Role of Aquaporins in a Composite Model of Water Transport in the Leaf
Abstract
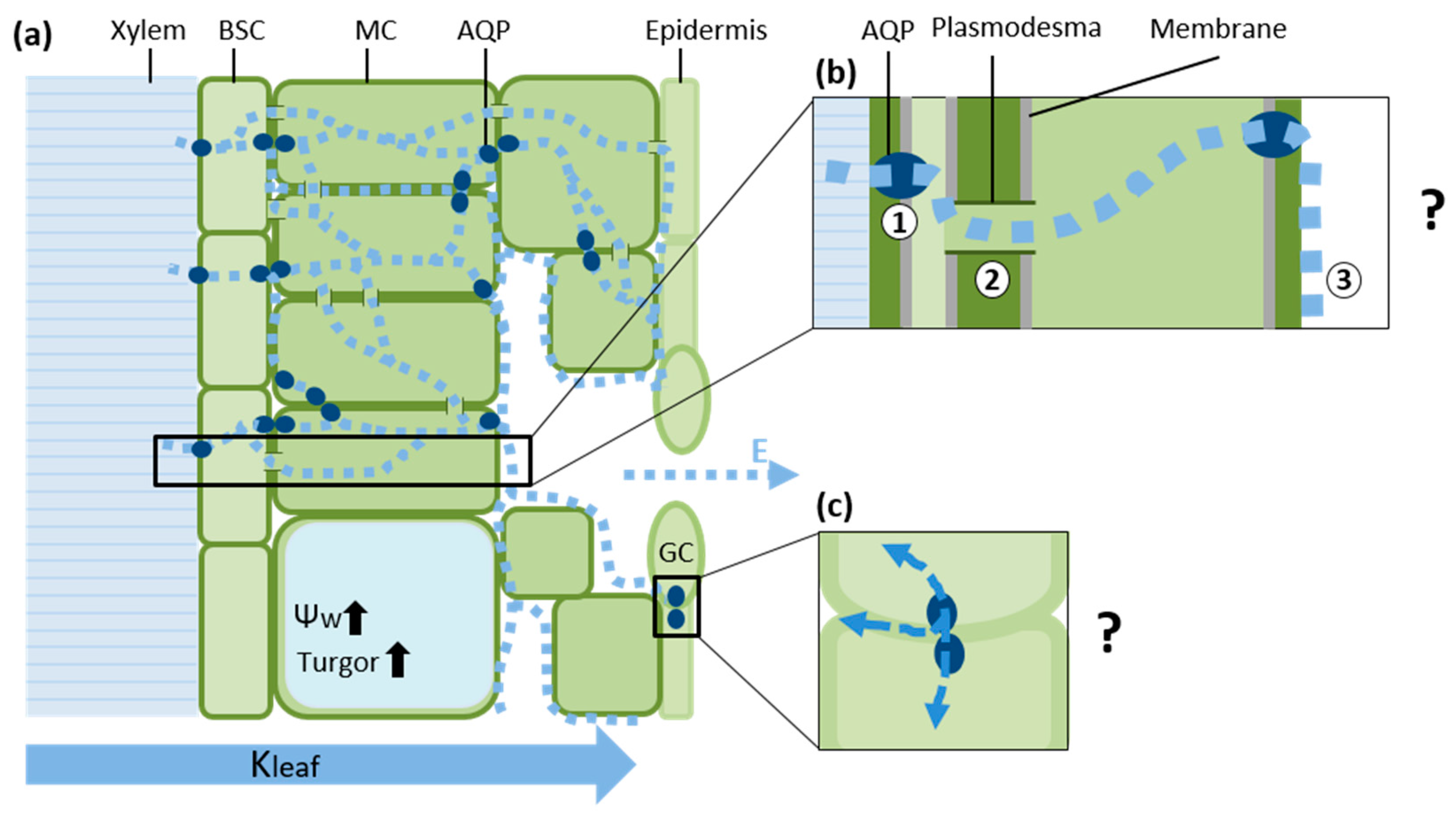
:1. A Composite Model of Water Transport in the Leaf
2. Hydraulic Regulation of the Xylem and Leaf Veins
3. The Leaf Vascular Bundle Sheath Cells (BSC) as a Selective Barrier
- No symplastic continuum appears to exist between the bundle sheath and the phloem and xylem [49].
- Hydraulic pressure builds up in the xylem, usually at night, and is released only from the hydathodes (as guttation drops) and not through the vascular tissue (i.e., the air spaces within the mesophyll are flooded) [50].
