms
3.2. Incubation of Aspergillus on Litchi Pericarp
3.3. Incubation of Aspergillus with the Purified Condensed Tannin
3.4. Measurements of Contents of Total Phenolics and Flavonoids
3.5. HPLC Analysis
3.6. DPPH Radical Scavenging Assay
3.7. Measurements of Total Tannin and Mean Polymerization Degree
3.8. ESI-TOF-MS Analysis
3.9. Phloroglucinolysis Analysis of Condensed Tannin
3.10. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| LPP | Litchi pericarp powder |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| DW | Dry weight |
| GAE | Gallic acid equivalent |
| RE | Rutin equivalent |
| HPLC-MS | High performance liquid chromatography electrospray-mass spectrometer |
| ESI-TOF-MS | Electrospray ionization-time of flight-mass spectrometry |
References
- Jiang, Y.; Duan, X.; Joyce, D.; Zhang, Z.; Li, J. Advances in understanding of enzymatic browning in harvested litchi fruit. Food Chem. 2004, 88, 443–446. [Google Scholar] [CrossRef]
- Fan, P.; Terrier, L.; Hay, A.E.; Marston, A.; Hostettmann, K. Antioxidant and enzyme inhibition activities and chemical profiles of Polygonum sachalinensis F. Schmidt ex Maxim (Polygonaceae). Fitoterapia 2010, 81, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Kampkötter, A.; Timpel, C.; Zurawski, R.F.; Ruhl, S.; Chovolou, Y.; Proksch, P.; Wätjen, W. Increase of stress resistance and lifespan of Caenorhabditis elegans by quercetin. Comp. Biochem. Phys. B 2008, 149, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, H.; Motamedshariaty, V.; Hadizadeh, F. Antidepressant effect of keamperol, a constituent of saffron (Crocus sativus) petal, in mice and rats. Pharmacologyonline 2007, 2, 367–370. [Google Scholar]
- Lee, Y.J.; Suh, K.S.; Choi, M.C.; Chon, S.; Oh, S.; Woo, J.T.; Kim, S.W.; Kim, J.W.; Kim, Y.S. Kaempferol protects HIT-T15 pancreatic beta cells from 2-deoxy-d-ribose-induced oxidative damage. Phytother. Res. 2010, 24, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Sarni-Manchado, P.; Le Roux, E.; Le Guernevé, C.; Le Lozano, Y.; Cheynier, V. Phenolic composition of litchi fruit pericarp. J. Agric. Food Chem. 2001, 48, 5995–6002. [Google Scholar] [CrossRef]
