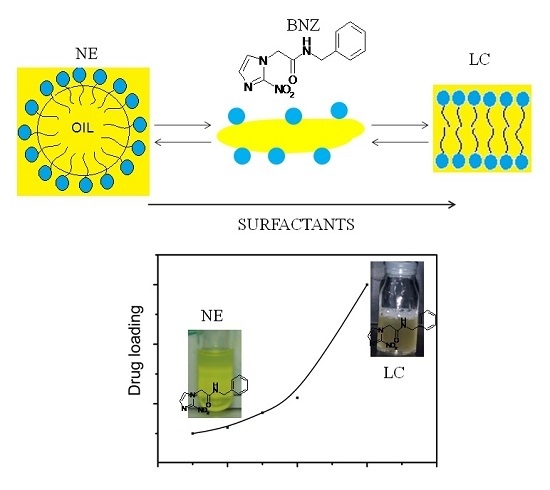
Phase Transitions of Isotropic to Anisotropic Biocompatible Lipid-Based Drug Delivery Systems Overcoming Insoluble Benznidazole Loading
Abstract
:1. Introduction
2. Results
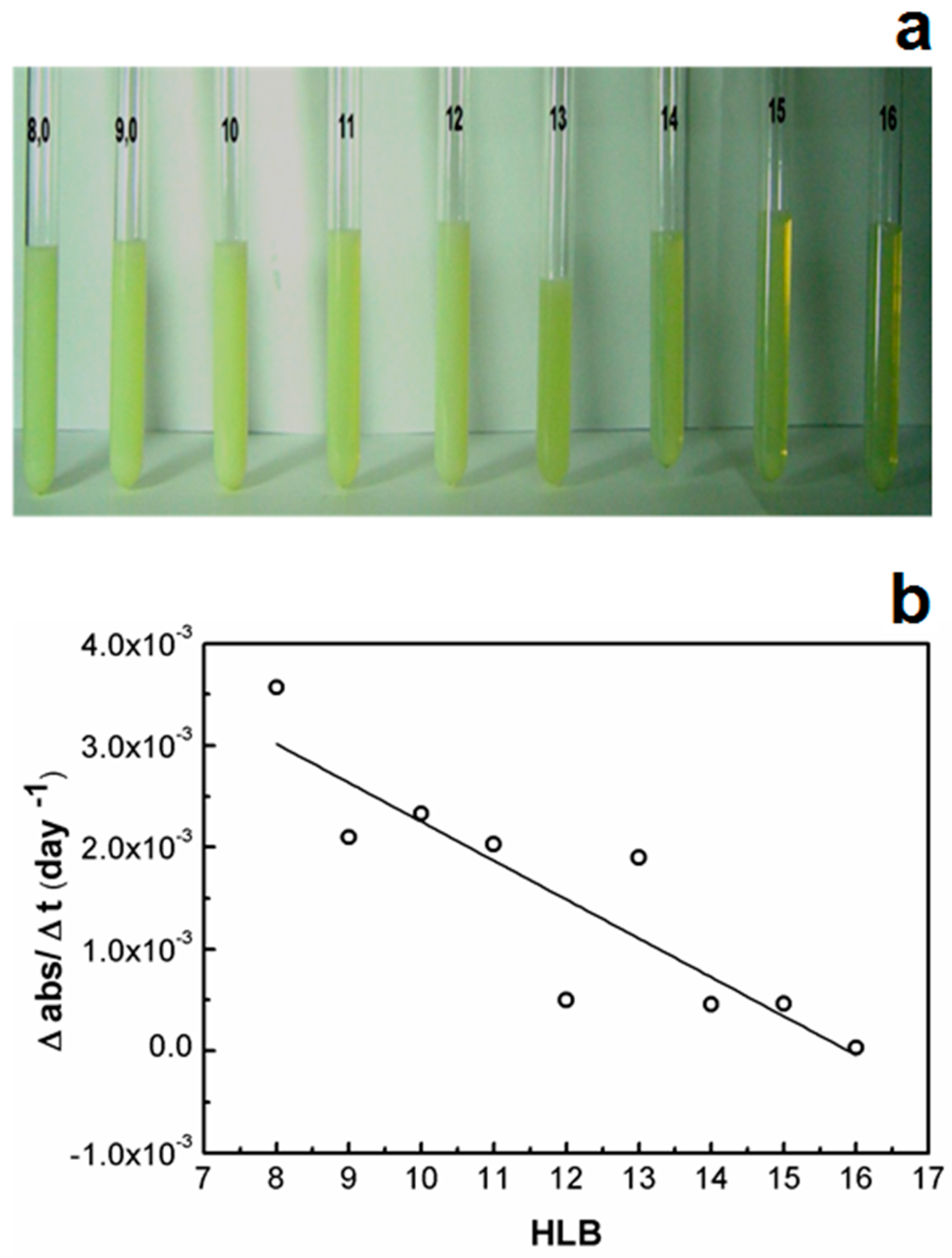
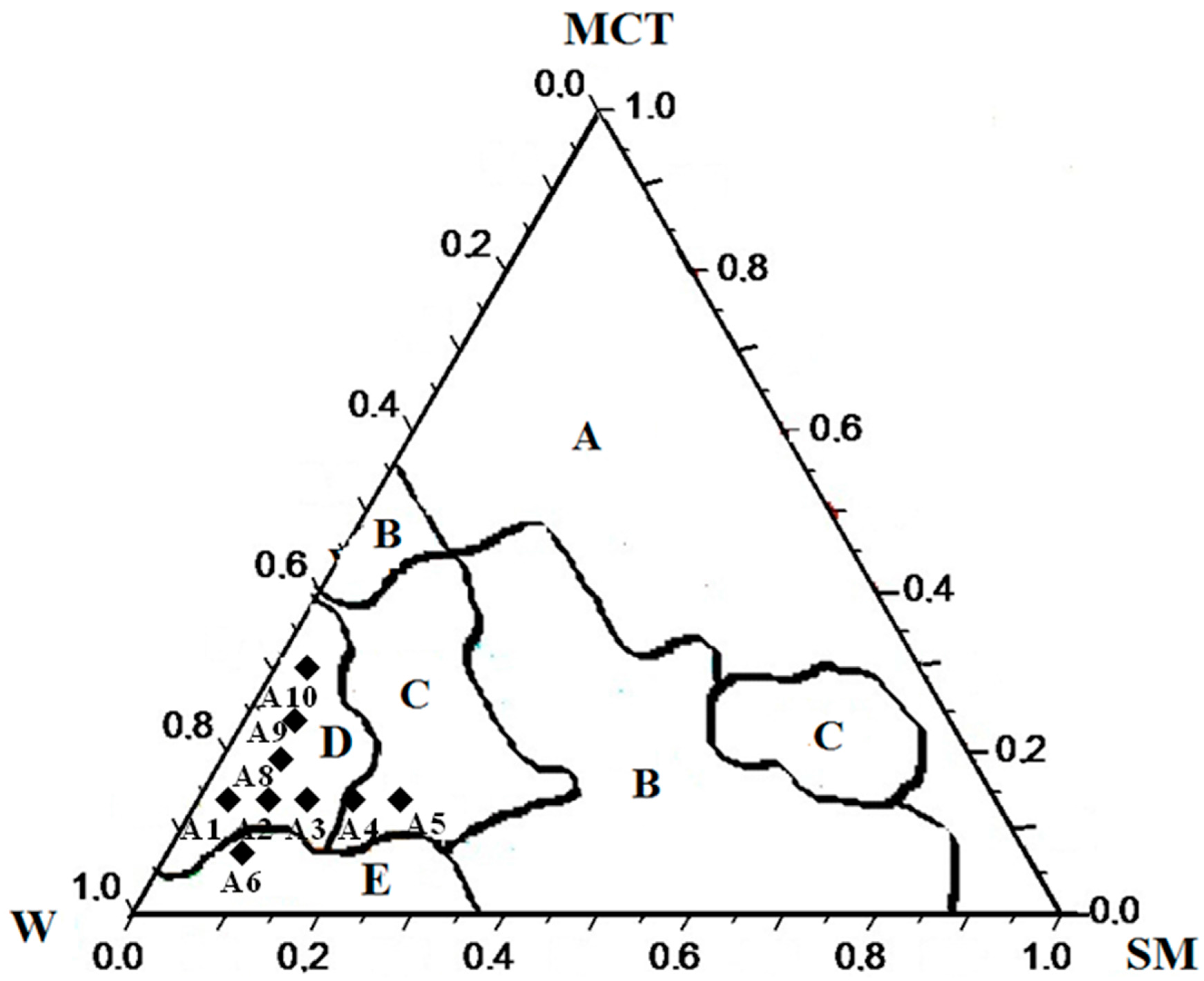
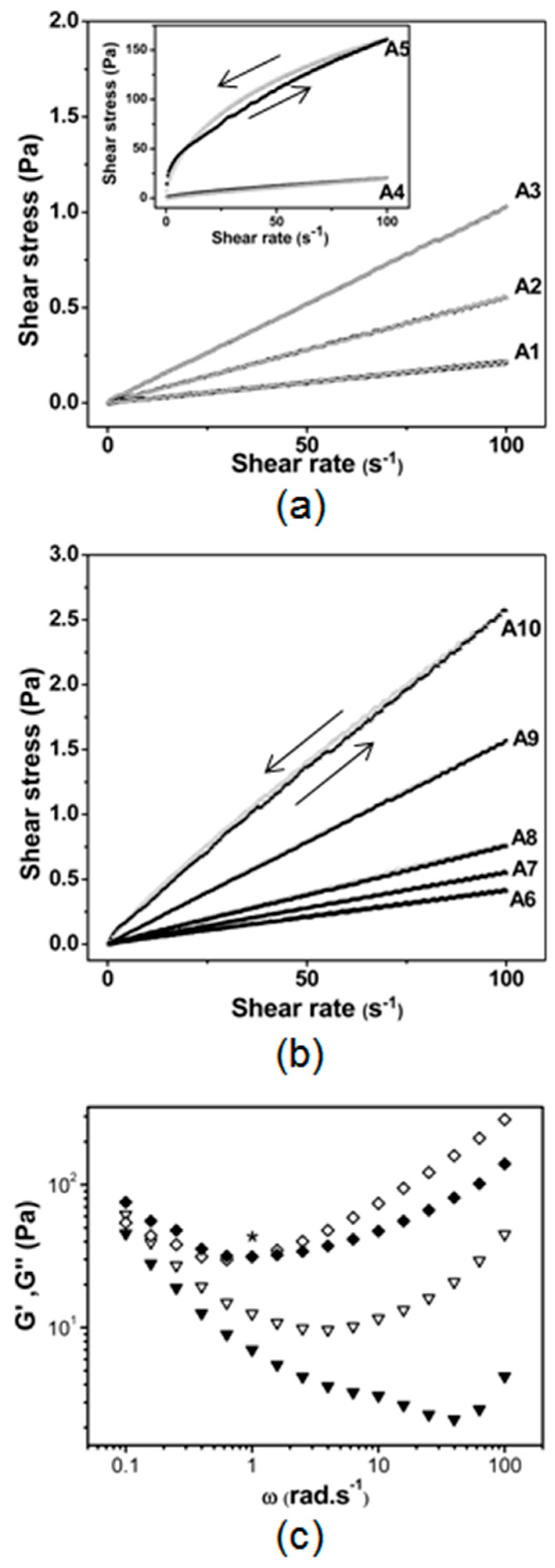
2.1. Effect of SOR and OWR on the Aspect and the Rheological and Optical Properties of LBDDS
2.2. Effect of SOR and OWR on Drug Loading
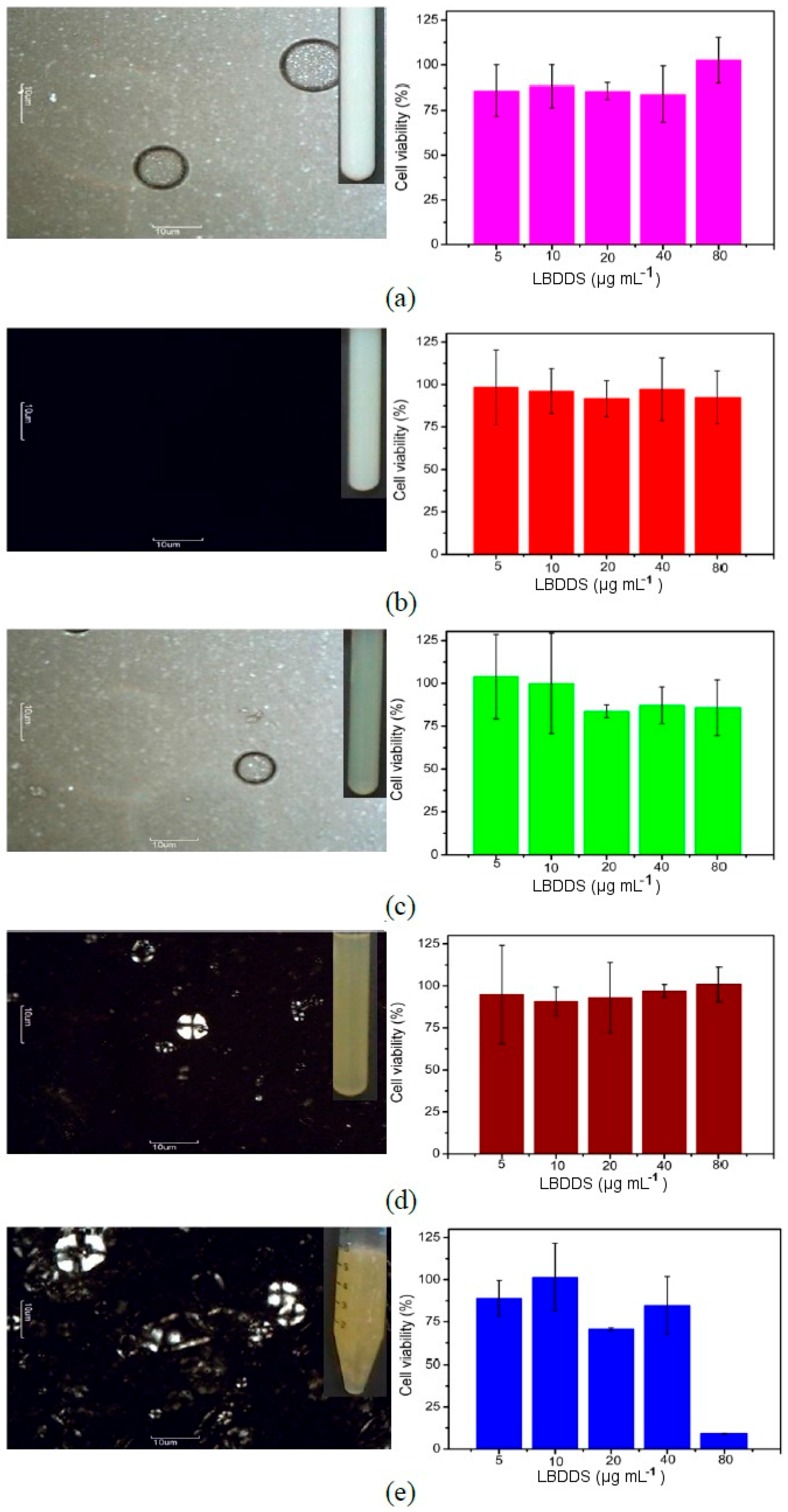
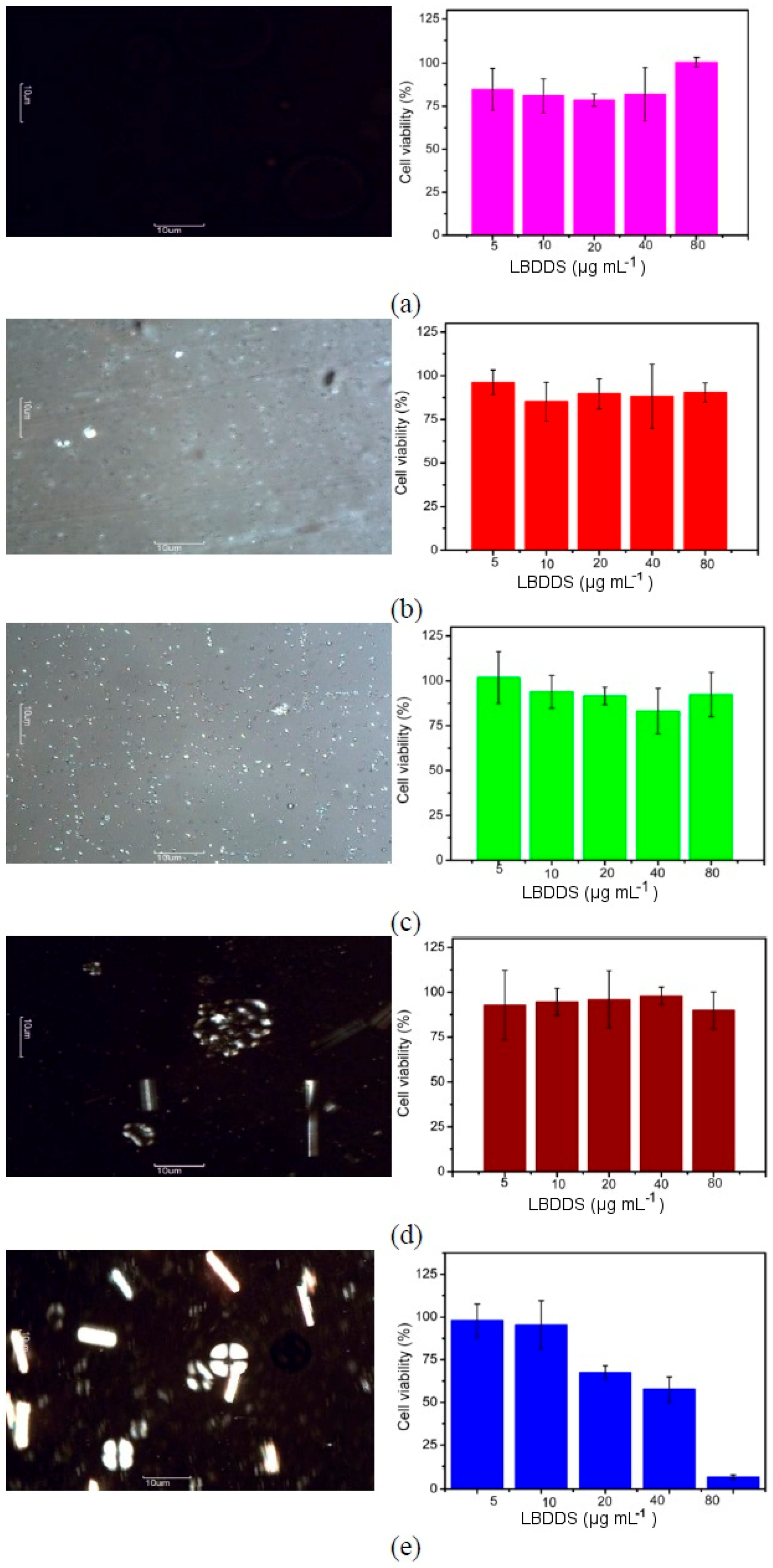
2.3. Cytotoxicity Assays
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Pseudo-Ternary Phase Diagram
4.3. Preparation of the Lipid-Based Drug Delivery Systems
4.4. Drug Loading into LBDDS
4.5. Rheology
4.6. Cross-Polarized Light Microscopy
4.7. Droplet Size and Zeta Potential Measurements
4.8. Cytotoxicity Assay
4.9. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| LBDDS | lipid-based drug delivery systems |
| BNZ | benznidazole |
| SOR | surfactant-to-oil ratio |
| OWR | oil-to-water ratio |
| SM | surfactant mixture |
| SPC | soy phosphatidylcholine |
| SO | sodium oleate |
| MCT | medium chain triglyceride |
| NE | nanoemulsion |
| LC | liquid crystals |
| HLB | hydrophilic lipophilic balance |
| O/W | oil-in-water |
| W/O | water-in-oil |
| CPLM | cross-polarized light microscopy |
| DLS | dynamic light scattering |
| ZP | Zeta potential |
| MTT | 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide |
| PdI | polydispersity index |
| EPI | emulsification by phase inversion |
References
- Zeeb, B.; Herz, E.; McClements, D.J.; Weiss, J. Impact of alcohols on the formation and stability of protein-stabilized nanoemulsions. J. Mol. Liq. 2014, 433, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Kaur, K.; Kumar, R.; Mehta, S.K. Nanoemulsion: A new medium to study the interactions and stability of curcumin with bovine serum albumin. J. Mol. Liq. 2015, 209, 62–70. [Google Scholar] [CrossRef]