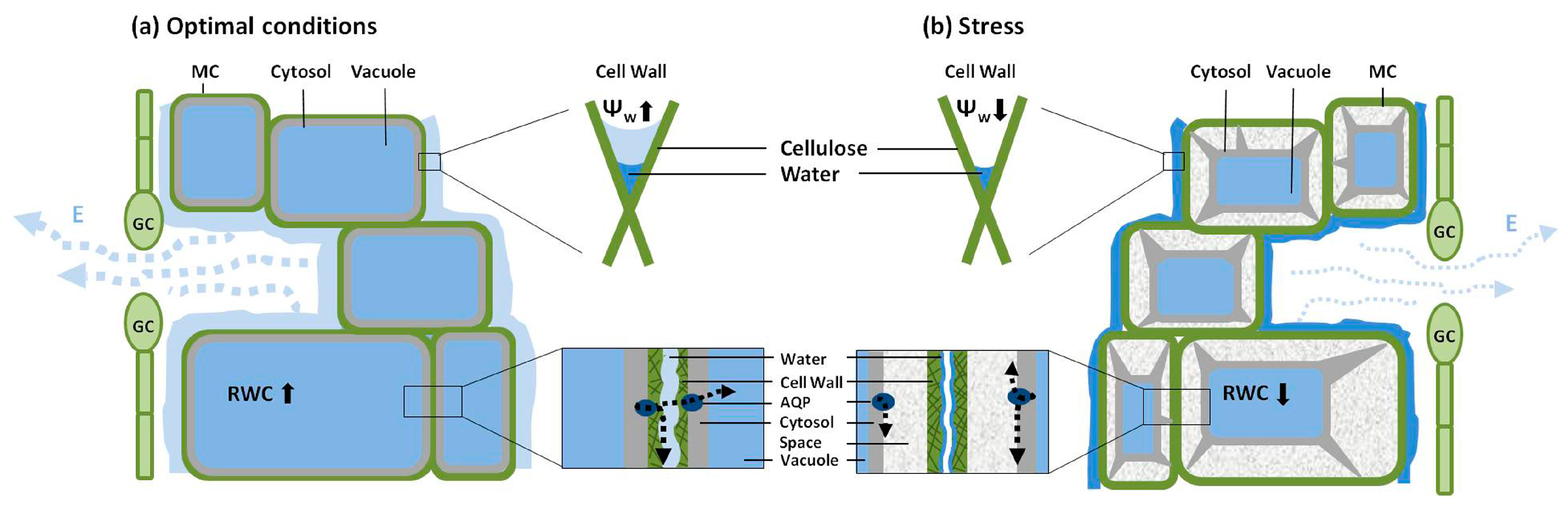
4. Hydraulic Properties of Mesophyll Cells
5. Permeability of Guard Cells to Water and Regulation of Stomatal Aperture
6. Possible Water-Related, Post-Translational Regulation of AQPs
7. Conclusions
Supplementary Materials
Acknowledgments
Conflicts of Interest
References
- Rensing, S.A.; Lang, D.; Zimmer, A.D.; Terry, A.; Salamov, A.; Shapiro, H.; Nishiyama, T.; Perroud, P.F.; Lindquist, E.A.; Kamisugi, Y.; et al. The physcomitrella genome reveals evolutionary insights into the conquest of land by plants. Science 2008, 319, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Brodribb, T.J.; Feild, T.S.; Jordan, G.J. Leaf maximum photosynthetic rate and venation are linked by hydraulics. Plant Physiol. 2007, 144, 1890–1898. [Google Scholar] [CrossRef] [PubMed]
- Brodribb, T.J.; Feild, T.S.; Sack, L. Viewing leaf structure and evolution from a hydraulic perspective. Funct. Plant Biol. 2010, 37, 488–498. [Google Scholar] [CrossRef]
- Brodribb, T.J.; McAdam, S.A.M. Passive origins of stomatal control in vascular plants. Science 2011, 331, 582–585. [Google Scholar] [CrossRef] [PubMed]
- Sack, L.; Holbrook, N.M. Leaf hydraulics. Annu. Rev. Plant Biol. 2006, 57, 361–381. [Google Scholar] [CrossRef] [PubMed]
- Boyce, C.K.; Brodribb, T.J.; Feild, T.S.; Zwieniecki, M.A. Angiosperm leaf vein evolution was physiologically and environmentally transformative. Proc. Biol. Sci. 2009, 276, 1771–1776. [Google Scholar] [CrossRef] [PubMed]
- Assouline, S.; Or, D. Plant water use efficiency over geological time—Evolution of leaf stomata configurations affecting plant gas exchange. PLoS ONE 2013, 8, e67757. [Google Scholar] [CrossRef] [PubMed]
- Nardini, A.; Salleo, S. Limitation of stomatal conductance by hydraulic traits: Sensing or preventing xylem cavitation? Trees 2000, 15, 14–24. [Google Scholar] [CrossRef]
- Sack, L.; Tyree, M.T.; Holbrook, N.M. Leaf hydraulic architecture correlates with regeneration irradiance in tropical rainforest trees. New Phytol. 2005, 167, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Richards, R.A. Selectable traits to increase crop photosynthesis and yield of grain crops. J. Exp. Bot. 2000, 51, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Cochard, H.; Venisse, J.-S.; Barigah, T.S.; Brunel, N.; Herbette, S.; Guilliot, A.; Tyree, M.T.; Sakr, S. Putative role of aquaporins in variable hydraulic conductance of leaves in response to light. Plant Physiol. 2007, 143, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Tyree, M.T.; Nardini, A.; Salleo, S.; Sack, L.; El Omari, B. The dependence of leaf hydraulic conductance on irradiance during HPFM measurements: Any role for stomatal response? J. Exp. Bot. 2005, 56, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Ben Baaziz, K.; Lopez, D.; Rabot, A.; Combes, D.; Gousset, A.; Bouzid, S.; Cochard, H.; Sakr, S.; Venisse, J.S. Light-mediated Kleaf induction and contribution of both the PIP1s and PIP2s aquaporins in five tree species: Walnut (Juglans regia) case study. Tree Physiol. 2012, 32, 423–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prado, K.; Maurel, C. Regulation of leaf hydraulics: From molecular to whole plant levels. Front. Plant Sci. 2013, 4, 255. [Google Scholar] [CrossRef] [PubMed]
- Prado, K.; Boursiac, Y.; Tournaire-Roux, C.; Monneuse, J.-M.; Postaire, O.; Da Ines, O.; Schäffner, A.R.; Hem, S.; Santoni, V.; Maurel, C. Regulation of Arabidopsis leaf hydraulics involves light-dependent phosphorylation of aquaporins in veins. Plant Cell 2013, 25, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
- Martre, P.; Morillon, R.; Barrieu, F.; North, G.B.; Nobel, P.S.; Chrispeels, M.J. Plasma membrane aquaporins play a significant role during recovery from water deficit. Plant Physiol. 2002, 130, 2101–2110. [Google Scholar] [CrossRef] [PubMed]
