Preparation and Characterization of a Polyclonal Antibody against Human Actin Filament-Associated Protein-120 kD
Abstract
:1. Introduction
2. Results
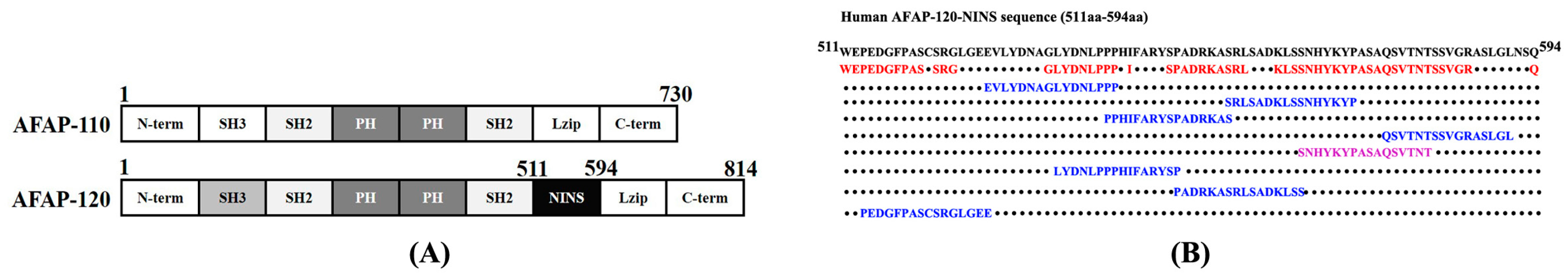
2.1. Sequence Analysis and AFAP-120 Protein Epitope Prediction
2.2. Identification and Purification of the Synthetic Epitope Peptide
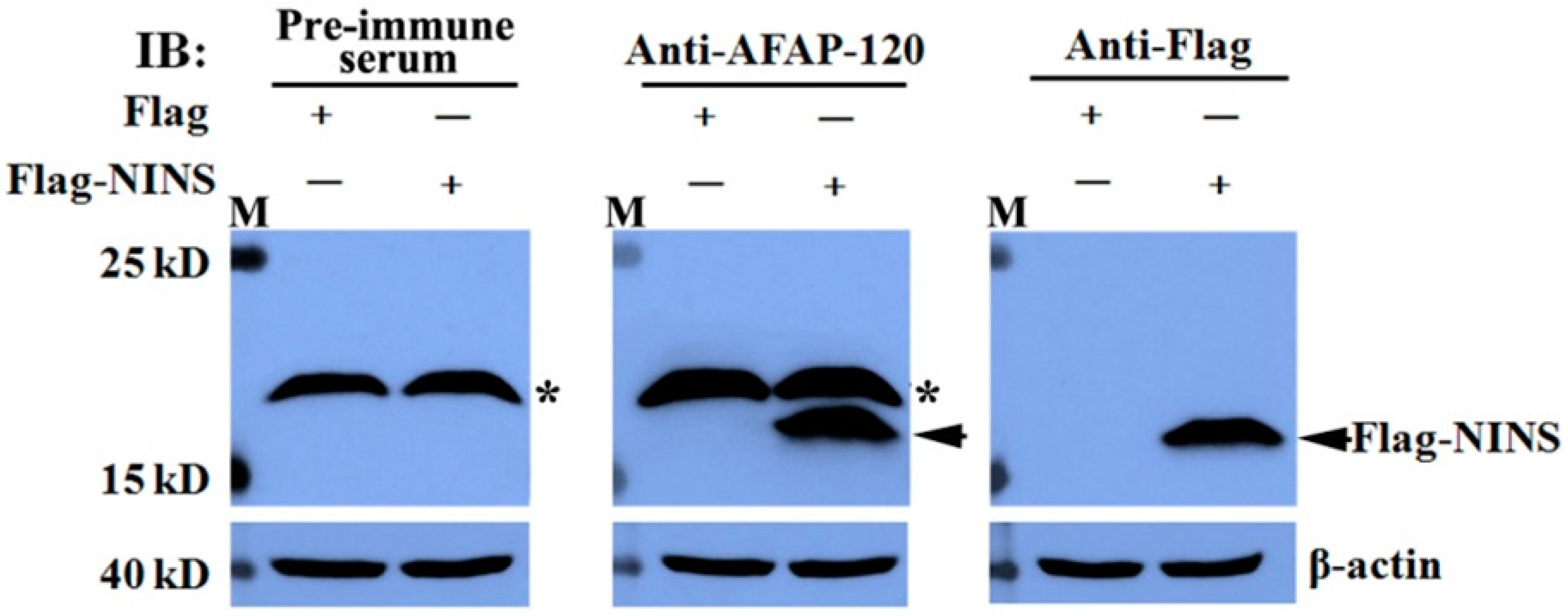
2.3. Recognization of the Anti-AFAP-120 Antibody to Human NINS Peptide
2.4. Recognization of the Anti-AFAP-120 Antibody to Human Denatured AFAP-120
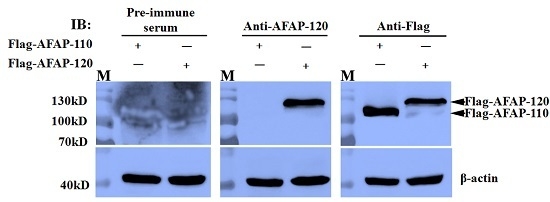
2.5. Reaction of the Anti-AFAP-120 Antibody with Native Human AFAP-120 Protein
3. Discussion
4. Materials and Methods
4.1. Plasmid Constructs
4.2. Sequence Analysis and B-Cell Epitopes Prediction of the AFAP-120 Protein
4.3. Immunization and Production of the AFAP120-Reactive Rabbit Polyclonal Antibody
4.4. Cell Culture and Transfection
4.5. Immunoprecipitation
4.6. Immunoblotting
4.7. Immunofluorescence
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AFAP-120 | actin filament-associated protein-120kD |
| AFAP-110 | actin filament-associated protein-110kD |
| NINS | neuronal insert |
| PKCα | protein kinase C alpha |
| Lzip | leucine zipper |
| MS | mass spectrum |
| HPLC | high performance liquid chromatography |
| FCA | Freund’s complete adjuvant |
| FIA | Freund’s incomplete adjuvant |
| SDS-PAGE | SDS–polyacrylamide gel electrophoresis |
| IB | immunoblotting |
| IP | immunoprecipitation |
References
- Flynn, D.C.; Leu, T.H.; Reynolds, A.B.; Parsons, J.T. Identification and sequence analysis of cDNAs encoding a 110-kilodalton actin filament-associated pp60src substrate. Mol. Cell. Biol. 1993, 13, 7892–7900. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Harder, J.; Flynn, D.C.; Lanier, L.M. AFAP120 regulates actin organization during neuronal differentiation. Differentiation 2009, 77, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Dorfleutner, A.; Cho, Y.; Vincent, D.; Cunnick, J.; Lin, H.; Weed, S.A.; Stehlik, C.; Flynn, D.C. Phosphorylation of AFAP-110 affects podosome lifespan in A7r5 cells. J. Cell Sci. 2008, 121, 2394–2405. [Google Scholar] [CrossRef] [PubMed]
