Intravenous Administration Is an Effective and Safe Route for Cancer Gene Therapy Using the Bifidobacterium-Mediated Recombinant HSV-1 Thymidine Kinase and Ganciclovir
Abstract
:1. Introduction
2. Results
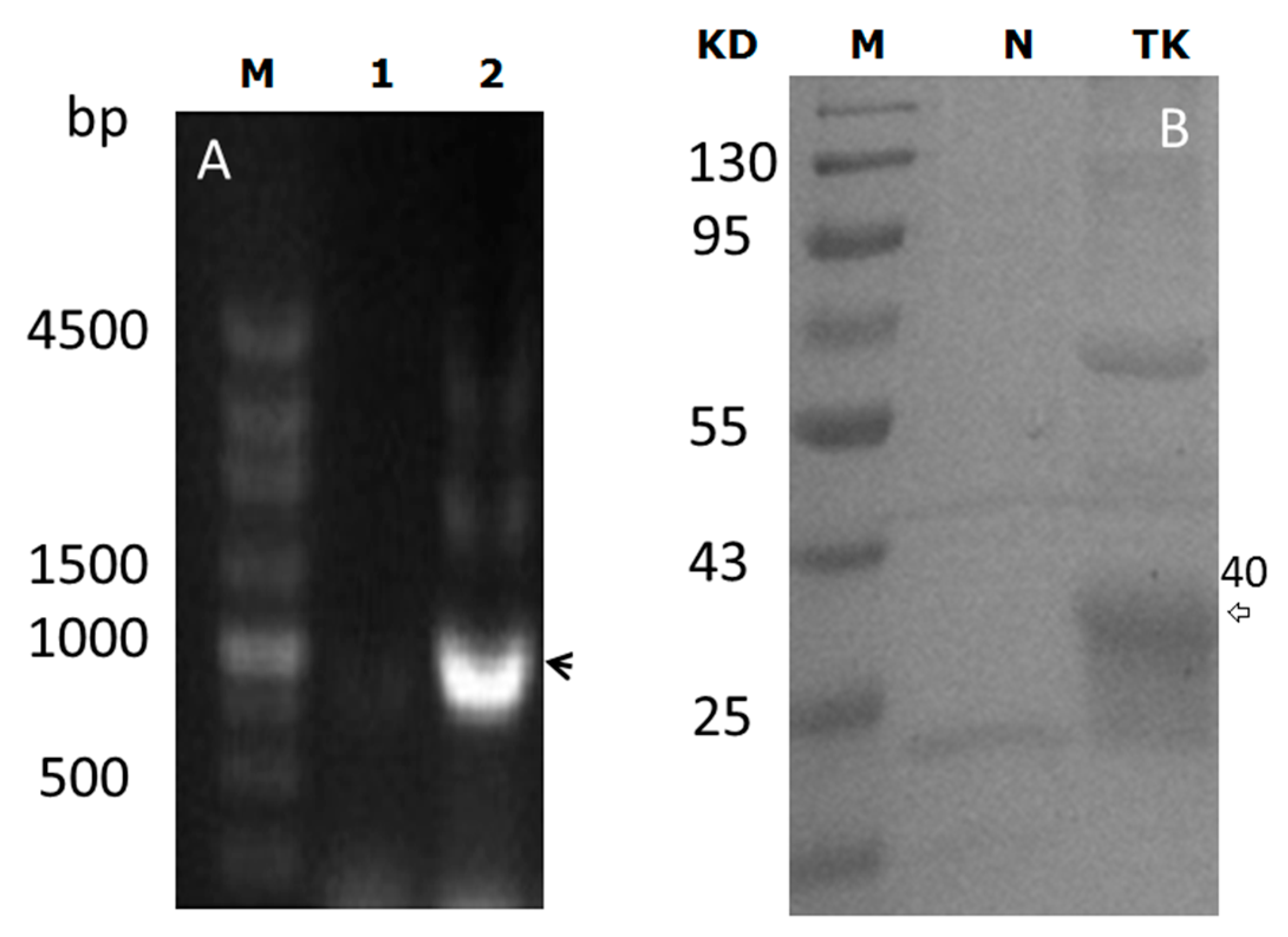
2.1. pBEX-tk Is Expressed in Bifidobacterium (BF)
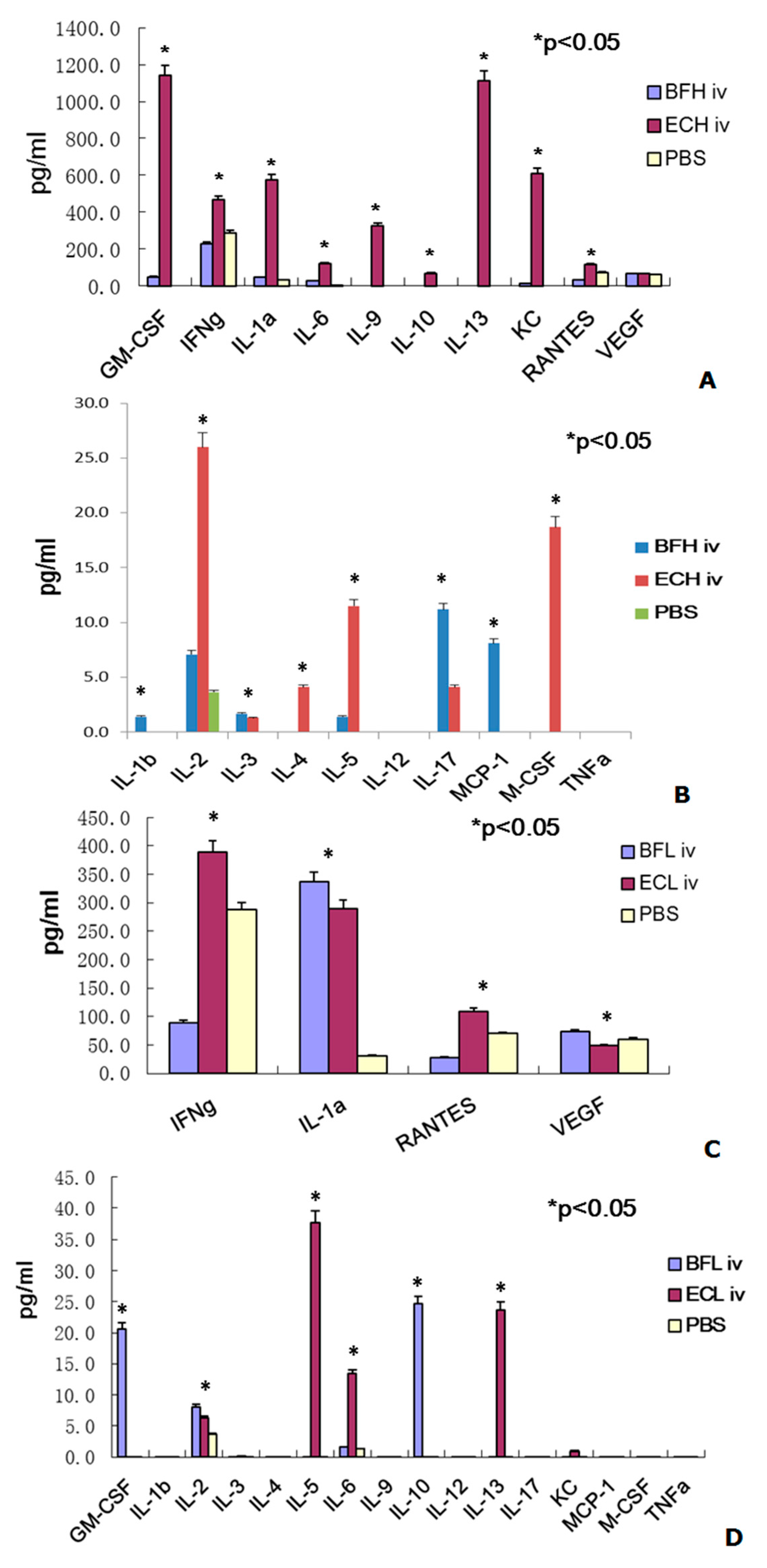
2.2. Administration of BF-rTK/GCV via Intravenous (IV) Only Minimally Induces Cytokine Expression
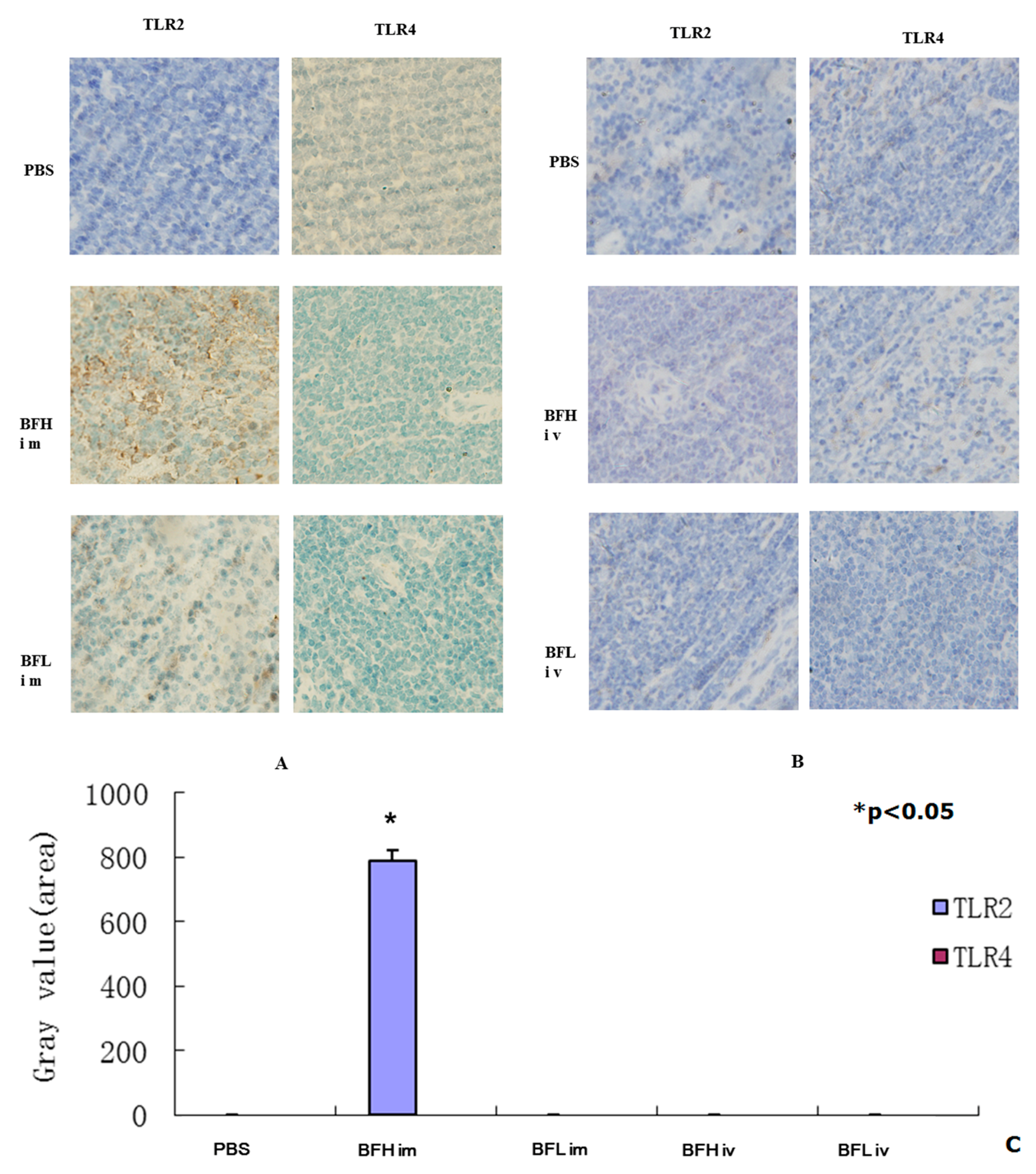
2.3. Intramuscular (IM) Injection of a High Dose of BF-rTK Induces TLR2 Expression
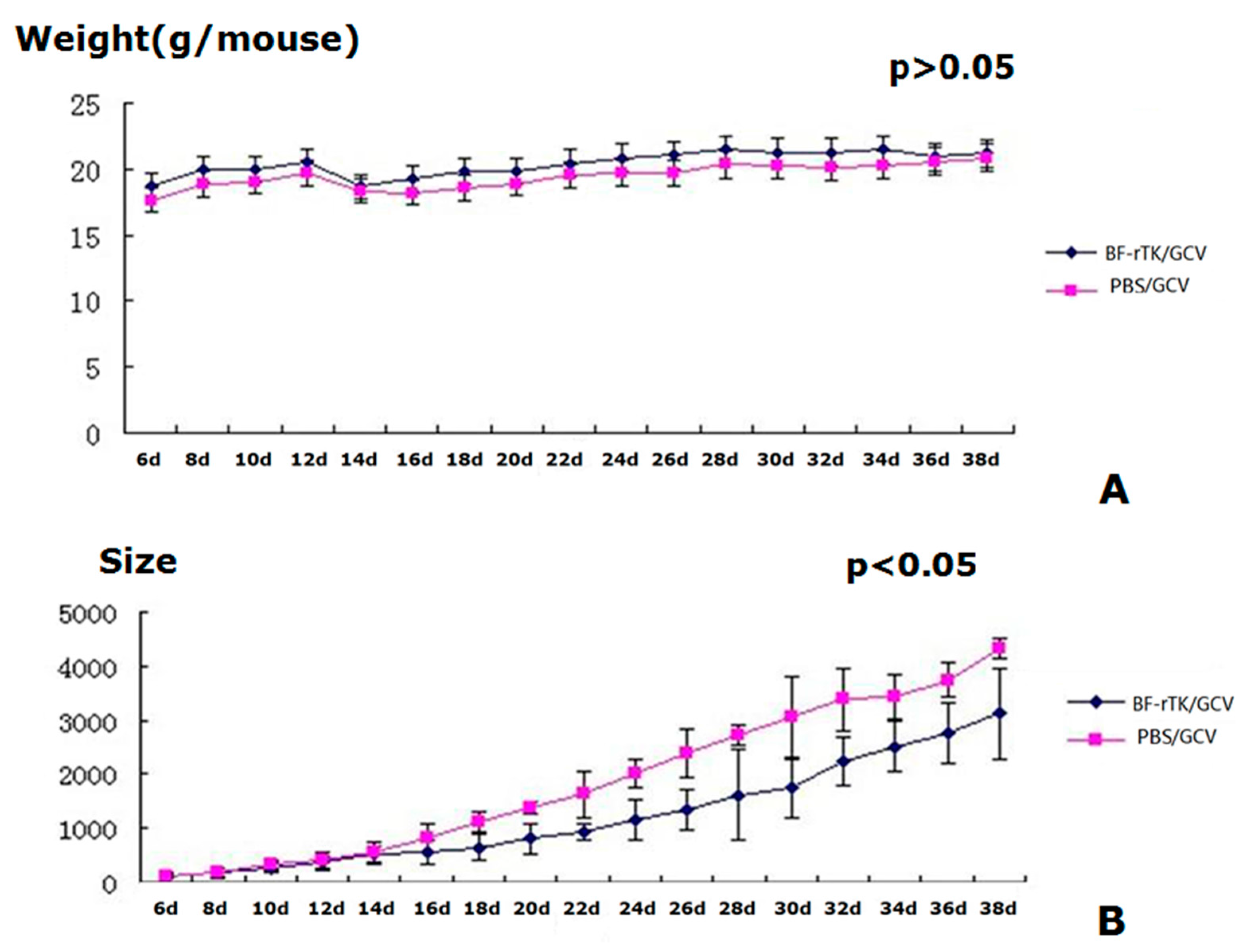
2.4. BF-rTK/GCV Treatment Significantly Inhibits Tumor Growth