- Scoffoni, C.; Rawls, M.; McKown, A.; Cochard, H.; Sack, L. Decline of leaf hydraulic conductance with dehydration: Relationship to leaf size and venation architecture. Plant Physiol. 2011, 156, 832–843. [Google Scholar] [CrossRef] [PubMed]
- Shatil-Cohen, A.; Attia, Z.; Moshelion, M. Bundle-sheath cell regulation of xylem-mesophyll water transport via aquaporins under drought stress: A target of xylem-borne ABA? Plant J. 2011, 67, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Tyree, M.T.; Service, U.F.; Forestry, A.; Box, P.O.; Burlington, S. The cohesion—Tension theory of sap ascent: Current controversies. J. Exp. Bot. 1997, 48, 1753–1765. [Google Scholar] [CrossRef]
- Steudle, E. Water uptake by roots: Effects of water deficit. J. Exp. Bot. 2000, 51, 1531–1542. [Google Scholar] [CrossRef] [PubMed]
- Buckley, T.N. The control of stomata by water balance. New Phytol. 2005, 168, 275–292. [Google Scholar] [CrossRef] [PubMed]
- Buckley, T.N.; Mott, K.A.; Farquhar, G.D. A hydromechanical and biochemical model of stomatal conductance. Plant Cell Environ. 2003, 26, 1767–1785. [Google Scholar] [CrossRef]
- Buckley, T.N. The contributions of apoplastic, symplastic and gas phase pathways for water transport outside the bundle sheath in leaves. Plant Cell Environ. 2014, 38, 7–22. [Google Scholar] [CrossRef] [PubMed]
- Attia, Z.; Domec, J.C.; Oren, R.; Way, D.A.; Moshelion, M. Growth and physiological responses of isohydric and anisohydric poplars to drought. J. Exp. Bot. 2015, 66, 4373–4381. [Google Scholar] [CrossRef] [PubMed]
- Tardieu, F.; Simonneau, T.; Parent, B. Modelling the coordination of the controls of stomatal aperture, transpiration, leaf growth, and abscisic acid: Update and extension of the Tardieu–Davies model. J. Exp. Bot. 2015, 66. [Google Scholar] [CrossRef] [PubMed]
- Tardieu, F.; Simonneau, T. Variability among species of stomatal control under fluctuating soil water status and evaporative demand: Modelling isohydric and anisohydric behaviours. J. Exp. Bot. 1998, 49, 419–432. [Google Scholar] [CrossRef]
- Brodribb, T.J.; Holbrook, N.M. Diurnal depression of leaf hydraulic conductance in a tropical tree species. Plant Cell Environ. 2004, 27, 820–827. [Google Scholar] [CrossRef]
- Perez-Martin, A.; Michelazzo, C.; Torres-Ruiz, J.M.; Flexas, J.; Fernandez, J.E.; Sebastiani, L.; Diaz-Espejo, A. Regulation of photosynthesis and stomatal and mesophyll conductance under water stress and recovery in olive trees: Correlation with gene expression of carbonic anhydrase and aquaporins. J. Exp. Bot. 2014, 65, 3143–3156. [Google Scholar] [CrossRef] [PubMed]
- Sade, N.; Moshelion, M. Plant aquaporins and abiotic stress. In Plant Aquaporins: From Transport to Signalling; Chaumont, F., Tyerman, S., Eds.; Spinger-Verlag: Berlin-Heidelberg, Germany, 2016. [Google Scholar]
- Tyerman, S.D.; Niemietz, C.M.; Bramley, H. Plant aquaporins: Multifunctional water and solute channels. Plant Cell Environ. 2002, 25, 173–194. [Google Scholar] [CrossRef] [PubMed]
- Maurel, C.; Verdoucq, L.; Luu, D.-T.; Santoni, V. Plant aquaporins: Membrane channels with multiple integrated functions. Annu. Rev. Plant Biol. 2008, 59, 595–624. [Google Scholar] [CrossRef] [PubMed]
- Kaldenhoff, R.; Ribas-Carbo, M.; Sans, J.F.; Lovisolo, C.; Heckwolf, M.; Uehlein, N. Aquaporins and plant water balance. Plant Cell Environ. 2008, 31, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Heinen, R.B.; Ye, Q.; Chaumont, F. Role of aquaporins in leaf physiology. J. Exp. Bot. 2009, 60, 2971–2985. [Google Scholar] [CrossRef] [PubMed]
- Steudle, E.; Peterson, C.A. How does water get through roots? J. Exp. Bot. 1998, 49, 775–788. [Google Scholar] [CrossRef]
- Roth-Nebelsick, A. Evolution and function of leaf venation architecture: A review. Ann. Bot. 2001, 87, 553–566. [Google Scholar] [CrossRef]
- Sack, L.; Dietrich, E.M.; Streeter, C.M.; Sánchez-Gómez, D.; Holbrook, N.M. Leaf palmate venation and vascular redundancy confer tolerance of hydraulic disruption. Proc. Natl. Acad. Sci. USA 2008, 105, 1567–1572. [Google Scholar] [CrossRef] [PubMed]