- Buyukozturk, F.; Benneyan, J.C.; Carrier, R.L. Impact of emulsion-based drug delivery systems on intestinal permeability and drug release kinetics. J. Control. Release 2010, 142, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Parmar, N.; Singla, N.; Amin, S.; Kohli, K. Study of cosurfactant effect on nanoemulsifying area and development of lercanidipine loaded (SNEDDS) self nanoemulsifying drug delivery system. Colloids Surf. B: Biointerfaces 2011, 86, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Constantinides, P.P.; Chaubal, M.V.; Shorr, R. Advances in lipid nanodispersions for parenteral drug delivery and targeting. Adv. Drug Deliv. Rev. 2008, 60, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Lamas, M.C.; Villaggi, L.; Nocito, I.; Bassani, G.; Leonardi, D.; Pascutti, F.; Serra, E.; Salomón, C.J. Development of parenteral formulations and evaluation of the biological activity of the trypanocide drug benznidazole. Int. J. Pharm. 2006, 307, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Urbina, J.A.; Docampo, R. Specific chemotherapy of Chagas disease: Controversies and advances. Trends Parasitol. 2003, 19, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Maximiano, F.P.; Costa, G.H.Y.; de Souza, J.; Cunha-Filho, M.S.S. Caracterização físico-química do fármaco antichagásico benznidazol. Quím. Nova 2010, 33, 1714–1719. (In Portuguese) [Google Scholar] [CrossRef]
- Streck, L.; de Araújo, M.M.; de Souza, I.; Fernandes-Pedrosa, M.F.; do Egito, E.S.T.; de Oliveira, A.G.; da Silva-Júnior, A.A. Surfactant–cosurfactant interactions and process parameters involved in the formulation of stable and small droplet-sized benznidazole-loaded soybean O/W emulsions. J. Mol. Liq. 2014, 196, 178–186. [Google Scholar] [CrossRef]
- Badran, M.M.; Taha, E.I.; Tayel, S.A. Al-Suwayeh, Ultra-fine self nanoemulsifying drug delivery system for transdermal delivery of meloxicam: Dependency on the type of surfactants. J. Mol. Liq. 2014, 190, 16–22. [Google Scholar] [CrossRef]
- Formariz, T.P.; Chiavacci, L.A.; Sarmento, V.H.V.; Santilli, C.V.; Egito, E.S.T.; Oliveira, A.G. Relationship between structural features and in vitro release of doxorubicin from biocompatible anionic microemulsion. Colloids Surf. B: Biointerfaces 2007, 60, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Ostertag, F.; Weiss, J.; McClements, D.J. Low-energy formation of edible nanoemulsions: Factors influencing droplet size produced by emulsion phase