Diet Quality and Cancer Outcomes in Adults: A Systematic Review of Epidemiological Studies
Abstract
:1. Introduction
2. Review Aims
3. Review Methods
3.1. Search Strategy
3.2. Eligibility Criteria
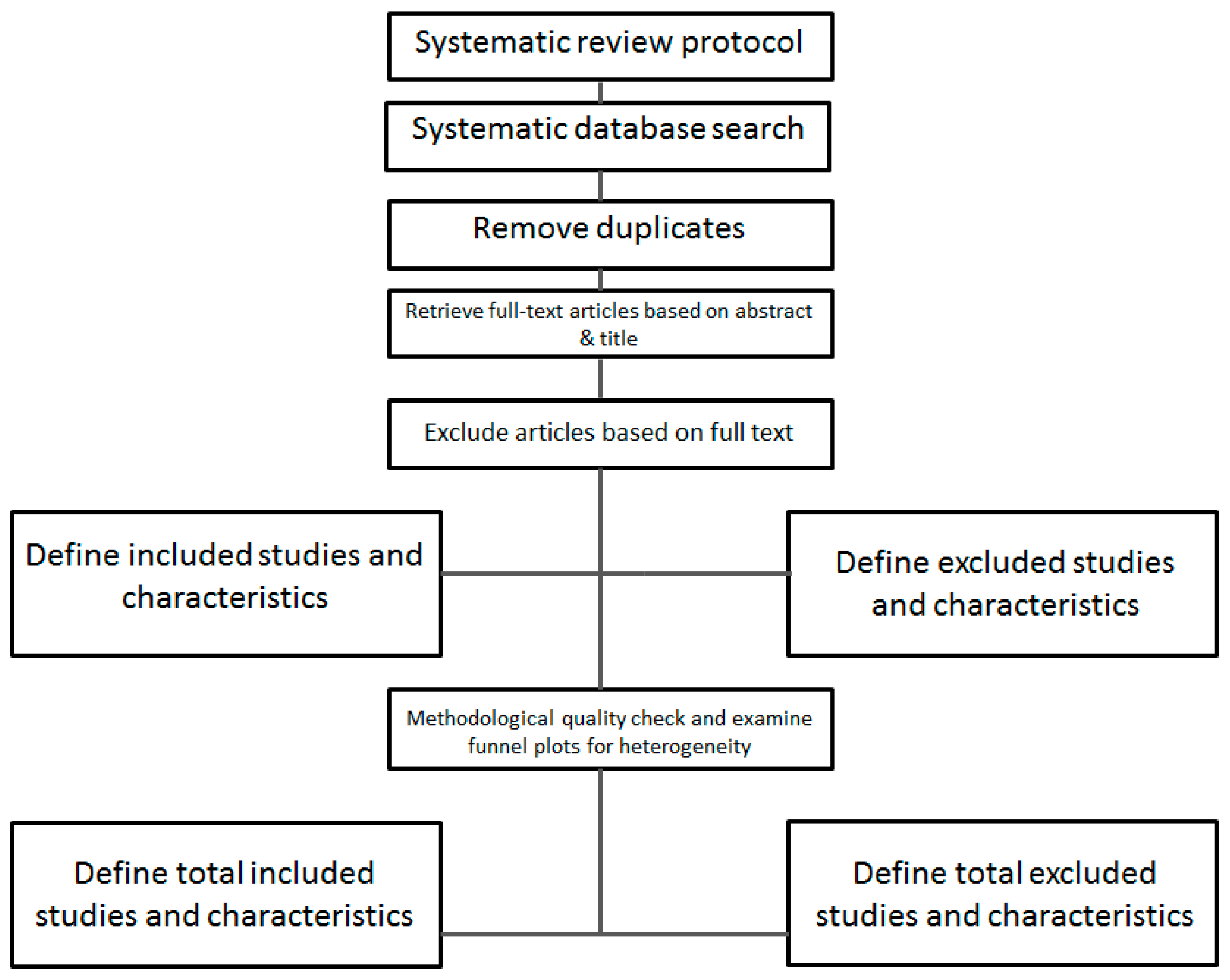
3.3. Selection Process
3.4. Quality Assessment of Included Studies
4. Results
4.1. Description of Studies
4.2. Diet Quality Scores
4.3. Diet Quality and Cancer Risk
4.4. Overall Cancer Risk
4.5. Breast Cancer Risk
4.6. Colorectal Cancer Risk
4.7. Gastric Cancer
4.8. Prostate Cancer
4.9. Head and Neck Cancer
4.10. Risk of Other Cancers
4.11. Diet Quality and Cancer Mortality
4.12. Diet Quality and Risk of Mortality from All-Sites Cancer
4.13. Mediterranean Diet Scores
4.14. Healthy Eating Index
4.15. The Recommended Food Score
4.16. Diet Diversity Scores
4.17. Other Diet Quality Scores Associated with Cancer Mortality Risk
4.18. Diet Quality Scores Associated with Increased Cancer Mortality Risk
4.19. Other Diet Quality Scores Not Associated with Cancer Mortality Risk
4.20. Diet Quality and Risk of Site-Specific Cancer Mortality
5. Discussion
6. Conclusions and Recommendations
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| Selection | |
|---|---|
1. Representativeness of the exposed cohort
| |
2. Selection of the non-exposed cohort
| |
3. Ascertainment of exposure
| |
4. Demonstration that outcome of interest was not present at start of study
| |
| Comparability | |
1. Comparability of cohorts on the basis of the design or analysis
| |
| Outcome | |
1. Assessment of outcome
| |
2. Was follow-up long enough for outcomes to occur
| |
3. Adequacy of follow-up of cohorts
| |
| Total Score | |
| Comments | |
References
- World Cancer Research Fund; American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective; American Institute for Cancer Research: Washington, DC, USA, 2007. [Google Scholar]
- Cancer Council. Cancer in Australia 2014. Available online: http://www.cancer.org.au/about-cancer/what-is-cancer/facts-and-figures.html (accessed on 5 March 2014).