- Nardini, A.; Salleo, S.; Andri, S. Circadian regulation of leaf hydraulic conductance in sunflower (Helianthus annuus L. cv Margot). Plant Cell Environ. 2005, 28, 750–759. [Google Scholar] [CrossRef]
- Lovisolo, C.; Schubert, A. Effects of water stress on vessel size and xylem hydraulic conductivity in Vitis vinifera L. J. Exp. Bot. 1998, 49, 693–700. [Google Scholar]
- Corcuera, L.; Camarero, J.J.; Gil-Pelegrín, E. Effects of a severe drought on Quercus ilex radial growth and xylem anatomy. Trees Struct. Funct. 2004, 18, 83–92. [Google Scholar]
- Plavcová, L.; Hacke, U.G. Phenotypic and developmental plasticity of xylem in hybrid poplar saplings subjected to experimental drought, nitrogen fertilization, and shading. J. Exp. Bot. 2012, 63, 6481–6491. [Google Scholar] [CrossRef] [PubMed]
- Schultz, H.R.; Matthews, M.A. Xylem development and hydraulic conductance in sun and shade shoots of grapevine (Vitis vinifera L.): Evidence that low light uncouples water transport capacity from leaf area. Planta 1993, 190, 393–406. [Google Scholar] [CrossRef]
- Nardini, A.; Gortan, E.; Salleo, S. Hydraulic efficiency of the leaf venation system in sun- and shade-adapted species. Funct. Plant Biol. 2005, 32, 953–961. [Google Scholar] [CrossRef]
- Atkinson, C.; Taylor, J.M. Effects of elevated CO2 on stem growth, vessel area and hydraulic conductivity of oak and cherry seedlings. New Phytol. 1996, 133, 617–626. [Google Scholar] [CrossRef]
- Zwieniecki, M.A.; Melcher, P.J.; Michele Holbrook, N.M. Hydrogel control of xylem hydraulic resistance in plants. Science 2001, 291, 1059–1062. [Google Scholar] [CrossRef] [PubMed]
- Nardini, A.; Salleo, S.; Jansen, S. More than just a vulnerable pipeline: Xylem physiology in the light of ion-mediated regulation of plant water transport. J. Exp. Bot. 2011, 62, 4701–4718. [Google Scholar] [CrossRef] [PubMed]
- Esau, K. Plant Anatomy, 1st ed.; John Wiley & Sons: Hoboken, NJ, USA; New York, NY, USA, 1953; pp. 429–432. [Google Scholar]
- Aloni, R.; Schwalm, K.; Langhans, M.; Ullrich, C.I. Gradual shifts in sites of free-auxin production during leaf-primordium development and their role in vascular differentiation and leaf morphogenesis in Arabidopsis. Planta 2003, 216, 841–853. [Google Scholar] [PubMed]
- Leegood, R.C. Roles of the bundle sheath cells in leaves of C3 plants. J. Exp. Bot. 2008, 59, 1663–1673. [Google Scholar] [CrossRef] [PubMed]
- Ache, P.; Bauer, H.; Kollist, H.; Al-Rasheid, K.A.S.; Lautner, S.; Hartung, W.; Hedrich, R. Stomatal action directly feeds back on leaf turgor: New insights into the regulation of the plant water status from non-invasive pressure probe measurements. Plant J. 2010, 62, 1072–1082. [Google Scholar] [CrossRef] [PubMed]
- Shatil-cohen, A.; Moshelion, M. The bundle sheath role as xylem-mesophyll barrier. Plant Signal. Behav. 2012, 7, 1088–1091. [Google Scholar] [CrossRef] [PubMed]
- Nardini, A.; Raimondo, F.; Lo Gullo, M.A.; Salleo, S. Leafminers help us understand leaf hydraulic design. Plant Cell Environ. 2010, 33, 1091–1100. [Google Scholar] [PubMed]
- Lersten, N.R.; Curtis, J.D. Anatomy and distribiution of foliar idioblasts in Scrophularia and Verbascum. Am. J. Bot. 1997, 84, 1638–1645. [Google Scholar] [CrossRef] [PubMed]
- Pilot, G.; Stransky, H.; Bushey, D. Overexpression of GLUTAMINE DUMPER1 leads to hypersecretion of glutamine from hydathodes of Arabidopsis leaves. Plant Cell 2004, 16, 1827–1840. [Google Scholar] [CrossRef] [PubMed]
- Shapira, O.; Khadka, S.; Israeli, Y.; Shani, U.; Schwartz, A. Functional anatomy controls ion distribution in banana leaves: Significance of Na+ seclusion at the leaf margins. Plant Cell Environ. 2009, 32, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Galvez-Valdivieso, G.; Fryer, M.J.; Lawson, T.; Slattery, K.; Truman, W.; Smirnoff, N.; Asami, T.; Davies, W.J.; Jones, A.M.; Baker, N.R.; et al. The high light response in Arabidopsis involves ABA signaling between vascular and bundle sheath cells. Plant Cell 2009, 21, 2143–2162. [Google Scholar] [CrossRef] [PubMed]