- Lodyga, M.; Bai, X.H.; Mourgeon, E.; Han, B.; Keshavjee, S.; Liu, M. Molecular cloning of actin filament-associated protein: A putative adaptor in stretch-induced Src activation. Am. J. Physiol. Lung Cell Mol. Physiol. 2002, 283, L265–L274. [Google Scholar] [CrossRef] [PubMed]
- Baisden, J.M.; Qian, Y.; Zot, H.M.; Flynn, D.C. The actin filament-associated protein AFAP-110 is an adaptor protein that modulates changes in actin filament integrity. Oncogene 2001, 20, 6435–6447. [Google Scholar] [CrossRef] [PubMed]
- Baisden, J.M.; Gatesman, A.S.; Cherezova, L.; Jiang, B.H.; Flynn, D.C. The intrinsic ability of AFAP-110 to alter actin filament integrity is linked with its ability to also activate cellular tyrosine kinases. Oncogene 2001, 20, 6607–6616. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Baisden, J.M.; Cherezova, L.; Summy, J.M.; Guappone-Koay, A.; Shi, X.; Mast, T.; Pustula, J.; Zot, H.G.; Mazloum, N.; et al. PC phosphorylation increases the ability of AFAP-110 to cross-link actin filaments. Mol. Biol. Cell 2002, 13, 2311–2322. [Google Scholar] [CrossRef] [PubMed]
- Guappone, A.C.; Flynn, D.C. The integrity of the SH3 binding motif of AFAP-110 is required to facilitate tyrosine phosphorylation by, and stable complex formation with, Src. Mol. Cell. Biochem. 1997, 175, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Guappone, A.C.; Weimer, T.; Flynn, D.C. Formation of a stable src-AFAP-110 complex through either an amino-terminal or a carboxy-terminal SH2-binding motif. Mol. Carcinog. 1998, 22, 110–119. [Google Scholar] [CrossRef]
- Qian, Y.; Baisden, J.M.; Zot, H.G.; Van Winkle, W.B.; Flynn, D.C. The carboxy terminus of AFAP-110 modulates direct interactions with actin filaments and regulates its ability to alter actin filament integrity and induce lamellipodia formation. Exp. Cell Res. 2000, 255, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Flynn, D.C.; Koay, T.C.; Humphries, C.G.; Guappone, A.C. AFAP-120. A variant form of the Src SH2/SH3-binding partner AFAP-110 is detected in brain and contains a novel internal sequence which binds to a 67-kDa protein. J. Biol. Chem. 1995, 270, 3894–3899. [Google Scholar] [PubMed]