- Collins, C.E.; Young, A.F.; Hodge, A. Diet quality is associated with higher nutrient intake and self-rated health in mid-aged women. J. Am. Coll. Nutr. 2008, 27, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Romaguera, D.; Vergnaud, A.C.; Peeters, P.H.; van Gils, C.H.; Chan, D.S.; Ferrari, P.; Romieu, I.; Jenab, M.; Slimani, N.; Clavel-Chapelon, F.; et al. Is concordance with World Cancer Research Fund/American Institute for Cancer Research guidelines for cancer prevention related to subsequent risk of cancer? Results from the EPIC study. Am. J. Clin. Nutr. 2012, 96, 150–163. [Google Scholar] [CrossRef] [PubMed]
- Bertuccio, P.; Rosato, V.; Andreano, A.; Ferraroni, M.; Decarli, A.; Edefonti, V.; La Vecchia, C. Dietary patterns and gastric cancer risk: A systematic review and meta-analysis. Ann. Oncol. 2013, 24, 1450–1458. [Google Scholar] [CrossRef] [PubMed]
- Magalhaes, B.; Peleteiro, B.; Lunet, N. Dietary patterns and colorectal cancer: Systematic review and meta-analysis. Eur. J. Cancer Prev. 2012, 21, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Brennan, S.F.; Cantwell, M.M.; Cardwell, C.R.; Velentzis, L.S.; Woodside, J.V. Dietary patterns and breast cancer risk: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2010, 91, 1294–1302. [Google Scholar] [CrossRef] [PubMed]
- Randi, G.; Edefonti, V.; Ferraroni, M.; La Vecchia, C.; Decarli, A. Dietary patterns and the risk of colorectal cancer and adenomas. Nutr. Rev. 2010, 68, 389–408. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.T.; Brown, L.S. Dietary Patterns and the Risk of Colorectal Cancer. Curr. Nutr. Rep. 2013, 2, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Bamia, C.; Lagiou, P.; Buckland, G.; Grioni, S.; Agnoli, C.; Taylor, A.J.; Dahm, C.C.; Overvad, K.; Olsen, A.; Tjonneland, A.; et al. Mediterranean diet and colorectal cancer risk: Results from a European cohort. Eur. J. Epidemiol. 2013, 28, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.T.; Hu, F.B.; Wu, K.; Chiuve, S.E.; Fuchs, C.S.; Giovannucci, E. The Mediterranean and Dietary Approaches to Stop Hypertension (DASH) diets and colorectal cancer. Am. J. Clin. Nutr. 2010, 92, 1429–1435. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.T.; Hu, F.B.; Hankinson, S.E.; Willett, W.C.; Holmes, M.D. Low-carbohydrate diets, dietary approaches to stop hypertension-style diets, and the risk of postmenopausal breast cancer. Am. J. Epidemiol. 2011, 174, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Reedy, J.; Mitrou, P.N.; Krebs-Smith, S.M.; Wirfält, E.; Flood, A.; Kipnis, V.; Leitzmann, M.; Mouw, T.; Hollenbeck, A.; Schatzkin, A.; et al. Index-based dietary patterns and risk of colorectal cancer: The NIH-AARP Diet and Health Study. Am. J. Epidemiol. 2008, 168, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Shahril, M.R.; Sulaiman, S.; Shaharudin, S.H.; Akmal, S.N. Healthy eating index and breast cancer risk among Malaysian women. Eur. J. Cancer Prev. 2013, 22, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Murtaugh, M.A.; Sweeney, C.; Giuliano, A.R.; Herrick, J.S.; Hines, L.; Byers, T.; Baumgartner, K.B.; Slattery, M.L. Diet patterns and breast cancer risk in Hispanic and non-Hispanic white women: The Four-Corners Breast Cancer Study. Am. J. Clin. Nutr. 2008, 87, 978–984. [Google Scholar] [PubMed]
- Chlebowski, R.T.; Blackburn, G.L.; Thomson, C.A.; Nixon, D.W.; Shapiro, A.; Hoy, M.K.; Goodman, M.T.; Giuliano, A.E.; Karanja, N.; McAndrew, P.; et al. Dietary fat reduction and breast cancer outcome: Interim efficacy results from the Women’s Intervention Nutrition Study. J. Natl. Cancer Inst. 2006, 98, 1767–1776. [Google Scholar] [CrossRef] [PubMed]
- Buckland, G.; Travier, N.; Cottet, V.; Gonzalez, C.A.; Lujan-Barroso, L.; Agudo, A.; Trichopoulou, A.; Lagiou, P.; Trichopoulos, D.; Peeters, P.H.; et al. Adherence to the mediterranean diet and risk of breast cancer in the European prospective investigation into cancer and nutrition cohort study. Int. J. Cancer 2013, 132, 2918–2927. [Google Scholar] [CrossRef] [PubMed]
- Bosire, C.; Stampfer, M.J.; Subar, A.F.; Park, Y.; Kirkpatrick, S.I.; Chiuve, S.E.; Hollenbeck, A.R.; Reedy, J. Index-based dietary patterns and the risk of prostate cancer in the NIH-AARP diet and health study. Am. J. Epidemiol. 2013, 177, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Praud, D.; Bertuccio, P.; Bosetti, C.; Turati, F.; Ferraroni, M.; La Vecchia, C. Adherence to the Mediterranean diet and gastric cancer risk in Italy. Int. J. Cancer 2013, 134, 2935–2941. [Google Scholar] [CrossRef] [PubMed]