- Frangne, N.; Maeshima, M.; Schäffner, A.R.; Mandel, T.; Martinoia, E.; Bonnemain, J.L. Expression and distribution of a vaculoar aquaporin in young and mature leaf tissues of Brassica napus in relation to water fluxes. Planta 2001, 212, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Sack, L.; Melcher, P.J.; Zwieniecki, M.A.; Holbrook, N.M. The hydraulic conductance of the angiosperm leaf lamina: A comparison of three measurement methods. J. Exp. Bot. 2002, 53, 2177–2184. [Google Scholar] [CrossRef] [PubMed]
- Scoffoni, C.; Pou, A.; Aasamaa, K.; Sack, L. The rapid light response of leaf hydraulic conductance: New evidence from two experimental methods. Plant Cell Environ. 2008, 31, 1803–1812. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.X.; Steudle, E. Light and turgor affect the water permeability (aquaporins) of parenchyma cells in the midrib of leaves of Zea mays. J. Exp. Bot. 2007, 58, 4119–4129. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Chung, G.C.; Zwiazek, J.J. Effects of irradiance on cell water relations in leaf bundle sheath cells of wild-type and transgenic tobacco (Nicotiana tabacum) plants overexpressing aquaporins. Plant Sci. 2009, 176, 248–255. [Google Scholar] [CrossRef]
- Pantin, F.; Monnet, F.; Jannaud, D. The dual effect of abscisic acid on stomata. New Phytol. 2013, 197, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Osborne, C.P.; Sack, L. Evolution of C4 plants: A new hypothesis for an interaction of CO2 and water relations mediated by plant hydraulics. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012, 367, 583–600. [Google Scholar] [CrossRef] [PubMed]
- Christmann, A.; Grill, E.; Huang, J. Hydraulic signals in long-distance signaling. Curr. Opin. Plant Biol. 2013, 16, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Sade, N.; Gallé, A.; Flexas, J.; Lerner, S.; Peleg, G.; Yaaran, A.; Moshelion, M. Differential tissue-specific expression of NtAQP1 in Arabidopsis thaliana reveals a role for this protein in stomatal and mesophyll conductance of CO2 under standard and salt-stress conditions. Planta 2014, 239, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, H.; Weller, G.; Toy, L.F.M.; Dennis, R.J. You’re so vein: Bundle sheath physiology, phylogeny and evolution in C3 and C4 plants. Plant Cell Environ. 2013, 36, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Sade, N.; Shatil-Cohen, A.; Attia, Z.; Maurel, C.; Boursiac, Y.; Kelly, G.; Granot, D.; Yaaran, A.; Lerner, S.; Moshelion, M. The role of plasma membrane aquaporins in regulating the bundle sheath-mesophyll continuum and leaf hydraulics. Plant Physiol. 2014, 166, 1609–1620. [Google Scholar] [CrossRef] [PubMed]
- Longstrethi, D.J.; Hartsock, T.L.; Nobel, P.S. Mesophyll cell properties for some C3 and C4 species with high photosynthetic rates. Physiol. Plant 1980, 48, 494–498. [Google Scholar] [CrossRef]
- Canny, M. Water loss from leaf mesophyll stripped of the epidermis. Funct. Plant Biol. 2012, 39, 421–434. [Google Scholar] [CrossRef]
- Zwieniecki, M.A.; Brodribb, T.J.; Holbrook, N.M. Hydraulic design of leaves: Insights from rehydration kinetics. Plant Cell Environ. 2007, 30, 910–921. [Google Scholar] [CrossRef] [PubMed]
- Buckley, T.N.; Sack, L.; Gilbert, M.E. The role of bundle sheath extensions and life form in stomatal responses to leaf water status. Plant Physiol. 2011, 156, 962–973. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Holbrook, N.M.; Zwieniecki, M.A. Cell-to-cell pathway dominates xylem-epidermis hydraulic connection in Tradescantia fluminensis (Vell. Conc.) leaves. Planta 2008, 227, 1311–1319. [Google Scholar] [CrossRef] [PubMed]