inversion. J. Colloid Interface Sci. 2012, 388, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Santiago, J.; Ananthapadmanabhan, K.P.; Tsaur, L.; Totland, C.; Somasundaran, P. Effects of fatty acids on polyelectrolyte–surfactant interactions: Implications for polymer-induced flocculation/dispersion in emulsion systems. Colloids Surf. A Physicochem. Eng. Asp. 2014, 461, 57–65. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Katare, O.P.; Singh, B. Optimized self nano-emulsifying systems of ezetimibe with enhanced bioavailability potential using long chain and medium chain triglycerides. Colloids Surf. B: Biointerfaces 2012, 100, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, P.; Feng, J.; Esquena, J.; Tadros, T.F.; Dederen, J.C.; Garcia, M.J.; Azemar, N.; Solans, C. The influence of surfactant mixing ratio on nano-emulsion formation by the pit method. J. Colloid Interface Sci. 2005, 285, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.M.; Aramaki, K. Effect of molecular weight of triglycerides on the formation and rheological behavior of cubic and hexagonal phase based gel emulsions. J. Colloid Interface Sci. 2009, 336, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Yu, L.; Xu, J.; Sun, D. Preparation of highly stable concentrated W/O nanoemulsions by PIC method at elevated temperature. Colloids Surf. A Physicochem. Eng. Asp. 2014, 447, 97–102. [Google Scholar] [CrossRef]
- Bali, V.; Ali, M.; Ali, J. Study of surfactant combinations and development of a novel nanoemulsion for minimising variations in bioavailability of ezetimibe. Colloids Surf. B: Biointerfaces 2010, 76, 410–420. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Leser, M.E.; Sher, A.A.; McClements, D.J. Formation and stability of emulsions using a natural small molecule surfactant: Quillaja saponin (Q-Naturale®). Food Hydrocoll. 2013, 30, 589–596. [Google Scholar] [CrossRef]
- Antunes, F.E.; Coppola, L.; Gaudio, D.; Nicotera, I.; Oliviero, C. Shear rheology and phase behaviour of sodium oleate/water mixtures. Colloids Surf. A Physicochem. Eng. Asp. 2007, 297, 95–104. [Google Scholar] [CrossRef]