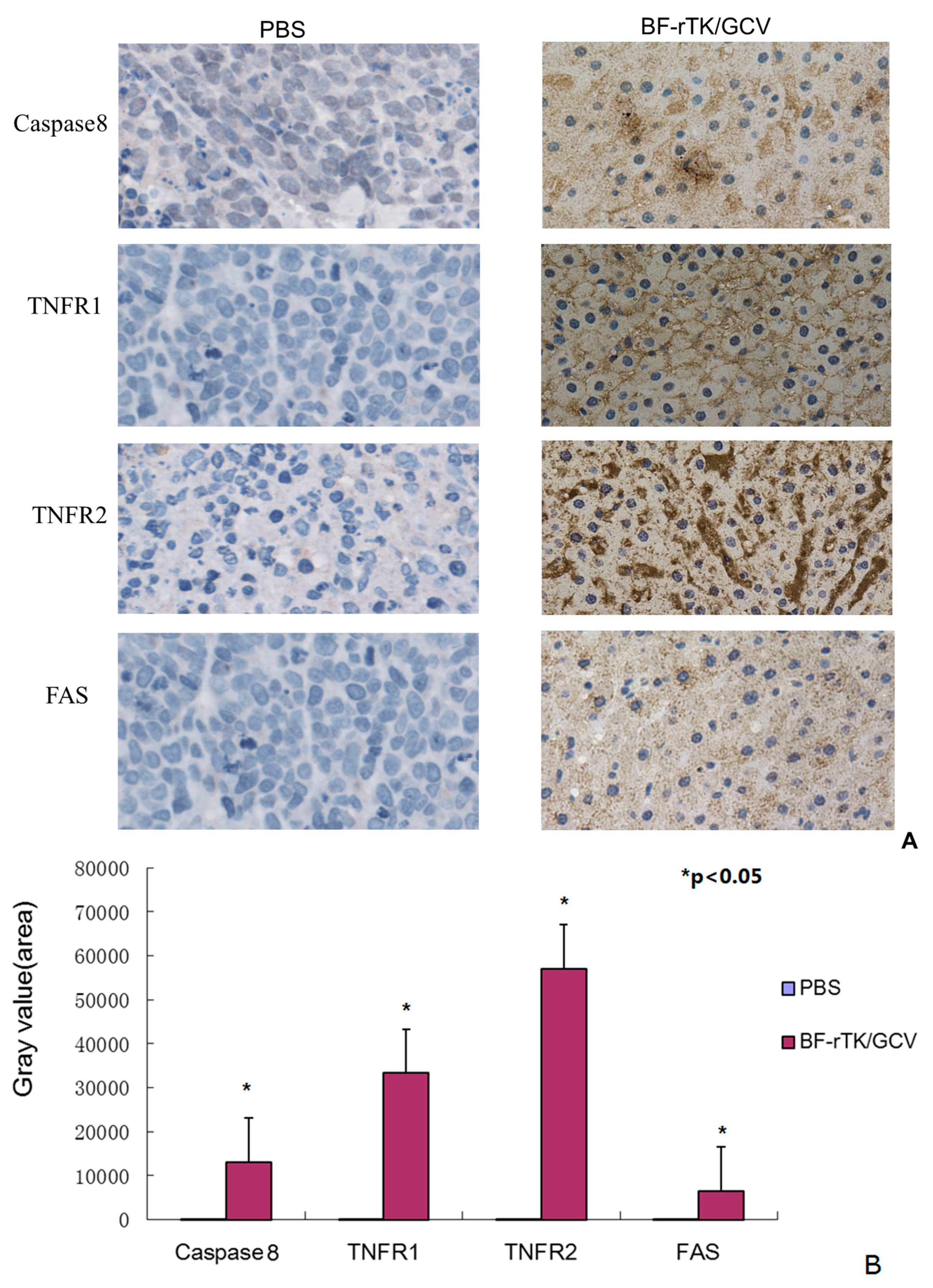
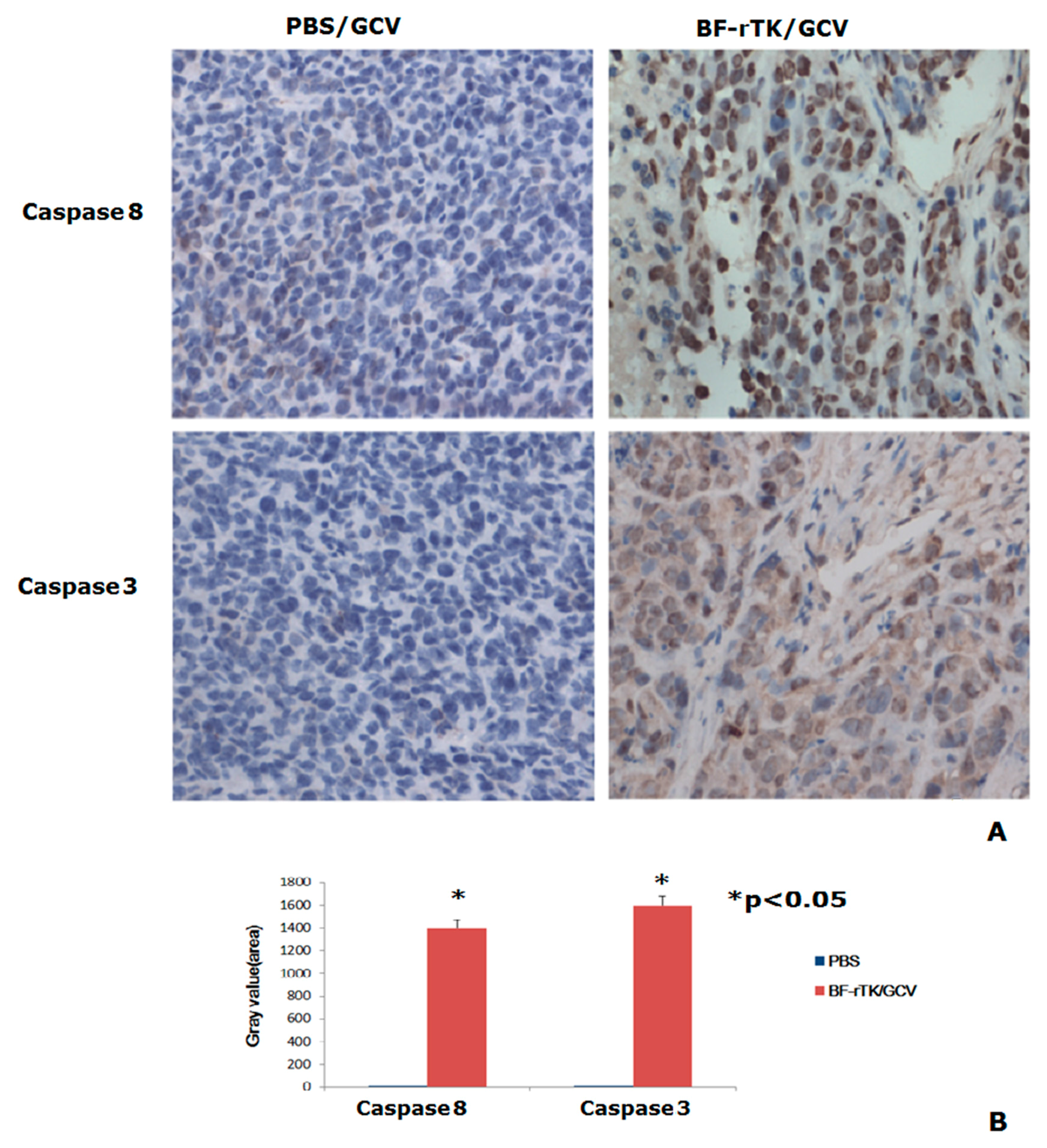
2.5. BF-rTK/GCV Significantly Upregulates Apoptosis-Associated Molecules Expression
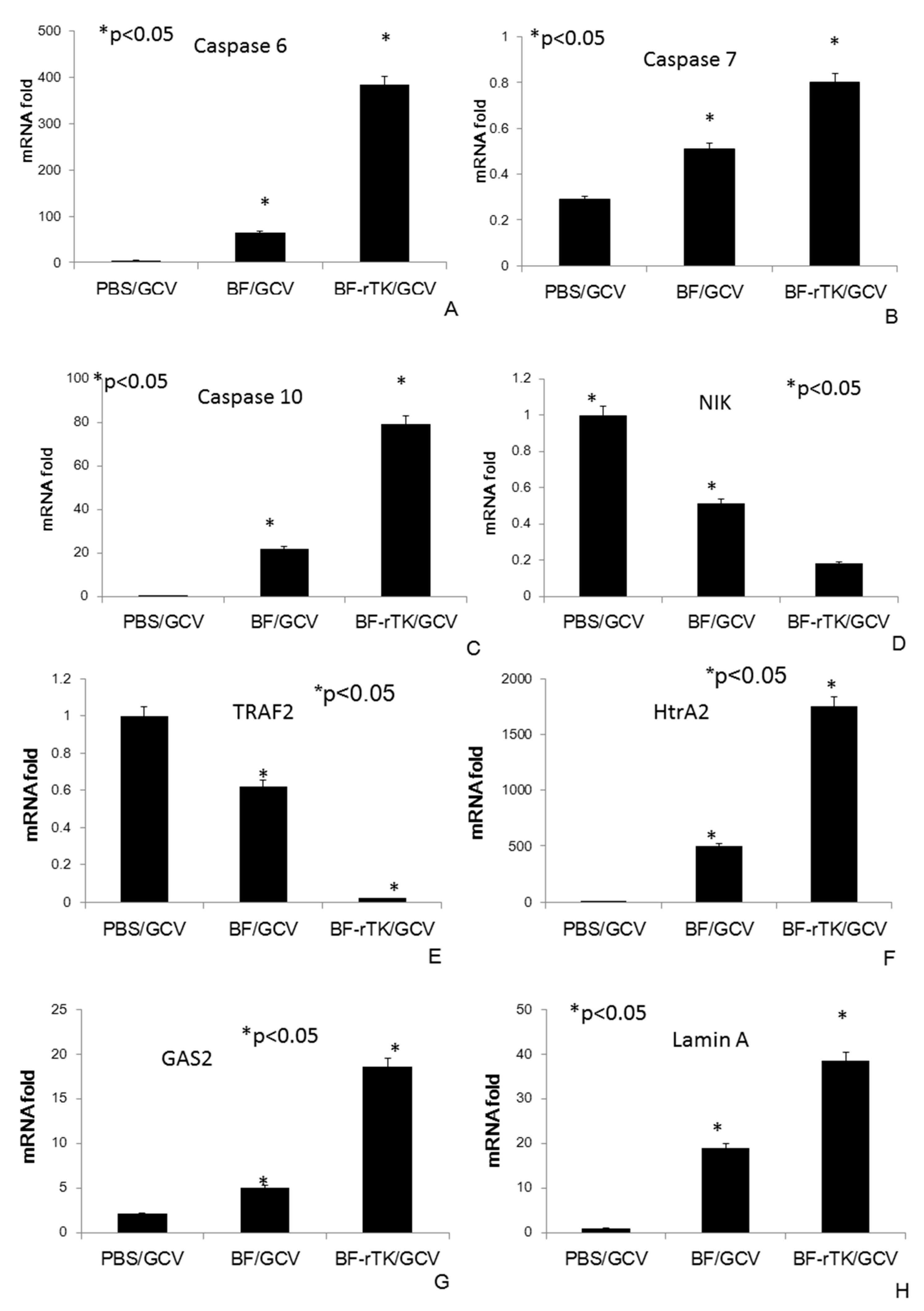
2.6. BF-rTK Regulates Caspase 8 Signaling Apoptosis-Associated Downstream Molecules in Intestinal Cancer
3. Discussion
4. Experimental Section
4.1. Construction of the BF-rTK Gene Therapy System
4.2. Experimental Animals
4.3. Cells and Cell Culture
4.4. Evaluation of the Safety of the BF-rTK/GCV System by a Cytokine Profile Assay
4.5. Immunohistochemical (IHC) Detection of Toll-Like Receptors Upregulated by BF-rTK/GCV Treatment