- Li, W.Q.; Park, Y.; Wu, J.W.; Goldstein, A.M.; Taylor, P.R.; Hollenbeck, A.R.; Freedman, N.D.; Abnet, C.C. Index-based dietary patterns and risk of head and neck cancer in a large prospective study. Am. J. Clin. Nutr. 2014, 99, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Samoli, E.; Lagiou, A.; Nikolopoulos, E.; Lagogiannis, G.; Barbouni, A.; Lefantzis, D.; Trichopoulos, D.; Brennan, P.; Lagiou, P. Mediterranean diet and upper aerodigestive tract cancer: The Greek segment of the Alcohol-Related Cancers and Genetic Susceptibility in Europe study. Br. J. Nutr. 2010, 104, 1369–1374. [Google Scholar] [CrossRef] [PubMed]
- National Health and Medical Research Council. Australian Dietary Guidelines; National Health and Medical Research Council: Canberra, Australian, 2013. [Google Scholar]
- Aljadani, H.M.; Patterson, A.; Sibbritt, D.; Hutchesson, M.J.; Jensen, M.E.; Collins, C.E. Diet quality, measured by fruit and vegetable intake, predicts weight change in young women. J. Obes. 2013, 2013, 525161. [Google Scholar] [CrossRef] [PubMed]
- Wolongevicz, D.M.; Zhu, L.; Pencina, M.J.; Kimokoti, R.W.; Newby, P.K.; D’Agostino, R.B.; Millen, B.E. Diet quality and obesity in women: The Framingham Nutrition Studies. Br. J. Nutr. 2010, 103, 1223–1229. [Google Scholar] [CrossRef] [PubMed]
- Schroder, H.; Fito, M.; Covas, M.I. Association of fast food consumption with energy intake, diet quality, body mass index and the risk of obesity in a representative Mediterranean population. Br. J. Nutr. 2007, 98, 1274–1280. [Google Scholar] [CrossRef] [PubMed]
- Centre for Reviews and Dissemination. Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Health Care; University of York: New York, NY, USA, 2008. [Google Scholar]
- Potter, J.; Collins, C.E.; Brown, L.; Byles, J. University of York Centre for Reviews and Dissemination. Available online: http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42014010317#.VGrmoU1O7IU (accessed on 27 June 2014).
- Akbaraly, T.N.; Ferrie, J.E.; Berr, C.; Brunner, E.J.; Head, J.; Marmot, M.G.; et al. Alternative Healthy Eating Index and mortality over 18 y of follow-up: Results from the Whitehall II cohort. Am. J. Clin. Nutr. 2011, 94, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Arem, H.; Reedy, J.; Sampson, J.; Jiao, L.; Hollenbeck, A.R.; Risch, H.; Mayne, S.T.; Stolzenberg-Solomon, R.Z. The Healthy Eating Index 2005 and risk for pancreatic cancer in the NIH-AARP study. J. Natl. Cancer Inst. 2013, 105, 1298–1305. [Google Scholar] [CrossRef] [PubMed]
- Ax, E.; Garmo, H.; Grundmark, B.; Bill-Axelson, A.; Holmberg, L.; Becker, W.; Zethelius, B.; Cederholm, T.; Sjögren, P. Dietary patterns and prostate cancer risk: Report from the population based ULSAM cohort study of Swedish men. Nutr. Cancer 2014, 66, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Berentzen, N.E.; Beulens, J.W.; Hoevenaar-Blom, M.P.; Kampman, E.; Bueno-de-Mesquita, H.B.; Romaguera-Bosch, D.; Peeters, P.H.M.; May, A.M. Adherence to the WHO’s healthy diet indicator and overall cancer risk in the EPIC-NL cohort. PLoS ONE 2013, 8, e70535. [Google Scholar] [CrossRef] [PubMed]
- Benetou, V.; Trichopoulou, A.; Orfanos, P.; Naska, A.; Lagiou, P.; Boffetta, P.; Trichopoulos, D. Conformity to traditional Mediterranean diet and cancer incidence: The Greek EPIC cohort. Br. J. Cancer 2008, 99, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Büchner, F.L.; Bueno-De-Mesquita, H.B.; Ros, M.M.; Kampman, E.; Egevad, L.; Overvad, K.; Tjãnneland, A.; Roswall, N.; Clavel-Chapelon, F.; Boutron-Ruault, M.C.; et al. Variety in vegetable and fruit consumption and risk of bladder cancer in the European Prospective Investigation into Cancer and Nutrition. Int. J. Cancer 2011, 128, 2971–2979. [Google Scholar] [CrossRef] [PubMed]
- Büchner, F.L.; Bueno-de-Mesquita, H.B.; Ros, M.M.; Overvad, K.; Dahm, C.C.; Hansen, L.; Tjønneland, A.; Clavel-Chapelon, F.; Boutron-Ruault, M.C.; Touillaud, M.; et al. Variety in fruit and vegetable consumption and the risk of lung cancer in the European Prospective Investigation into Cancer and Nutrition. Cancer Epidemiol. Biomark. Prev. 2010, 19, 2278–2286. [Google Scholar] [CrossRef] [PubMed]
- Buckland, G.; Agudo, A.; Lujan, L.; Jakszyn, P.; Bueno-de-Mesquita, H.B.; Palli, D.; Boeing, H.; Carneiro, F.; Krogh, V.; Sacerdote, C.; et al. Adherence to a Mediterranean diet and risk of gastric adenocarcinoma within the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort study. Am. J. Clin. Nutr. 2010, 91, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Buckland, G.; Agudo, A.; Travier, N.; Huerta, J.M.; Cirera, L.; Tormo, M.J.; Navarro, C.; Chirlaque, M.D.; Moreno-Iribas, C.; Ardanaz, E.; et al. Adherence to the Mediterranean diet reduces mortality in the Spanish cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC-Spain). Br. J. Nutr. 2011, 106, 1581–1591. [Google Scholar] [CrossRef] [PubMed]