- Pund, S.; Thakur, R.; More, U.; Joshi, A. Lipid based nanoemulsifying resveratrol for improved physicochemical characteristics, in vitro cytotoxicity and in vivo antiangiogenic efficacy. Colloids Surf. B: Biointerfaces 2014, 120, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Pestana, K.C.; Formariz, T.P.; Franzini, C.M.; Sarmento, V.H.V.; Chiavacci, L.A.; Scarpa, M.V.; Egito, E.S.T.; Oliveira, A.G. Oil-in-water lecithin-based microemulsions as a potential delivery system for amphotericin B. Colloids Surf. B: Biointerfaces 2008, 66, 253–259. [Google Scholar] [CrossRef] [PubMed]
- You, Q.; Zhang, Y.; Wang, H.; Fan, H.; Guo, J.; Li, M. The Formation of pH-Sensitive Wormlike Micelles in Ionic Liquids Driven by the Binding Ability of Anthranilic Acid. Int. J. Mol. Sci. 2015, 16, 28146–28155. [Google Scholar] [CrossRef] [PubMed]
- Tadros, T. Application of rheology for assessment and prediction of the long-term physical stability of emulsions. Adv. Colloid Interfac Sci. 2004, 108–109, 227–258. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, E.; Golding, M. Rheology of Sodium Caseinate Stabilized Oil-in-Water Emulsions. J. Colloid Interface Sci. 1997, 191, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, G.; Zaritzky, N.; Califano, A. Rheological analysis of emulsion-filled gels based on high acyl gellan gum. Food Hydrocoll. 2013, 30, 672–680. [Google Scholar] [CrossRef]
- Moschakis, T.; Murray, B.S.; Biliaderis, C.G. Modifications in stability and structure of whey protein-coated o/w emulsions by interacting chitosan and gum arabic mixed dispersions. Food Hydrocoll. 2010, 24, 8–17. [Google Scholar] [CrossRef]
- Formariz, T.P.; Chiavacci, L.A.; Scarpa, M.V.; Silva-Júnior, A.A.; Egito, E.S.T.; Terrugi, C.H.B.; Franzini, C.M.; Sarmento, V.H.V.; Oliveira, A.G. Structure and viscoelastic behavior of pharmaceutical biocompatible anionic microemulsions containing the antitumoral drug compound doxorubicin. Colloids Surf. B: Biointerfaces 2010, 77, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Goloub, T.P.; Pugh, R.J. The role of the surfactant head group in the emulsification process: Binary (nonionic–ionic) surfactant mixtures. J. Colloid Interface Sci. 2005, 291, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Saiki, Y.; Horn, R.G.; Prestidge, C.A. Droplet structure instability in concentrated emulsions. J. Colloid Interface Sci. 2008, 320, 569–574. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J.; Dungan, S.R. Light scattering study of solubilization of emulsion droplets by non-ionic surfactant solutions. Colloids Surf. A Physicochem. Eng. Asp. 1995, 104, 127–135. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; Rojas-Graü, M.A.; Soliva-Fortuny, R.; Martín-Belloso, O. Effect of processing parameters on physicochemical characteristics of microfluidized lemongrass essential oil-alginate nanoemulsions. Food Hydrocoll. 2013, 30, 401–407. [Google Scholar] [CrossRef]
- Fernandez, P.; André, V.; Rieger, J.; Kühnle, A. Nano-emulsion formation by emulsion phase inversion. Colloids Surf. A Physicochem. Eng. Asp. 2004, 251, 53–58. [Google Scholar] [CrossRef]
- Guttoff, M.; Saberi, A.H.; McClements, D.J. Formation of vitamin D nanoemulsion-based delivery systems by spontaneous emulsification: Factors affecting particle size and stability. Food Chem. 2015, 171, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Thomas, N.; Llertz, A.M.; Graf, A.; Rades, T. Influence of lipid composition and drug load on the in vitro performance of self-nanoemulsifying drug delivery systems. J. Pharm. Sci. 2012, 101, 1721–1731. [Google Scholar] [CrossRef] [PubMed]
- Djekic, L.; Primorac, M.; Filipic, S.; Agbaba, D. Investigation of surfactant/cosurfactant synergism impact on ibuprofen solubilization capacity and drug release characteristics of nonionic microemulsions. Int. J. Pharm. 2012, 433, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Formariz, T.P.; Sarmento, V.H.V.; Silva-Junior, A.A.; Scarpa, M.V.; Santilli, C.V.; Oliveira, A.G. Doxorubicin biocompatible O/W microemulsion stabilized by mixed surfactant containing soya phosphatidylcholine. Colloids Surf. B: Biointerfaces 2006, 51, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Formariz, T.P.; Chiavacci, L.A.; Sarmento, V.H.V.; Franzini, C.M.; Silva, A.A.; Scarpa, M.V.; Santilli, C.V.; Egito, E.S.T.; Oliveira, A.G. Structural changes of biocompatible neutral microemulsions stabilized by mixed surfactant containing soya phosphatidylcholine and their relationship with doxorubicin release. Colloids Surf. B: Biointerfaces 2008, 63, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Rhue, R.D. Effect of Interfacial Alcohol Concentrations on Oil Solubilization by Sodium Dodecyl Sulfate Micelles. J. Colloid Interface Sci. 2000, 228, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Nam, Y.S.; Kim, J.