4.6. Establishment of Xenograft Tumor Models and Assessment of the Efficacy of Inhibition Tumor Growth by BF-rTK/GCV Treatment
4.7. IHC Detection of Apoptosis-Associated Molecular Markers Activated by BF-rTK/GCV Treatment
4.8. RNA Isolation and Quantitative RT-PCR
4.9. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tu, H.; Sun, L.; Dong, X.; Gong, Y.; Xu, Q.; Jing, J.; Long, Q.; Flanders, W.D.; Bostick, R.M.; Yuan, Y. Temporal changes in serum biomarkers and risk for progression of gastric precancerous lesions: A longitudinal study. Int. J. Cancer 2015, 136, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, J.; Wang, Z.; Yu, C.; Zhang, Z.P. Effectiveness of local injection of lentivirus-delivered stathmin1 and stathmin1 shrna in human gastric cancer xenograft mouse. J. Gastroenterol. Hepatol. 2014, 29, 1685–1691. [Google Scholar] [CrossRef] [PubMed]
- Higashi, K.; Hazama, S.; Araki, A.; Yoshimura, K.; Iizuka, N.; Yoshino, S.; Noma, T.; Oka, M. A novel cancer vaccine strategy with combined IL-18 and HSV-TK gene therapy driven by the hTERT promoter in a murine colorectal cancer model. Int. J. Oncol. 2014, 45, 1412–1420. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Huang, L. Recent advances in nonviral vectors for gene delivery. Acc. Chem. Res. 2012, 45, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Z.; Gemeinhart, R.A. Progress in microRNA delivery. J. Control. Release 2013, 172, 962–974. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.F. The death of Jesse Gelsinger: New evidence of the influence of money and prestige in human research. Am. J. Law Med. 2010, 36, 295–325. [Google Scholar] [PubMed]
- Stolberg, S.G. The biotech death of Jesse Gelsinger. N. Y. Times Mag. 1999, 28, 136–140. [Google Scholar]
- Cronin, M.; Stanton, R.M.; Francis, K.P.; Tangney, M. Bacterial vectors for imaging and cancer gene therapy: A review. Cancer Gene Ther. 2012, 19, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.F.; Zhu, L.P.; Hu, B.; Fu, G.F.; Zhang, H.Y.; Wang, J.J.; Xu, G.X. A new expression plasmid in Bifidobacterium longum as a delivery system of endostatin for cancer gene therapy. Cancer Gene Ther. 2007, 14, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; He, Y.; Zhou, S.; Ma, Y.; Liu, G. A novel Bifidobacterium infantis-mediated TK/GCV suicide gene therapy system exhibits antitumor activity in a rat model of bladder cancer. J. Exp. Clin. Cancer Res. 2009, 28. [Google Scholar] [CrossRef] [PubMed]
- Kieback, D.G. Adenovirus-mediated thymidine kinase gene therapy and coxsackie adenovirus receptor expression in ovarian cancer cells. Cancer Genom. Proteom. 2008, 5, 311–318. [Google Scholar]
- Yao, X.L.; Nakagawa, S.; Gao, J.Q. Current targeting strategies for adenovirus vectors in cancer gene therapy. Curr. Cancer Drug Targets 2011, 11, 810–825. [Google Scholar] [CrossRef] [PubMed]