- Clump, D.A.; Clem, R.; Qian, Y.; Guappone-Koay, A.; Berrebi, A.S.; Flynn, D.C. Protein expression levels of the Src activating protein AFAP are developmentally regulated in brain. J. Neurobiol. 2003, 54, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Harder, J.; Xu, X.; Letourneau, P.; Lanier, L.M. The actin cross-linking protein AFAP120 regulates axon elongation in a tyrosine phosphorylation-dependent manner. Neurosci. Lett. 2008, 444, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Dorfleutner, A.; Stehlik, C.; Zhang, J.; Gallick, G.E.; Flynn, D.C. AFAP-110 is required for actin stress fiber formation and cell adhesion in MDA-MB-231 breast cancer cells. J. Cell. Physiol. 2007, 213, 740–749. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Gatesman, A.S.; Baisden, J.M.; Zot, H.G.; Cherezova, L.; Qazi, I.; Mazloum, N.; Lee, M.Y.; Guappone-Koay, A.; Flynn, D.C. Analysis of the role of the leucine zipper motif in regulating the ability of AFAP-110 to alter actin filament integrity. J. Cell. Biochem. 2004, 91, 602–620. [Google Scholar] [CrossRef] [PubMed]
- Gatesman, A.; Walker, V.G.; Baisden, J.M.; Weed, S.A.; Flynn, D.C. Protein kinase Calpha activates c-Src and induces podosome formation via AFAP-110. Mol. Cell. Biol. 2004, 24, 7578–7597. [Google Scholar] [CrossRef] [PubMed]
- Crimaldi, L.; Courtneidge, S.A.; Gimona, M. Tks5 recruits AFAP-110, p190RhoGAP, and cortactin for podosome formation. Exp. Cell. Res. 2009, 315, 2581–2592. [Google Scholar] [CrossRef] [PubMed]
- Walker, V.G.; Ammer, A.; Cao, Z.; Clump, A.C.; Jiang, B.H.; Kelley, L.C.; Weed, S.A.; Zot, H.; Flynn, D.C. PI3K activation is required for PMA-directed activation of cSrc by AFAP-110. Am. J. Physiol. Cell. Physiol. 2007, 293, C119–C132. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Park, S.I.; Artime, M.C.; Summy, J.M.; Shah, A.N.; Bomser, J.A.; Dorfleutner, A.; Flynn, D.C.; Gallick, G.E. AFAP-110 is overexpressed in prostate cancer and contributes to tumorigenic growth by regulating focal contacts. J. Clin. Investig. 2007, 117, 2962–2973. [Google Scholar] [CrossRef] [PubMed]