-W.; Park, J.; Shim, J.; Lee, J.S.; Han, S.H. Tocopheryl acetate nanoemulsions stabilized with lipid–polymer hybrid emulsifiers for effective skin delivery. Colloids Surf. B: Biointerfaces 2012, 94, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Sznitowska, M.; Dabrowska, E.A.; Janicki, S. Solubilizing potential of submicron emulsions and aqueous dispersions of lecithin. Int. J. Pharm. 2002, 246, 203–206. [Google Scholar] [CrossRef]
- De Melo, P.N.; Barbosa, E.G.; de Caland, L.B.; Carpegianni, H.; Garnero, C.; Longhi, M.; Fernades-Pedrosa, M.F.; Silva-Júnior, A.A. Host–guest interactions between benznidazole and beta-cyclodextrin in multicomponent complex systems involving hydrophilic polymers and triethanolamine in aqueous solution. J. Mol. Liq. 2013, 186, 147–156. [Google Scholar] [CrossRef]
- Han, L.; Zhou, X. Synthesis and characterization of liposomes nano-composite-particles with hydrophobic magnetite as a MRI probe. Appl. Surf. Sci. 2016, 376, 252–260. [Google Scholar] [CrossRef]
- Benzaria, A.; Gràcia-Julià, A.; Picart-Palmade, L.; Hue, P.; Chevalier-Lucia, D.; Marti-Mestres, G.; Hodor, N.; Dumay, E. UHPH-processed O/W submicron emulsions stabilised with a lipid-based surfactant: Physicochemical characteristics and behaviour on in vitro TC7-cell monolayers and ex vivo pig’s ear skin. Colloids Surf. B: Biointerfaces 2014, 116, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, X.; Zhao, L.; Almásy, L.; Garamus, V.M.; Willumeit, R.; Zou, A. Preparation and characterization of a nanostructured lipid carrier for a poorly soluble drug. Colloids Surf. A Physicochem. Eng. Asp. 2014, 455, 36–43. [Google Scholar] [CrossRef]
- Shakeel, F.; Haq, N.; Al-Dhfyan, A.; Alanazi, F.K.; Alsarra, I.A. Double w/o/w nanoemulsion of 5-fluorouracil for self-nanoemulsifying drug delivery system. J. Mol. Liq. 2014, 200 (Part B), 183–190. [Google Scholar] [CrossRef]