- Buckland, G.; Ros, M.M.; Roswall, N.; Bueno-De-Mesquita, H.B.; Travier, N.; Tjonneland, A.; Kiemeney, L.A.; Sacerdote, C.; Tumino, R.; Ljungberg, B.; et al. Adherence to the Mediterranean diet and risk of bladder cancer in the EPIC cohort study. Int. J. Cancer 2014, 134, 2504–2511. [Google Scholar] [CrossRef] [PubMed]
- Cade, J.E.; Taylor, E.F.; Burley, V.J.; Greenwood, D.C. Does the Mediterranean dietary pattern or the Healthy Diet Index influence the risk of breast cancer in a large British cohort of women? Eur. J. Clin. Nutr. 2011, 65, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Chiuve, S.E.; Fung, T.T.; Rimm, E.B.; Hu, F.B.; McCullough, M.L.; Wang, M.; Stampfer, M.J.; Willett, W.C. Alternative dietary indices both strongly predict risk of chronic disease. J. Nutr. 2012, 142, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Couto, E.; Boffetta, P.; Lagiou, P.; Ferrari, P.; Buckland, G.; Overvad, K.; Dahm, C.C.; Tjønneland, A.; Olsen, A.; Clavel-Chapelon, F.; et al. Mediterranean dietary pattern and cancer risk in the EPIC cohort. Br. J. Cancer. 2011, 104, 1493–1499. [Google Scholar] [CrossRef] [PubMed]
- Couto, E.; Sandin, S.; Lof, M.; Ursin, G.; Adami, H.-O.; Weiderpass, E. Mediterranean dietary pattern and risk of breast cancer. PLoS ONE 2013, 8, e55374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuenca-Garcia, M.; Artero, E.G.; Sui, X.; Lee, D.-C.; Hebert, J.R.; Blair, S.N. Dietary indices, cardiovascular risk factors and mortality in middle-aged adults: Findings from the Aerobics Center Longitudinal Study. Ann. Epidemiol. 2014, 24, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Drake, I.; Gullberg, B.; Sonestedt, E.; Wallstrom, P.; Persson, M.; Hlebowicz, J.; Nilsson, J.; Hedblad, B.; Wirfalt, E. Scoring models of a diet quality index and the predictive capability of mortality in a population-based cohort of Swedish men and women. Public Health Nutr. 2013, 16, 468–478. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, A.L.; Dewar, R.A.; Veugelers, P.J. Diet quality and cancer incidence in Nova Scotia, Canada. Nutr. Cancer 2002, 43, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.T.; Hu, F.B.; McCullough, M.L.; Newby, P.K.; Willett, W.C.; Holmes, M.D. Diet quality is associated with the risk of estrogen receptor-negative breast cancer in postmenopausal women. J. Nutr. 2006, 136, 466–472. [Google Scholar] [PubMed]
- Fung, T.T.; van Dam, R.M.; Hankinson, S.E.; Stampfer, M.; Willett, W.C.; Hu, F.B. Low-carbohydrate diets and all-cause and cause-specific mortality: Two cohort studies. Ann. Int. Med. 2010, 153, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Huijbregts, P.; Feskens, E.J.M.; Räsänen, L.; Fidanza, F.; Nissinen, A.; Menotti, A.; Kromhout, D. Dietary pattern and 20 year mortality in elderly men in Finland, Italy, and the Netherlands: Longitudinal cohort study. Br. Med. J. 1997, 315, 18. [Google Scholar] [CrossRef]
- Jarvandi, S.; Davidson, N.O.; Schootman, M. Increased risk of colorectal cancer in type 2 diabetes is independent of diet quality. PLoS ONE 2013, 8, e74616. [Google Scholar] [CrossRef] [PubMed]
- Jeurnink, S.M.; Büchner, F.L.; Bueno-De-Mesquita, H.B.; Siersema, P.D.; Boshuizen, H.C.; Numans, M.E.; Dahm, C.C.; Overvad, K.; Tjønneland, A.; Roswall, N.; et al. Variety in vegetable and fruit consumption and the risk of gastric and esophageal cancer in the European prospective investigation into cancer and nutrition. Int. J. Cancer 2012, 131, E963–E973. [Google Scholar] [CrossRef] [PubMed]
- Kaluza, J.; Håkansson, N.; Brzozowska, A.; Wolk, A. Diet quality and mortality: A population-based prospective study of men. Eur. J. Clin. Nutr. 2009, 63, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Kant, A.K.; Schatzkin, A.; Ziegler, R.G. Dietary diversity and subsequent cause-specific mortality in the NHANES I epidemiologic follow-up study. J. Am. Coll. Nutr. 1995, 14, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Kant, A.K.; Schatzkin, A.; Graubard, B.I.; Schairer, C. A prospective study of diet quality and mortality in women. JAMA 2000, 283, 2109–2115. [Google Scholar] [CrossRef] [PubMed]
- Kant, A.K.; Leitzmann, M.F.; Park, Y.; Hollenbeck, A.; Schatzkin, A. Patterns of recommended dietary behaviors predict subsequent risk of mortality in a large cohort of men and women in the United States. J. Nutr. 2009, 139, 1374–1380. [Google Scholar] [CrossRef] [PubMed]