- Ross, K.A. Concepts important in understanding the health benefits of phenolics in fruits and vegetables: $ and non-extractable phenolics and the influence of cell wall polysaccharides. Res. Health Nutr. 2014, 2, 29–43. [Google Scholar]
- Tarascou, I.; Souquet, J.M.; Mazauric, J.P.; Carrillo, S.; Coq, S.; Canon, F.; Fulcrand, H.; Cheynier, V. The hidden face of food phenolic composition. Arch. Biochem. Biophys. 2010, 501, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Sun, S.; Zhang, S. Enhanced production of total flavones and exopolysaccharides via Vitreoscilla hemoglobin biosynthesis in Phellinus igniarius. Bioresour. Technol. 2011, 102, 1747–1751. [Google Scholar] [CrossRef] [PubMed]
- Mateos-Martín, M.L.; Pérez-Jiménez, J.; Fuguet, E.; Torres, J.L. Non-extractable proanthocyanidins from grapes are a source of bioavailable (epi) catechin and derived metabolites in rats. Brit. J. Nutr. 2012, 108, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Han, B.Z.; Rombouts, F.M.; Nout, M.J.R. A Chinese fermented soybean food. Int. J. Food Microbiol. 2001, 65, 1–10. [Google Scholar] [CrossRef]
- Mugula, J.K.; Nnko, S.A.M.; Narvhus, J.A.; Sørhaug, T. Microbiological and fermentation characteristics of togwa, a Tanzanian fermented food. Int. J. Food Microbiol. 2003, 80, 187–199. [Google Scholar] [CrossRef]
- Parvez, S.; Malik, K.A.; Kang, S.A.; Kim, H.Y. Probiotics and their fermented food products are beneficial for health. J. Appl. Microbiol. 2006, 100, 1171–1185. [Google Scholar] [CrossRef] [PubMed]
- Stanton, C.; Ross, R.P.; Fitzgerald, G.F.; Sinderen, D.V. Fermented functional foods based on probiotics and their biogenic metabolites. Curr. Opin. Biotechnol. 