- Lateef, S.S.; Gupta, S.; Jayathilaka, L.P.; Krishnanchettiar, S.; Huang, J.S.; Lee, B.S. An improved protocol for coupling synthetic peptides to carrier proteins for antibody production using DMF to solubilize peptides. J. Biomol. Tech. 2007, 18, 173–176. [Google Scholar] [PubMed]
- Saha, S.; Raghava, G.P. Prediction of continuous B-cell epitopes in an antigen using recurrent neural network. Proteins 2006, 65, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Larsen, J.E.; Lund, O.; Nielsen, M. Improved method for predicting linear B-cell epitopes. Immunome Res. 2006, 2, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
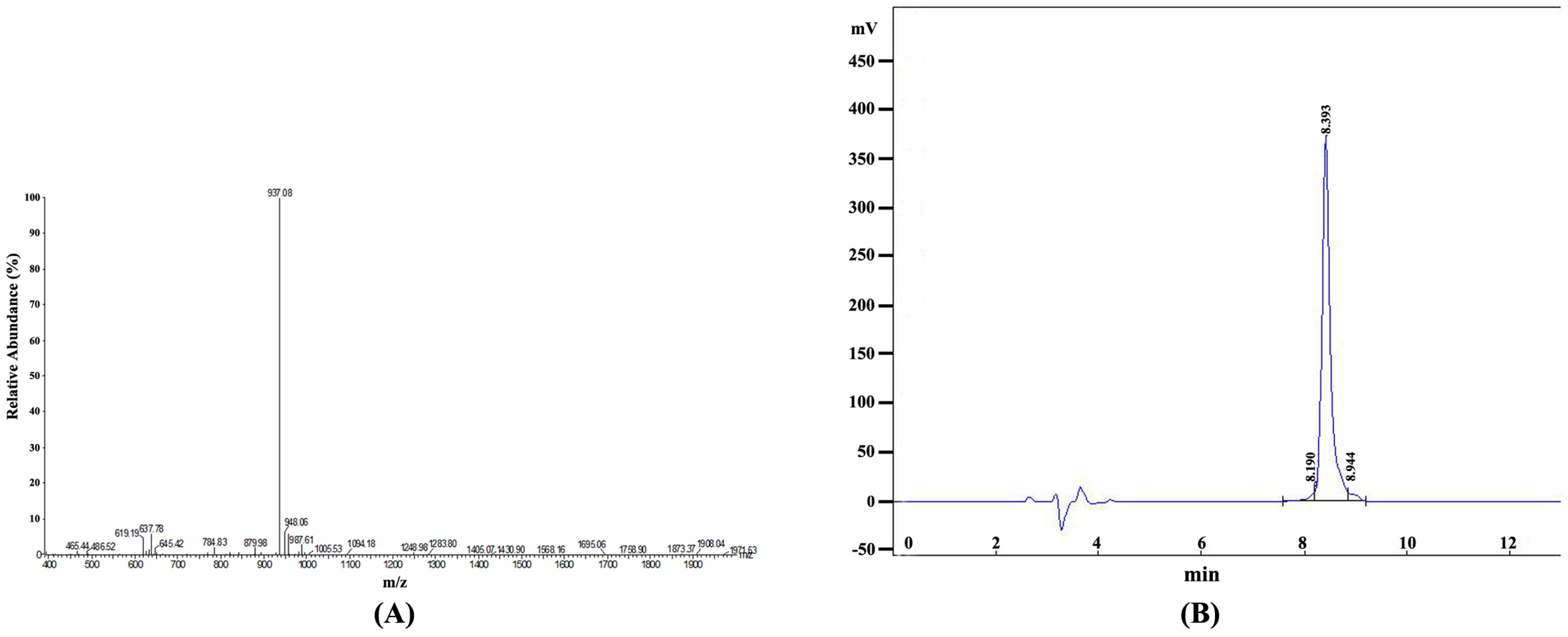


| No. | Retention Time | Peak Area | Content (%) |
|---|---|---|---|
| 1 | 8.190 | 118,254 | 2.6289 |
| 2 | 8.393 | 4,283,028 | 95.2168 |
| 3 | 8.944 | 96,906 | 2.1543 |
| Total | 4,498,188 | 100 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Liu, Y.; Guo, J.; Tang, T.; Gao, J.; Huang, T.; Wang, B.; Liu, S. Preparation and Characterization of a Polyclonal Antibody against Human Actin Filament-Associated Protein-120 kD. Int. J. Mol. Sci. 2016, 17, 942. https://doi.org/10.3390/ijms17060942
Chen Y, Liu Y, Guo J, Tang T, Gao J, Huang T, Wang B, Liu S. Preparation and Characterization of a Polyclonal Antibody against Human Actin Filament-Associated Protein-120 kD. International Journal of Molecular Sciences. 2016; 17(6):942. https://doi.org/10.3390/ijms17060942
Chicago/Turabian StyleChen, Yujian, Yong Liu, Jiayu Guo, Tao Tang, Jian Gao, Tao Huang, Bin Wang, and Shaojun Liu. 2016. "Preparation and Characterization of a Polyclonal Antibody against Human Actin Filament-Associated Protein-120 kD" International Journal of Molecular Sciences 17, no. 6: 942. https://doi.org/10.3390/ijms17060942
APA StyleChen, Y., Liu, Y., Guo, J., Tang, T., Gao, J., Huang, T., Wang, B., & Liu, S. (2016). Preparation and Characterization of a Polyclonal Antibody against Human Actin Filament-Associated Protein-120 kD. International Journal of Molecular Sciences, 17(6), 942. https://doi.org/10.3390/ijms17060942