- Postaire, O.; Tournaire-Roux, C.; Grondin, A.; Boursiac, Y.; Morillon, R.; Schäffner, A.R.; Maurel, C. A PIP1 aquaporin contributes to hydrostatic pressure-induced water transport in both the root and rosette of Arabidopsis. Plant Physiol. 2010, 152, 1418–1430. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.G.; Sieber, H.; Kammerloher, W.; Schaffner, A.R.; Institut, P.; Gottingen, U.; Karspüle, U.; Gottingen, D.; Germany, H.S. PIPl Aquaporins sre concentrated in plasmalemmasomes of Arabidopsis thaliana mesophyl. Plant Physiol. 1996, 111, 645–649. [Google Scholar] [PubMed]
- Morillon, R.; Chrispeels, M.J. The role of ABA and the transpiration stream in the regulation of the osmotic water permeability of leaf cells. Proc. Natl. Acad. Sci. USA 2001, 98, 14138–14143. [Google Scholar] [CrossRef] [PubMed]
- Voicu, M.C.; Cooke, J.E.K.; Zwiazek, J.J. Aquaporin gene expression and apoplastic water flow in bur oak (Quercus macrocarpa) leaves in relation to the light response of leaf hydraulic conductance. J. Exp. Bot. 2009, 60, 4063–4075. [Google Scholar] [CrossRef] [PubMed]
- Voicu, M.C.; Zwiazek, J.J.; Tyree, M.T. Light response of hydraulic conductance in bur oak (Quercus macrocarpa) leaves. Tree Physiol. 2008, 28, 1007–1015. [Google Scholar] [CrossRef] [PubMed]
- Katsuhara, M.; Koshio, K.; Shibasaka, M.; Hayashi, Y.; Hayakawa, T.; Kasamo, K. Over-expression of a Barley aquaporin increased the shoot/root ratio and raised salt sensitivity in Transgenic riceplants. Plant Cell Physiol. 2003, 44, 1378–1383. [Google Scholar] [CrossRef] [PubMed]
- Oparka, K.J.; Prior, D.A.M.; Crawford, J.W. Behaviour of plasma membrane, cortical ER and plasmodesmata during plasmolysis of onion epidermal cells. Plant Cell Environ. 1994, 17, 163–171. [Google Scholar] [CrossRef]
- Canut, H.; Carrasco, A.; Galaud, J.P.; Cassan, C.; Bouyssou, H.; Vita, N.; Ferrara, P.; Pont-Lezica, R. High affinity RGD-binding sites at the plasma membrane of Arabidopsis thaliana links the cell wall. Plant J. 1998, 16, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Nobel, P.S. Physicochemical and Environmental Plant Physiology, 3rd ed.; Elsevier Academic Press: Burlington, MA, USA, 1999; pp. 87–91. [Google Scholar]
- Taiz, L.; Zeiger, E. Plant Physiology, 5th ed.; Sinauer Associates: Sunderland, MA, USA, 2010; pp. 90–96. [Google Scholar]
- Bartlett, M.K.; Scoffoni, C.; Sack, L. The determinants of leaf turgor loss point and prediction of drought tolerance of species and biomes: A global meta-analysis. Ecol. Lett. 2012, 15, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Pierce, M.; Raschke, K. Correlation between loss of turgor and accumulation of abscisic acid in detached leaves. Planta 1980, 148, 174–182. [Google Scholar] [CrossRef] [PubMed]
- McAdam, S.A.M.; Brodribb, T.J. Linking turgor with ABA biosynthesis: Implications for stomatal responses to vapour pressure deficit across land plants. Plant Physiol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Cowan, I.R. Oscillations in stomatal conductance and plant functioning associated with stomatal conductance: Observations and a model. Planta 1972, 106, 185–219. [Google Scholar] [CrossRef] [PubMed]
- Franks, P.J.; Farquhar, G.D. The mechanical diversity of stomata and its significance in gas-exchange control. Plant Physiol. 2006, 143, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Sakurai-Ishikawa, J.; Murai-Hatano, M.; Hayashi, H.; Ahamed, A.; Fukushi, K.; Matsumoto, T.; Kitagawa, Y. Transpiration from shoots triggers diurnal changes in root aquaporin expression. Plant Cell Environ. 2011, 34, 1150–1163. [Google Scholar] [CrossRef] [PubMed]
- Sack, L.; Scoffoni, C. Measurement of Leaf Hydraulic Conductance and Stomatal Conductance and their Responses to Irradiance and Dehydration Using the Evaporative Flux Method (EFM). Available online: http://www.jove.com/video/4179/measurement-leaf-hydraulic-conductance-stomatal-conductance-their (accessed on 18 May 2016).