2005, 16, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Zhu, Q.; Wen, L.; Yang, B.; Jiang, G.; Gao, H.; Chen, F.; Jiang, Y. Production of quercetin, kaempferol and their glycosidic derivatives from the aqueous-organic extracted residue of litchi pericarp with Aspergillus awamori. Food Chem. 2014, 145, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; He, J.; Jiang, Y.; Wu, F.; Wang, H.; Wu, D.; Sun, J.; Zhang, D.; Qu, H.; Yang, B. Production of nigragillin and dihydrophaseic acid by biotransformation of litchi pericarp with Aspergillus awamori and their antioxidant activities. J. Funct. Foods 2014, 7, 278–286. [Google Scholar] [CrossRef]
- Lin, S.; Wen, L.; Yang, B.; Jiang, G.; Shi, J.; Chen, F.; Jiang, Y. Improved growth of Lactobacillus bulgaricus and Streptococcus thermophilus as well as increased antioxidant activity by biotransforming litchi pericarp polysaccharide with Aspergillus awamori. BioMed. Res. Int. 2013. [Google Scholar] [CrossRef] [PubMed]
- Mccrae, S.I.; Leith, K.M.; Gordon, A.H.; Wood, T.M. Xylan-degrading enzyme system produced by the fungus Aspergillus awamori: Isolation and characterization of a feruloyl esterase and a p-coumaroyl esterase. Enzym. Microb. Tech. 1994, 16, 826–834. [Google Scholar] [CrossRef]
- Smith, D.C.; Wood, T.M. Xylanase production by Aspergillus awamori development of a medium and optimization of the fermentation parameters for the production of extracellular xylanase and beta-xylosidase while maintaining low protease production. Biotechnol. Bioeng. 1991, 38, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, L.M.F.; Oliveira, R.A.; Bon, E.P.D.S. Cellulases, xylanases, β-glucosidase and ferulic acid esterase produced by Trichoderma and Aspergillus act synergistically in the hydrolysis of sugarcane bagasse. Biochem. Eng. J. 2010, 51, 72–78. [Google Scholar] [CrossRef]
- Prasad, K.N.; Yang, B.; Zhao, M.; Ruenroengklin, N.; Jiang, Y. Application of ultrasonication or high-pressure extraction of flavonids from litchi fruit pericarp. J. Food Process Eng. 2009, 32, 828–843. [Google Scholar] [CrossRef]
- Zhang, D.; Quantick, P.C.; Grigor, J.M. Changes in phenolic compounds in Litchi (Litchi chinensis Sonn.) fruit during postharvest storage. Postharvest Biol. Technol. 2000, 19, 165–172. [Google Scholar] [CrossRef]