- Kappeler, R.; Eichholzer, M.; Rohrmann, S. Meat consumption and diet quality and mortality in NHANES III. Eur. J. Clin. Nutr. 2013, 67, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Kenfield, S.A.; Dupre, N.; Richman, E.L.; Stampfer, M.J.; Chan, J.M.; Giovannucci, E.L. Mediterranean diet and prostate cancer risk and mortality in the health professionals follow-up study. Eur. Urol. 2014, 65, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Knoops, K.T.B.; de Groot, L.C.P.G.M.; Kromhout, D.; Perrin, A.; Moreiras-Varela, O.; Menotti, A.; van Staveren, W.A. Mediterranean Diet, Lifestyle Factors, and 10-Year Mortality in Elderly European Men and Women. J. Am. Med. Assoc. 2004, 292, 6. [Google Scholar] [CrossRef] [PubMed]
- Kyro, C.; Skeie, G.; Loft, S.; Overvad, K.; Christensen, J.; Tjonneland, A.; Olsen, A. Adherence to a healthy Nordic food index is associated with a lower incidence of colorectal cancer in women: The Diet, Cancer and Health cohort study. Br. J. Nutr. 2013, 109, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Lagiou, P.; Trichopoulos, D.; Sandin, S.; Lagiou, A.; Mucci, L.; Wolk, A.; Weiderpass, E.; Adami, H.O. Mediterranean dietary pattern and mortality among young women: A cohort study in Sweden. Br. J. Nutr. 2006, 96, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Lagiou, P.; Sandin, S.; Weiderpass, E.; Lagiou, A.; Mucci, L.; Trichopoulos, D.; Adami, H.O. Low carbohydrate-high protein diet and mortality in a cohort of Swedish women. J. Intern. Med. 2007, 261, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Huang, Y.C.; Su, H.H.; Lee, M.Z.; Wahlqvist, M.L. A simple food quality index predicts mortality in Elderly Taiwanese. J. Nutr. Health Aging 2011, 15, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Park, Y.; Wu, J.W.; Ren, J.; Goldstein, A.M.; Taylor, P.R.; Hollenbeck, A.R.; Freedman, N.D.; Abnet, C.C. Index-based Dietary Patterns and Risk of Esophageal and Gastric Cancer in a Large Cohort Study. Clin. Gastrol. Hepat. 2013, 11, 1130–1136. [Google Scholar] [CrossRef] [PubMed]
- Li, W.Q.; Park, Y.; McGlynn, K.A.; Hollenbeck, A.R.; Taylor, P.R.; Goldstein, A.M.; Freedman, N.D. Index-based dietary patterns and risk of incident hepatocellular carcinoma and mortality from chronic liver disease in a prospective study. Hepatology 2014, 60, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Mai, V.; Kant, A.K.; Flood, A.; Lacey, J.V., Jr.; Schairer, C.; Schatzkin, A. Diet quality and subsequent cancer incidence and mortality in a prospective cohort of women. Int. J. Epidemiol. 2005, 34, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Gonzalez, M.A.; Guillen-Grima, F.; de Irala, J.; Ruiz-Canela, M.; Bes-Rastrollo, M.; Beunza, J.J.; Lopez del Burgo, C.; Toledo, E.; Carlos, S.; Sanchez-Villegas, A. The Mediterranean diet is associated with a reduction in premature mortality among middle-aged adults. J. Nutr. 2012, 142, 1672–1678. [Google Scholar] [CrossRef] [PubMed]
- McCullough, M.L.; Feskanich, D.; Rimm, E.B.; Giovannucci, E.L.; Ascherio, A.; Variyam, J.N.; Spiegelman, D.; Stampfer, M.J.; Willett, W.C. Adherence to the Dietary Guidelines for Americans and risk of major chronic disease in men. Am. J. Clin. Nutr. 2000, 72, 1223–1231. [Google Scholar] [PubMed]
- McCullough, M.L.; Feskanich, D.; Stampfer, M.J.; Rosner, B.A.; Hu, F.B.; Hunter, D.J.; Variyamm, J.N.; Colditz, G.A.; Willett, W.C. Adherence to the Dietary Guidelines for Americans and risk of major chronic disease in women. Am. J. Clin. Nutr. 2000, 72, 1214–1222. [Google Scholar] [PubMed]
- McCullough, M.L.; Feskanich, D.; Stampfer, M.J.; Giovannucci, E.L.; Rimm, E.B.; Hu, F.B.; Spiegelman, D.; Hunter, D.J.; Colditz, G.A.; Willett, W.C. Diet quality and major chronic disease risk in men and women: Moving toward improved dietary guidance. Am. J. Clin. Nutr. 2002, 76, 1261–1271. [Google Scholar] [PubMed]
- Michels, K.; Wolk, A. A prospective study of variety of healthy foods and mortality in women. Int. J. Epidemiol. 2002, 31, 7. [Google Scholar] [CrossRef]
- Miller, P.E.; Cross, A.J.; Subar, A.F.; Krebs-Smith, S.M.; Park, Y.; Powell-Wiley, T.; Hollenbeck, A.; Reedy, J. Comparison of 4 established DASH diet indexes: Examining associations of index scores and colorectal cancer. Am. J. Clin. Nutr. 2013, 98, 794–803. [Google Scholar] [CrossRef] [PubMed]