- Roelfsema, M.R.G.; Hedrich, R. In the light of stomatal opening: New insights into “the Watergate”. New Phytol. 2005, 167, 665–691. [Google Scholar] [CrossRef] [PubMed]
- Munemasa, S.; Hauser, F.; Park, J.; Waadt, R.; Brandt, B.; Schroeder, J.I. Mechanisms of abscisic acid-mediated control of stomatal aperture. Curr. Opin. Plant Biol. 2015, 28, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.; Mcainsh, M.; Gray, J. Calcium-based signalling systems in guard cells. New Phytol. 2001, 151, 109–120. [Google Scholar] [CrossRef]
- Kim, T.H.; Böhmer, M.; Hu, H.; Nishimura, N.; Schroeder, J.I. Guard cell signal transduction network: Advances in understanding abscisic acid, CO2, and Ca2+ signaling. Annu. Rev. Plant Biol. 2010, 61, 561–591. [Google Scholar] [CrossRef] [PubMed]
- Erwee, M.G.; Goodwin, P.B. Cell-cell communication in the leaves of cyanea and other plants Commelina. Planl Cell Environ. 1985, 8, 173–178. [Google Scholar] [CrossRef]
- Willmer, C.M.; Sexton, R. Stomata and plasmodesmata. Protoplasma 1979, 100, 113–124. [Google Scholar] [CrossRef]
- Meckel, T.; Gall, L.; Semrau, S.; Homann, U.; Thiel, G. Guard cells elongate: Relationship of volume and surface area during stomatal movement. Biophys. J. 2007, 92, 1072–1080. [Google Scholar] [CrossRef] [PubMed]
- Sarda, X.; Tousch, D.; Ferrare, K.; Legrand, E.; Dupuis, J.M.; Casse-Delbart, F.; Lamaze, T. Two TIP-like genes encoding aquaporins are expressed in sunflower guard cells. Plant J. 1997, 12, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Heinen, R.B.; Bienert, G.P.; Cohen, D.; Chevalier, A.S.; Uehlein, N.; Hachez, C.; Kaldenhoff, R.; Le Thiec, D.; Chaumont, F. Expression and characterization of plasma membrane aquaporins in stomatal complexes of Zea mays. Plant Mol. Biol. 2014, 86, 335–350. [Google Scholar] [CrossRef] [PubMed]
- Grondin, A.; Rodrigues, O.; Verdoucq, L.; Merlot, S.; Leonhardt, N.; Maurel, C. Aquaporins contribute to ABA-triggered stomatal closure through OST1-mediated phosphorylation. Plant Cell 2015, 27, 1945–1954. [Google Scholar] [CrossRef] [PubMed]
- MacRobbie, E.A.C. Osmotic effects on vacuolar ion release in guard cells. Proc. Natl. Acad. Sci. USA 2006, 103, 1135–1140. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.M.; Zhang, X.Y.; Tang, Q.L.; Wang, G.X. Extracellular calcium is involved in stomatal movement through the regulation of water channels in broad bean. Plant Growth Regul. 2006, 50, 79–83. [Google Scholar] [CrossRef]
- Shope, J.C.; Mott, K.A. Membrane trafficking and osmotically induced volume changes in guard cells. J. Exp. Bot. 2006, 57, 4123–4131. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Steudle, E.; Hartung, W. Gating of water channels (aquaporins) in cortical cells of young corn roots by mechanical stimuli (pressure pulses): Effects of ABA and of HgCl2. J. Exp. Bot. 2004, 55, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Soveral, G.; Madeira, A.; Loureiro-Dias, M.C.; Moura, T.F. Membrane tension regulates water transport in yeast. Biochim. Biophys. Acta Biomembr. 2008, 1778, 2573–2579. [Google Scholar] [CrossRef] [PubMed]
- Soveral, G.; Macey, R.I.; Moura, T.F. Membrane stress causes inhibition of water channels in brush border membrane vesicles from kidney proximal tubule. Biol Cell 1997, 89, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Ozu, M.; Dorr, R.A.; Gutiérrez, F.; Teresa Politi, M.; Toriano, R. Human AQP1 is a constitutively open channel that closes by a membrane-tension-mediated mechanism. Biophys. J. 2013, 104, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Törnroth-Horsefield, S.; Wang, Y.; Hedfalk, K.; Johanson, U.; Karlsson, M.; Tajkhorshid, E.; Neutze, R.; Kjellbom, P. Structural mechanism of plant aquaporin gating. Nature 2006, 439, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Johansson, I.; Larsson, C.; Ek, B.; Kjellbom, P. The major integral proteins of spinach leaf plasma membranes are putative aquaporins and are phosphorylated in response to Ca2+ and apoplastic water potential. Plant Cell 1996, 8, 1181–1191. [Google Scholar] [CrossRef] [PubMed]