- Le Roux, E.; Doco, T.; Sarni-Manchado, P.; Lozano, Y.; Cheynier, V. A-type proanthocyanidins from pericarp of Litchi chinensis. Phytochemistry 1998, 48, 1251–1258. [Google Scholar] [CrossRef]
- Kostrzewa-Susłow, E.; Dmochowska-Gładysz, J.; Janeczko, T. Microbial transformation of selected flavanones as a method of increasing the antioxidant properties. Z. Naturforsch. C 2010, 65, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Nikkuni, S.; Nakajima, H.; Hoshina, S.I.; Ohno, M.; Suzuki, C.; Kashiwagi, Y.; Mori, K. Evolutionary relationships among Aspergillus oryzae and related species based on the sequences of 18S rRNA genes and internal transcribed spacers. J. Gen. Appl. Microbiol. 1998, 44, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Jara, P.J.; Josep, L.T. Analysis of nonextractable phenolic compounds in foods: The current state of the art. J. Agric. Food Chem. 2011, 59, 12713–12724. [Google Scholar]
- Bourvellec, C.L.; Renard, C.M.G.C.; Bouchet, B. Non-covalent interaction between procyanidins and apple cell wall material. Part III: Study on model polysaccharides. BBA-Gen. Subj. 2005, 1725, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xie, B.; Cao, S.; Yang, E.; Xu, X.; Guo, S. A-type procyanidins from Litchi chinensis pericarp with antioxidant activity. Food Chem. 2007, 105, 1446–1451. [Google Scholar] [CrossRef]
- Zhou, H.C.; Lin, Y.M.; Li, Y.Y.; Li, M.; Wei, S.D. Antioxidant properties of polymeric proanthocyanidins from fruit stones and pericarps of Litchi chinensis Sonn. Food Res. Int. 2011, 44, 613–620. [Google Scholar] [CrossRef]
- Reddy, U.D.C.; Chawla, A.S.; Deepak, M.; Singh, D.; Handa, S.S. High pressure liquid chromatographic determination of bergenin and (+)-afzelechin from different parts of Paashaanbhed (Bergenia ligulata yeo). Phytochem. Anal. 1999, 10, 44–47. [Google Scholar] [CrossRef]