- Mitrou, P.N.; Kipnis, V.; Thiébaut, A.C.M.; Reedy, J.; Subar, A.F.; Wirfält, E.; Flood, A.; Mouw, T.; Hollenbeck, A.R.; Leitzmann, M.F.; et al. Mediterranean dietary pattern and prediction of all-cause mortality in a US population: Results from the NIH-AARP diet and health study. Arch. Int. Med. 2007, 167, 2461–2468. [Google Scholar] [CrossRef] [PubMed]
- Mursu, J.; Steffen, L.M.; Meyer, K.A.; Duprez, D.; Jacobs, D.R., Jr. Diet quality indexes and mortality in postmenopausal women: The Iowa Women’s Health Study. Am. J. Clin. Nutr. 2013, 98, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Ueshima, H.; Okamura, T.; Kadowaki, T.; Hayakawa, T.; Kita, Y.; Abbott, R.D.; Okayama, A. A Japanese diet and 19-year mortality: National integrated project for prospective observation of non-communicable diseases and its trends in the aged, 1980. Br. J. Nutr. 2009, 101, 1696–1705. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, L.M.; Winkvist, A.; Brustad, M.; Jansson, J.-H.; Johansson, I.; Lenner, P.; Lindahl, B.; van Guelpen, B. A traditional Sami diet score as a determinant of mortality in a general northern Swedish population. Int. J. Circumpolar Health 2012, 71, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, L.M.; Winkvist, A.; Eliasson, M.; Jansson, J.H.; Hallmans, G.; Johansson, I.; Lindahl, B.; Lenner, P.; van Guelpen, B. Low-carbohydrate, high-protein score and mortality in a northern Swedish population-based cohort. Eur. J. Clin. Nutr. 2012, 66, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, L.M.; Winkvist, A.; Johansson, I.; Lindahl, B.; Hallmans, G.; Lenner, P.; van Guelpen, B. Low-carbohydrate, high-protein diet score and risk of incident cancer; A prospective cohort study. Nutr. J. 2013, 12, 58. [Google Scholar] [CrossRef] [PubMed]
- Reedy, J.; Krebs-Smith, S.M.; Miller, P.E.; Liese, A.D.; Kahle, L.L.; Park, Y.; Subar, A.F. Higher diet quality is associated with decreased risk of all-cause, cardiovascular disease, and cancer mortality among older adults. J. Nutr. 2014, 144, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Seymour, J.D.; Calle, E.E.; Flagg, E.W.; Coates, R.J.; Ford, E.S.; Thun, M.J. Diet Quality Index as a predictor of short-term mortality in the American Cancer Society Cancer Prevention Study II Nutrition Cohort. Am. J. Epidemiol. 2003, 157, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Tognon, G.; Nilsson, L.M.; Lissner, L.; Johansson, I.; Hallmans, G.; Lindahl, B.; Winkvist, A. The mediterranean diet score and mortality are inversely associated in adults living in the subarctic region. J. Nutr. 2012, 142, 1547–1553. [Google Scholar] [CrossRef] [PubMed]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean diet and survival in a Greek population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef] [PubMed]
- Trichopoulou, A.; Bamia, C.; Lagiou, P.; Trichopoulos, D. Conformity to traditional Mediterranean diet and breast cancer risk in the Greek EPIC (European Prospective Investigation into Cancer and Nutrition) cohort. Am. J. Clin. Nutr. 2010, 92, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Van Dam, R.M.; Li, T.; Spiegelman, D.; Franco, O.H.; Hu, F.B. Combined impact of lifestyle factors on mortality: Prospective cohort study in US women. BMJ 2008, 337, 742–745. [Google Scholar]
- Vormund, K.; Braun, J.; Rohrmann, S.; Bopp, M.; Ballmer, P.; Faeh, D. Mediterranean diet and mortality in Switzerland: An alpine paradox? Eur. J. Nutr. 2014, 54, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Von Ruesten, A.; Illner, A.K.; Buijsse, B.; Heidemann, C.; Boeing, H. Adherence to recommendations of the German food pyramid and risk of chronic diseases: Results from the EPIC-Potsdam study. Eur. J. Clin. Nutr. 2010, 64, 1251–1259. [Google Scholar] [CrossRef] [PubMed]
- Zarrin, R.; Ibiebele, T.I.; Marks, G.C. Development and validity assessment of a diet quality index for australians. Asia Pac. J. Clin. Nutr. 2013, 22, 177–187. [Google Scholar] [PubMed]
- Trichopoulou, A.; Kouris-Blazos, A.; Wahlqvist, M.L.; Gnardellis, C.; Lagiou, P.; Polychronopoulos, E.; Vassilakou, T.; Lipworth, L. Trichopoulos, D. Diet and overall survival in elderly people. BMJ 1995, 311, 1457–1460. [Google Scholar] [CrossRef] [PubMed]
- McCullough, M.L.; Willett, W.C. Evaluating adherence to recommended diets in adults: The Alternate Healthy Eating Index. Public Health Nutr. 2006, 9. [Google Scholar] [CrossRef]
- Ferlay, J.; Soerjomataram, I.; Ervik, M.; Dikshit, R.; Eser, S.; Mathers, C. Cancer Incidence and Mortality Worldwide World Cancer Research Fund International, 2014. Available online: Http://www.wcrf.org/int/cancer-facts-figures/data-cancer-frequency-country (accessed on 7 June 2016).