- Garzoni, L.R.; Caldera, A.; Meirelles, M.N.L.; de Castro, S.L.; Docampo, R.; Meints, G.A.; Oldfield, E.; Urbina, J.A. Selective in vitro effects of the farnesyl pyrophosphate synthase inhibitor risedronate on Trypanosoma cruzi. Int. J. Antimicrob. Agents 2004, 23, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Martínez, C.; Correa-Basurto, J.; Nieto-Meneses, R.; Márquez-Navarro, A.; Aguilar-Suárez, R.; Montero-Cortes, M.D.; Nogueda-Torres, B.; Suárez-Contreras, E.; Galindo-Sevilla, N.; Rojas-Rojas, A.; et al. Design, synthesis and biological evaluation of quinazoline derivatives as anti-trypanosomatid and anti-plasmodial agents. Eur. J. Med. Chem. 2015, 96, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Wong-Baeza, C.; Nogueda-Torres, B.; Serna, M.; Meza-Toledo, S.; Baeza, I.; Wong, C. Trypanocidal effect of the benzyl ester of N-propyl oxamate: A bi-potential prodrug for the treatment of experimental Chagas disease. BMC Pharmacol. Toxicol. 2015, 16, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Mayer, S.; Weiss, J.; McClements, D.J. Behavior of vitamin E acetate delivery systems under simulated gastrointestinal conditions: Lipid digestion and bioaccessibility of low-energy nanoemulsions. J. Colloid Interface Sci. 2013, 404, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Streck, L.; dos Santos, K.S.C.R.; Fernandes-Pedrosa, M.F.; Oliveira, A.G.; Silva-Júnior, A.A. Validação de método analítico por espectrofotometria UV para sistema emulsionado lipídico contendo benznidazol. Quím Nova 2011, 34, 1459–1463. (In Portuguese) [Google Scholar] [CrossRef]

| Samples | Diameter (nm ± SD) | PdI | ZP (mV ± SD) | n | k (Pa s) | |
|---|---|---|---|---|---|---|
| Free BNZ-loaded LBDDS | A1 (SOR = 0.5) | 113.9 ± 0.7 | 0.20 | −61.8 ± 1.4 | 0.99 | 0.002 |
| A2 (SOR = 1.0) | 87.7 ± 0.4 | 0.17 | −60.6 ± 11.0 | 0.99 | 0.007 | |
| A3 (SOR = 1.5) | 72.3 ± 0.5 | 0.22 | −62.7 ± 3.3 | 1.01 | 0.014 | |
| A4 (SOR = 2.0) | ND * | ND * | ND * | 0.81 | 0.488 | |
| A5 (SOR = 2.5) | ND * | ND * | ND * | 0.53 | 13.49 | |
| A6 (OWR = 0.06) | ||||||
| A7 (OWR = 0.12) | 110.2 ± 0.7 | 0.28 | −53.6 ± 6.6 | 0.97 | 0.007 | |
| A8 (OWR = 0.20) | 87.7 ± 0.4 | 0.17 | −37.7 ± 11.0 | 0.99 | 0.007 | |
| A9 (OWR = 0.30) | 130.8 ± 2.4 | 0.26 | −72.4 ± 3.3 | 1.01 | 0.009 | |