- Groenewoud, G.; Hundt, H.K.L. The microbial metabolism of (+)-catechin to two novel diarylpropan-2-ol metabolites in vitro. Xenobiotica 1984, 14, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Groenewoud, G.; Hundt, H.K.L. The microbial metabolism of condensed (+)-catechins by rat-caecal microflora. Xenobiotica 1986, 16, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Miller, J.M. Tandem mass spectrometry of the B-type procyanidins in wine and B-type dehydrodicatechins in an autoxidation mixture of (+)-catechin and (−)-epicatechin. J. Mass Spectrom. 2003, 38, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Chunmei, L.; Rachael, L.; Trombley, J.D.; Shufen, X.; Jie, Y.; Yan, T.; Reed, J.D.; Hagerman, A.E. High molecular weight persimmon (Diospyros kaki L.) proanthocyanidin: A highly galloylated, A-linked tannin with an unusual flavonol terminal unit, myricetin. J. Agric. Food Chem. 2010, 58, 9033–9042. [Google Scholar]
- Osman, A.M.; Wong, K.K.Y. Laccase (EC 1.10.3.2) catalyses the conversion of procyanidin B-2 (epicatechin dimer) to type A-2. Tetrahedron Lett. 2007, 48, 1163–1167. [Google Scholar] [CrossRef]
- Kondo, K.; Kurihara, M.; Fukuhara, K.; Tanaka, T.; Suzuki, T.; Miyata, N.; Toyoda, M. Conversion of procyanidin B-type (catechin dimer) to A-type: Evidence for abstraction of C-2 hydrogen in catechin during radical oxidation. Tetrahedron Lett. 2000, 41, 485–488. [Google Scholar] [CrossRef]
- Akagi, T.; Suzuki, Y.; Ikegami, A.; Kamitakahara, H.; Takano, T.; Nakatsubo, F.; Yonemori, K. Condensed tannin composition analysis in persimmon (Diospyros kaki Thunb.) fruit by acid catalysis in the presence of excess Phloroglucinol. J. Jpn. Soc. Hortic. Sci. 2010, 79, 275–281. [Google Scholar] [CrossRef]
- Quettier-Deleu, C.; Gressier, B.; Vasseur, J.; Dine, T.; Brunet, C.; Luyckx, M.; Cazin, M.; Cazin, J.C.; Bailleul, F.; Trotin, F. Phenolic compounds and antioxidant activities of buckwheat (Fagopyrum esculentum Moench) hulls and flour. J. Ethnopharmacol. 2000, 72, 35–42. [Google Scholar] [CrossRef]
- Jia, Z.; Tang, M.; Wu, J. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar]
- Yang, Z.; Zhai, W. Identification and antioxidant activity of anthocyanins extracted from the seed and cob of purple corn (Zea mays L.). Innov. Food Sci. Emerg. 2010, 11, 169–176. [Google Scholar] [CrossRef]