- Imamura, F.; Micha, R.; Khatibzadeh, S.; Fahimi, S.; Shi, P.; Powles, J.; Mozaffarian, D. Dietary quality among men and women in 187 countries in 1990 and 2010: A systematic assessment. Lancet Glob. Health 2015, 3, e132–e142. [Google Scholar] [CrossRef]
- National Cancer Institute. Cancer Statistics; National Cancer Institute: Bethesda, MD, USA, 2016. [Google Scholar]
- Chlebowski, R.; Blackburn, G.; Elashoff, R.; Thomson, C.; Goodman, M.; Shapiro, A.; Giuliano, A.; Karanja, N.; Hoy, M.; Nixon, D. (Eds.) Dietary fat reduction in postmenopausal women with primary breast cancer: Phase III Women’s Intervention Nutrition Study (WINS). J. Clin. Oncol. 2005, 23, 10.
- Carvalho, K.M.B.D.; Dutra, E.S.; Pizato, N.; Gruezo, N.D.; Ito, M.K. Diet quality assessment indexes. Rev. Nutr. 2014, 27, 605–617. [Google Scholar] [CrossRef]
- Boyd, N.; Stone, J.; Vogt, K.; Connelly, B.; Martin, L.; Minkin, S. Dietary fat and breast cancer risk revisited: A meta-analysis of the published literature. Br. J. Cancer 2003, 89, 1672–1685. [Google Scholar] [CrossRef] [PubMed]
- Levine Morgan, E.; Suarez Jorge, A.; Brandhorst, S.; Balasubramanian, P.; Cheng, C.-W.; Madia, F.; Fontana, L.; Mirisola Mario, G.; Guevara-Aguirre, J.; Wan, J.; et al. Low Protein Intake Is Associated with a Major Reduction in IGF-1, Cancer, and Overall Mortality in the 65 and Younger but Not Older Population. Cell Metab. 2014, 19, 407–417. [Google Scholar] [CrossRef] [PubMed]

| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Studies reporting on a predefined diet quality score and cancer risk 1 | Studies using factor analysis or clustering methods for dietary assessment |
| Studies reporting on a predefined diet quality score and cancer mortality 1 | Melanoma |
| Studies conducted in high income countries 2 Studies published in the English language | Studies published in a language other than English |
| Adult populations | Studies in animals |
| Prevalence or cross-sectional study design & cohort studies | Health conditions that might influence diet quality (e.g., diabetes) |
| Title | Study | Location | Cohort | Diet Quality Score |
|---|---|---|---|---|
| 1 | Akbaraly TN, et al. (2011) [28] | UK | Whitehall II cohort | AHEI |
| 2 | Arem H., et al., (2013) [29] | USA | NIH-AARP study | HEI-2005 |
| 3 | Ax E., et al., (2013) [30] | Sweden | ULSAM | MMDS; LCHP Score |
| 4 | Bamia C, et al., (2013) [10] | Northern and Southern Europe | EPIC | MMDS; CSMMDS |
| 5 | Berentzen NE., et al., (2013) [31] | Netherlands | EPIC-NL | HDI |
| 6 | Benetou V., et al., (2008) [32] | Greece | EPIC | MMDS |
| 7 | Bosire C., et al., (2013) [18] | USA | NIH-AARP | HEI-2005; aMED; AHEI-2010 |
| 8 | Buchner FL, et al., (2011) [33] | Northern and Southern Europe | EPIC | DDS |
| 9 | Buchner FL., et al., (2010) [34] | Northern and Southern Europe | EPIC | DDS |
| 10 | Buckland G., et al., (2010) [35] | Northern and Southern Europe | EPIC | rMED |
| 11 | Buckland G., et al., (2011) [36] | Spain | EPIC—Spain | rMED |
| 12 | Buckland G., et al., (2013) [17] | Northern and Southern Europe | EPIC | arMED |
| 13 | Buckland G., et al., (2014) [37] | Northern and Southern Europe | EPIC | rMED |
| 14 | Cade JE, et al., (2011) [38] | UK | UKWCS | MMDS; HDI |
| 15 | Chiuve SE, et al., (2012) [39] | USA | NHS HPFS | HEI; AHEI-2010 |
| 16 | Couto E., et al., (2011) [40] | Northern and Southern Europe | EPIC | MMDS |
| 17 | Couto E., et al., (2013) [41] | Sweden | Swedish WLH cohort | MMDS |
| 18 | Cuenca-Garcia M., et al., (2014) [42] | USA | ACLS | Ideal Diet Index (IDI); MMDS; DQI |
| 19 | Drake I., et al., (2012) [43] | Sweden | Malmö Diet and Cancer cohort | DQI-SNR |
| 20 | Fitzgerald AL, et al., (2002) [44] | Canada | Nova Scotia Nutrition Survey | Diet quality score based on the Nova Scotia DRIs |
| 21 | Fung TT, et al., (2006) [45] | USA | NHS | HEI-f; AHEI; DQIR; RFS; aMED |
| 22 | Fung TT., et al., (2010) [11] | USA | NHS HPFS | aMED; DASH score |
| 23 | Fung TT., et al., (2010) [46] | USA | NHS HPFS | LCHP |
| 24 | Fung TT., et al., (2011) [12] | USA | NHS | LCHP score; DASH score |
| 25 | Huijbregts P., et al., (1997) [47] | Northern and Southern Europe | Seven Countries Study | Healthy diet indicator (HDI) |
| 26 | Jarvandi S., et al., (2013) [48] | USA | NIH-AARP Diet and Health Study | HEI-2005 |