| A10 (OWR = 0.40) | 74.4 ± 1.0 | 0.21 | −89.2 ± 13.9 | 0.99 | 0.031 | |
| BNZ-loaded LBDDS | A1 (SOR = 0.5) | 136.7 ± 0.2 | 0.16 | −69.4 ± 6.9 | 1.02 | 0.001 |
| A2 (SOR = 1.0) | 90.3 ± 0.4 | 0.16 | −70.5 ± 7.0 | 0.91 | 0.009 | |
| A3 (SOR = 1.5) | 89.4 ± 1.1 | 0.20 | −60.8 ± 4.0 | 0.99 | 0.015 | |
| A4 (SOR = 2.0) | ND * | ND * | ND * | 0.89 | 0.219 | |
| A5 (SOR = 2.5) | ND * | ND * | ND * | 0.65 | 3.106 | |
| A6 (OWR = 0.06) | ||||||
| A7 (OWR = 0.12) | 81.6 ± 0.7 | 0.21 | −64.2 ± 4.6 | 1.01 | 0.003 | |
| A8 (OWR = 0.20) | 90.3 ± 0.4 | 0.16 | −70.6 ± 7.0 | 0.91 | 0.009 | |
| A9 (OWR = 0.30) | 80.7 ± 0.3 | 0.21 | −89.8 ± 1.7 | 1.01 | 0.008 | |
| A10 (OWR = 0.40) | 164.6 ± 3.6 | 0.24 | −96.7 ± 4.0 | 1.03 | 0.009 | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Streck, L.; Sarmento, V.H.V.; Machado, P.R.L.; Farias, K.J.S.; Fernandes-Pedrosa, M.F.; Da Silva-Júnior, A.A. Phase Transitions of Isotropic to Anisotropic Biocompatible Lipid-Based Drug Delivery Systems Overcoming Insoluble Benznidazole Loading. Int. J. Mol. Sci. 2016, 17, 981. https://doi.org/10.3390/ijms17070981
Streck L, Sarmento VHV, Machado PRL, Farias KJS, Fernandes-Pedrosa MF, Da Silva-Júnior AA. Phase Transitions of Isotropic to Anisotropic Biocompatible Lipid-Based Drug Delivery Systems Overcoming Insoluble Benznidazole Loading. International Journal of Molecular Sciences. 2016; 17(7):981. https://doi.org/10.3390/ijms17070981
Chicago/Turabian StyleStreck, Letícia, Víctor H. V. Sarmento, Paula R. L. Machado, Kleber J. S. Farias, Matheus F. Fernandes-Pedrosa, and Arnóbio Antônio Da Silva-Júnior. 2016. "Phase Transitions of Isotropic to Anisotropic Biocompatible Lipid-Based Drug Delivery Systems Overcoming Insoluble Benznidazole Loading" International Journal of Molecular Sciences 17, no. 7: 981. https://doi.org/10.3390/ijms17070981
APA StyleStreck, L., Sarmento, V. H. V., Machado, P. R. L., Farias, K. J. S., Fernandes-Pedrosa, M. F., & Da Silva-Júnior, A. A. (2016). Phase Transitions of Isotropic to Anisotropic Biocompatible Lipid-Based Drug Delivery Systems Overcoming Insoluble Benznidazole Loading. International Journal of Molecular Sciences, 17(7), 981. https://doi.org/10.3390/ijms17070981