- Nakajima, K.; Yoshie-Stark, Y.; Ogushi, M. Comparison of ACE inhibitory and DPPH radical scavenging activities of fish muscle hydrolysates. Food Chem. 2009, 114, 844–851. [Google Scholar] [CrossRef]
- Bate-Smith, E.C. Phytochemistry of proanthocyanidins. Phytochemistry 1975, 14, 1107–1113. [Google Scholar] [CrossRef]
- Butler, L.G.; Price, M.L.; Brotherton, J.E. Vanillin assay for proanthocyanidins (condensed tannins): Modification of the solvent for estimation of the degree of polymerization. J. Agric. Food Chem. 1981, 30, 1087–1089. [Google Scholar] [CrossRef]
- Kennedy, J.A.; Jones, G.P. Analysis of proanthocyanidin cleavage products following acid-catalysis in the presence of excess phloroglucinol. J. Agric. Food Chem. 2001, 49, 1740–1746. [Google Scholar] [CrossRef] [PubMed]
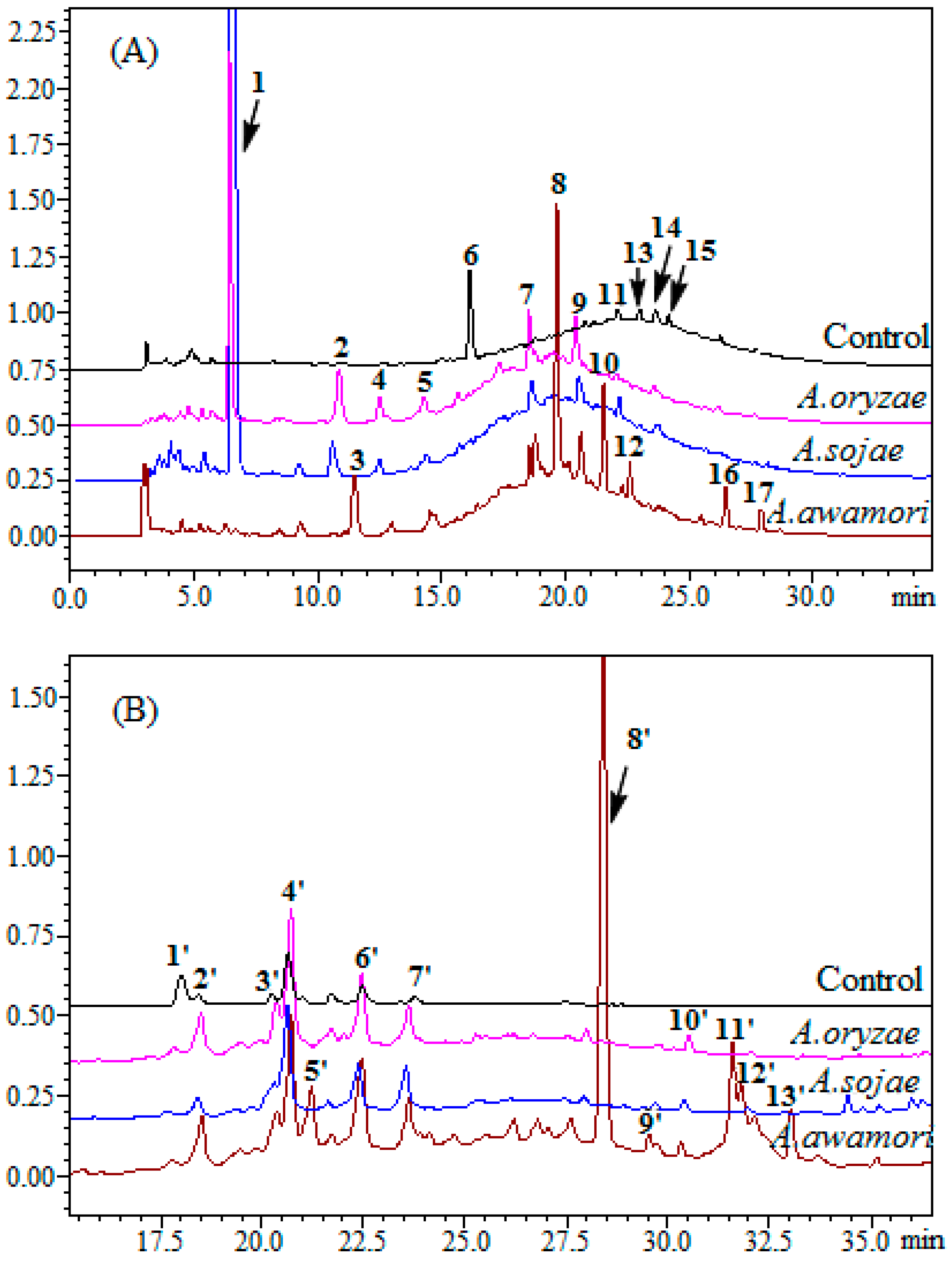
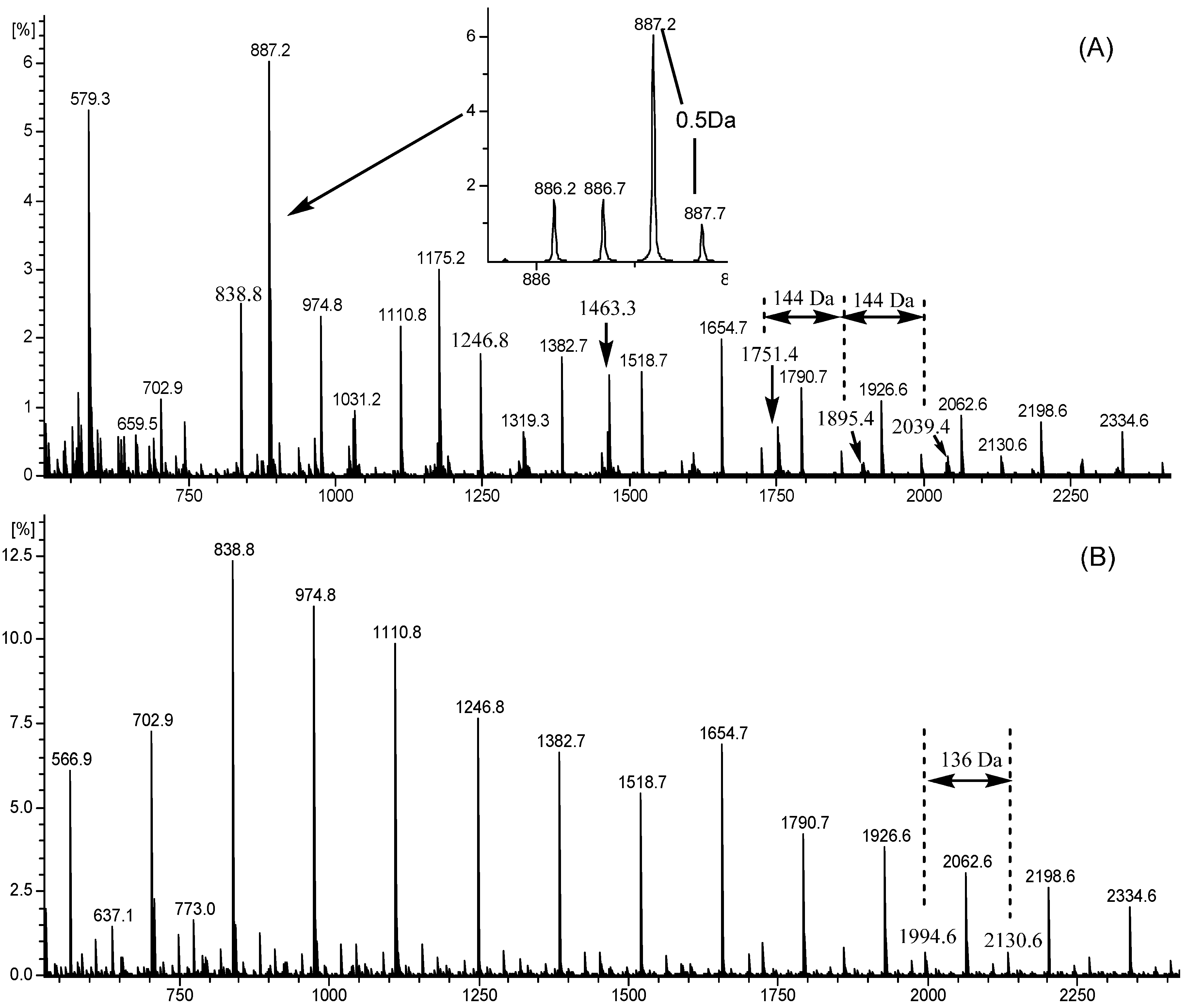

| Polymers | Proposed Ionization Type | Charge (CH) | A Type Linkage (AL) | Proposed Subunit | Calculated Masses * (m/z) | Observed Masses (m/z) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| H | Na | K | Af | Ca | Ga | NT | T | ||||
| 1-mers | 1 | 0 | 0 | 1 | 0 | 0 | 2 | 0 | 579 | 579.3 | - |
| 3-mers | 0 | 1 | 0 | 1 | 0 | 3 | 0 | 0 | 838 | 838.8 | 838.8 |
| 1 | 0 | 0 | 1 | 1 | 0 | 3 | 0 | 865 | 865.2 | 865.2 | |
| 0 | 1 | 0 | 1 | 0 | 0 | 2 | 1 | 903 | 903.2 | - | |
| 0 | 1 | 0 | 0 | 1 | 0 | 0 | 3 | 937 | 937.4 | - | |
| 4-mers | 0 | 1 | 0 | 0 | 1 | 4 | 0 | 0 | 1110 | 1110.8 | 1110.8 |
| 0 | 1 | 0 | 1 | 1 | 0 | 0 | 4 | 1382 | 1382.7 | 1382.7 | |
| 5-mers | 0 | 0 | 1 | 1 | 0 | 0 | 5 | 0 | 1479 | 1479.3 | - |
| 1 | 0 | 0 | 1 | 1 | 0 | 0 | 5 | 1518 | 1518.7 | 1518.7 | |
| 6-mers | 0 | 2 | 0 | 2 | 1 | 0 | 6 | 0 | 887 | 887.2 | - |
| 0 | 1 | 1 | 2 | 2 | 0 | 6 | 0 | 894 | 894.2 | - | |
| 0 | 0 | 2 | 0 | 0 | 0 | 6 | 0 | 954 | 954.2 | - | |
| 0 | 1 | 0 | 1 | 0 | 6 | 0 | 0 | 1654 | 1654.7 | 1654.7 | |
| 1 | 0 | 0 | 1 | 1 | 0 | 2 | 4 | 1790 | 1790.7 | 1790.7 | |
| 1 | 0 | 0 | 1 | 2 | 0 | 3 | 3 | 1926 | 1926.6 | 1926.6 | |
| 7-mers | 2 | 0 | 0 | 2 | 2 | 0 | 3 | 4 | 1039 | 1039.2 | - |
| 0 | 2 | 0 | 2 | 0 | 0 | 7 | 0 | 1183 | 1183.2 | - | |