| 27 | Jeurnink SM., et al., (2012) [49] | Northern and Southern Europe | EPIC | DDS |
| 28 | Kaluza, J., et al., (2009) [50] | Sweden | Cohort of Swedish Men | RFS; Non-RFS |
| 29 | Kant AK., et al., (1995) [51] | USA | NHANES Epidemiologic Follow-Up Study | DDS |
| 30 | Kant AK., et al., (2000) [52] | USA | BCDDP | RFS |
| 31 | Kant AK, et al., (2009) [53] | USA | NIH-American Association of Retired Persons cohort | Dietary behaviour score (DBS) |
| 32 | Kappeler R., et al., (2013) [54] | USA | NHANES III | HEI |
| 33 | Kenfield SA., et al., (2014) [55] | USA | HPFS | MMDS; aMED |
| 34 | Knoops KTB., et al., (2004) [56] | Northern and Southern Europe | HALE (European cohort) Survey in Europe on Nutrition and the Elderly: a concerned Action (SENECA) Finland, Italy the Netherlands elderly (FINE) study | MMDS |
| 35 | Kyro C., et al., (2013) [57] | Denmark | Diet, Cancer and Health cohort | Nordic food index |
| 36 | Lagiou P., et al., (2006) [58] | Sweden | Scandinavian Women’s Lifestyle and Health Cohort | MMDS |
| 37 | Lagiou P., et al., (2007) [59] | Sweden | Scandinavian Women’s Lifestyle and Health Cohort | LCHP |
| 38 | Lee M, et al., (2011) [60] | Taiwan | The Elderly Nutrition and Health Survey | ODI-R; DDS |
| 39 | Li W., et al., (2013) [61] | USA | NIH-AARP Diet and Health Study | HEI-2005; aMED |
| 40 | Li W., et al., (2014a) [62] | USA | NIH-AARP Diet and Health Study | HEI-2010; aMED |
| 41 | Li W., et al., (2014b) [20] | USA | NIH-AARP Diet and Health Study | HEI-2005; aMED |
| 42 | Mai V., et al., (2005) [63] | USA | BCDDP | RFS |
| 43 | Martinez-Gonzalez MA., (2012) [64] | Spain | Seguimiento Universidad de Navarra (SUN) Project | MMDS |
| 44 | McCullough ML., et al., (2000a) [65] | USA | HPFS | HEI-f |
| 45 | McCullough ML., et al., (2000b) [66] | USA | NHS | HEI-f |
| 46 | McCullough ML., et al., (2002) [67] | USA | NHS HPFS | AHEI; RFS |
| 47 | Michels KB., & Wolk A. (2002) [68] | Sweden | Mammography Screening Cohort | RFS; Non-RFS |
| 48 | Miller PE., et al., (2013) [69] | USA | NIH-AARP | DASH |
| 49 | Mitrou PN, et al., (2007) [70] | USA | NIH-AARP | tMED; aMED |
| 50 | Mursu J., et al., (2013) [71] | USA | Iowa Women’s Health Study | AHEI-2010 |
| 51 | Nakamura Y., et al., (2009) [72] | Japan | National Integrated Project for Prospective Observation of Non-Communicable Diseases and its Trends in the Aged | Reduced-salt Japanese diet score |
| 52 | Nilsson LM., et al., (2012a) [73] | Sweden | VIP | Traditional Sami Diet Score |
| 53 | Nilsson LM., et al., (2012b) [74] | Sweden | VIP | LCHP score |
| 54 | Nilsson LM., et al., (2013) [75] | Sweden | VIP | LCHP score |
| 55 | Reedy J., et al., (2008) [13] | USA | NIH-AARP | HEI-2005; AHEI; MMDS; RFS |
| 56 | Reedy J., et al., (2013) [76] | USA | NIH-AARP | HEI-2010; AHEI-2010; aMED; DASH score |
| 57 | Seymour JD., et al., (2003) [77] | USA | American Cancer Society Cancer Prevention Study II Nutrition Cohort | DQI |
| 58 | Tognon G., et al., (2012) [78] | Sweden | VIP | mMDS |
| 59 | Trichopoulou A., et al., (2003) [79] | Greece | EPIC | MMDS |
| 60 | Trichopoulou A., et al., (2010) [80] | Greece | EPIC | MMDS |
| 61 | van Dam RM., et al., (2008) [81] | USA | NHS | AHEI |
| 62 | Vormund K., et al., (2014) [82] | Switzerland | Longitudinal cohort | MDS |
| 63 | Von Rueston A., et al., (2010) [83] | Germany | EPIC-Potsdam | GFPI |
| 64 | Zarrin R., et al., (2013) [84] | Australia | Nambour Skin Cancer study | Aussie-DQI |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Potter, J.; Brown, L.; Williams, R.L.; Byles, J.; Collins, C.E. Diet Quality and Cancer Outcomes in Adults: A Systematic Review of Epidemiological Studies. Int. J. Mol. Sci. 2016, 17, 1052. https://doi.org/10.3390/ijms17071052
Potter J, Brown L, Williams RL, Byles J, Collins CE. Diet Quality and Cancer Outcomes in Adults: A Systematic Review of Epidemiological Studies. International Journal of Molecular Sciences. 2016; 17(7):1052. https://doi.org/10.3390/ijms17071052
Chicago/Turabian StylePotter, Jennifer, Leanne Brown, Rebecca L. Williams, Julie Byles, and Clare E. Collins. 2016. "Diet Quality and Cancer Outcomes in Adults: A Systematic Review of Epidemiological Studies" International Journal of Molecular Sciences 17, no. 7: 1052. https://doi.org/10.3390/ijms17071052