- Azad, A.K.; Sawa, Y.; Ishikawa, T.; Shibata, H. Phosphorylation of plasma membrane aquaporin regulates temperature-dependent opening of tulip petals. Plant Cell Physiol. 2004, 45, 608–617. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, S.I.; Yoshimura, S.; Shinzaki, Y.; Maeshima, M.; Miyake, C. Deactivation of aquaporins decreases internal conductance to CO2 diffusion in tobacco leaves grown under long-term drought. Funct. Plant Biol. 2008, 35, 553–564. [Google Scholar] [CrossRef]
- Kline, K.G.; Barrett-Wilt, G.A.; Sussman, M.R. In planta changes in protein phosphorylation induced by the plant hormone abscisic acid. Proc. Natl. Acad. Sci. USA 2010, 107, 15986–15991. [Google Scholar] [CrossRef] [PubMed]
- Turgeman, T.; Shatil-Cohen, A.; Moshelion, M.; Teper-Bamnolker, P.; Skory, C.D.; Lichter, A.; Eshel, D. The role of aquaporins in pH-dependent germination of Rhizopus delemar spores. PLoS ONE 2016, 11, e0150543. [Google Scholar] [CrossRef] [PubMed]
- Verdoucq, L.; Grondin, A.; Maurel, C. Structure–function analysis of plant aquaporin At PIP2;1 gating by divalent cations and protons. Biochem. J. 2008, 415, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Gerbeau, P.; Amodeo, G.; Henzler, T.; Santoni, V.; Ripoche, P.; Maurel, C. The water permeability of Arabidopsis plasma membrane is regulated by divalent cations and pH. Plant J. 2002, 30, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Vera-Estrella, R. Novel regulation of aquaporins during osmotic stress. Plant Physiol. 2004, 135, 2318–2329. [Google Scholar] [CrossRef] [PubMed]
- Boursiac, Y.; Chen, S.; Luu, D.; Sorieul, M.; Van Den Dries, N.; Maurel, C. Early effects of salinity on water transport in arabidopsis roots. Molecular and cellular features of aquaporin expression 1. Plant Physiol. 2005, 139, 790–805. [Google Scholar] [CrossRef] [PubMed]
- Fetter, K. Interactions between plasma membrane aquaporins modulate their water channel activity. Plant Cell Online 2004, 16, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Temmei, Y.; Uchida, S.; Hoshino, D.; Kanzawa, N.; Kuwahara, M.; Sasaki, S.; Tsuchiya, T. Water channel activities of Mimosa pudica plasma membrane intrinsic proteins are regulated by direct interaction and phosphorylation. FEBS Lett. 2005, 579, 4417–4422. [Google Scholar] [CrossRef] [PubMed]
- Hachez, C.; Zelazny, E.; Chaumont, F. Modulating the expression of aquaporin genes in planta: A key to understand their physiological functions? Biochim. Biophys. Acta Biomembr. 2006, 1758, 1142–1156. [Google Scholar] [CrossRef] [PubMed]
- Otto, B.; Uehlein, N.; Sdorra, S.; Fischer, M.; Ayaz, M.; Belastegui-Macadam, X.; Heckwolf, M.; Lachnit, M.; Pede, N.; Priem, N.; et al. Aquaporin tetramer composition modifies the function of tobacco aquaporins. J. Biol. Chem. 2010, 285, 31253–31260. [Google Scholar] [CrossRef] [PubMed]
- Leitão, L.; Prista, C.; Loureiro-Dias, M.C.; Moura, T.F.; Soveral, G. The grapevine tonoplast aquaporin TIP2;1 is a pressure gated water channel. Biochem. Biophys. Res. Commun. 2014, 450, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Wiera, B.; Steudle, E. A cohesion/tension mechanism explains the gating of water channels (aquaporins) in Chara internodes by high concentration. J. Exp. Bot. 2004, 55, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Muhr, J.; Steudle, E. A cohesion/tension model for the gating of aquaporins allows estimation of water channel pore volumes in Chara. Plant Cell Environ. 2005, 28, 525–535. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yaaran, A.; Moshelion, M. Role of Aquaporins in a Composite Model of Water Transport in the Leaf. Int. J. Mol. Sci. 2016, 17, 1045. https://doi.org/10.3390/ijms17071045
Yaaran A, Moshelion M. Role of Aquaporins in a Composite Model of Water Transport in the Leaf. International Journal of Molecular Sciences. 2016; 17(7):1045. https://doi.org/10.3390/ijms17071045
Chicago/Turabian StyleYaaran, Adi, and Menachem Moshelion. 2016. "Role of Aquaporins in a Composite Model of Water Transport in the Leaf" International Journal of Molecular Sciences 17, no. 7: 1045. https://doi.org/10.3390/ijms17071045