| 1 | 0 | 0 | 1 | 2 | 0 | 4 | 3 | 2062 | 2062.6 | 2062.6 | |
| 8-mers | 0 | 2 | 0 | 2 | 1 | 0 | 6 | 2 | 1191 | 1191.2 | - |
| 9-mers | 2 | 0 | 0 | 2 | 1 | 0 | 6 | 3 | 1319 | 1319.3 | - |
| 0 | 1 | 1 | 2 | 0 | 0 | 9 | 0 | 1327 | 1327.3 | - | |
| 0 | 2 | 0 | 2 | 0 | 1 | 9 | 0 | 1450 | 1450.7 | 1450.7 | |
| 10-mers | 0 | 2 | 0 | 2 | 1 | 0 | 10 | 0 | 1463 | 1463.3 | - |
| 0 | 2 | 0 | 2 | 1 | 0 | 9 | 1 | 1471 | 1471.3 | - | |
| 11-mers | 0 | 2 | 0 | 2 | 1 | 2 | 9 | 0 | 1586 | 1586.7 | 1586.7 |
| 0 | 2 | 0 | 2 | 1 | 0 | 9 | 2 | 1615 | 1615.3 | - | |
| 12-mers | 0 | 2 | 0 | 2 | 1 | 3 | 9 | 0 | 1722 | 1722.7 | 1722.7 |
| 0 | 2 | 0 | 2 | 1 | 0 | 12 | 0 | 1751 | 1751.4 | - | |
| 13-mers | 0 | 2 | 0 | 2 | 1 | 4 | 9 | 0 | 1858 | 1858.7 | 1858.7 |
| 0 | 2 | 0 | 2 | 1 | 0 | 13 | 0 | 1895 | 1895.4 | - | |
| 14-mers | 0 | 2 | 0 | 2 | 1 | 5 | 9 | 0 | 1994 | 1994.6 | 1994.6 |
| 0 | 2 | 0 | 2 | 1 | 0 | 14 | 0 | 2039 | 2039.4 | - | |
| 15-mers | 0 | 2 | 0 | 2 | 1 | 6 | 9 | 0 | 2130 | 2130.6 | 2130.6 |
| 0 | 2 | 0 | 2 | 1 | 0 | 15 | 0 | 2183 | 2183.5 | - | |
| 16-mers | 0 | 2 | 0 | 2 | 1 | 7 | 9 | 0 | 2266 | 2266.6 | 2266.6 |
| 0 | 2 | 0 | 2 | 1 | 0 | 16 | 0 | 2327 | 2327.5 | - | |
| 17-mers | 0 | 2 | 0 | 2 | 1 | 8 | 9 | 0 | 2402 | 2402.6 | 2402.6 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, S.; Li, Q.; Yang, B.; Duan, X.; Zhang, M.; Shi, J.; Jiang, Y. Transformation of Litchi Pericarp-Derived Condensed Tannin with Aspergillus awamori. Int. J. Mol. Sci. 2016, 17, 1067. https://doi.org/10.3390/ijms17071067
Lin S, Li Q, Yang B, Duan X, Zhang M, Shi J, Jiang Y. Transformation of Litchi Pericarp-Derived Condensed Tannin with Aspergillus awamori. International Journal of Molecular Sciences. 2016; 17(7):1067. https://doi.org/10.3390/ijms17071067
Chicago/Turabian StyleLin, Sen, Qing Li, Bao Yang, Xuewu Duan, Mingwei Zhang, John Shi, and Yueming Jiang. 2016. "Transformation of Litchi Pericarp-Derived Condensed Tannin with Aspergillus awamori" International Journal of Molecular Sciences 17, no. 7: 1067. https://doi.org/10.3390/ijms17071067