- Guan, G.F.; Zhao, M.; Liu, L.M.; Jin, C.S.; Sun, K.; Zhang, D.J.; Yu, D.J.; Cao, H.W.; Lu, Y.Q.; Wen, L.J. Salmonella typhimurium mediated delivery of apoptin in human laryngeal cancer. Int. J. Med. Sci. 2013, 10, 1639–1648. [Google Scholar] [CrossRef] [PubMed]
- Zaiss, A.K.; Muruve, D.A. Immunity to adeno-associated virus vectors in animals and humans: A continued challenge. Gene Ther. 2008, 15, 808–816. [Google Scholar] [CrossRef] [PubMed]
- Suerth, J.D.; Labenski, V.; Schambach, A. Alpharetroviral vectors: From a cancer-causing agent to a useful tool for human gene therapy. Viruses 2014, 6, 4811–4838. [Google Scholar] [CrossRef] [PubMed]
- Gregory, S.M.; Nazir, S.A.; Metcalf, J.P. Implications of the innate immune response to adenovirus and adenoviral vectors. Future Virol. 2011, 6, 357–374. [Google Scholar] [CrossRef] [PubMed]
- Marshall, E. Gene therapy death prompts review of adenovirus vector. Science 1999, 286, 2244–2245. [Google Scholar] [CrossRef] [PubMed]
- Koski, G. The clinical research process: Building a system in harmony with its users. Cancer Treat. Res. 2007, 132, 275–290. [Google Scholar] [PubMed]
- Karlsson, H.; Hessle, C.; Rudin, A. Innate immune responses of human neonatal cells to bacteria from the normal gastrointestinal flora. Infect. Immun. 2002, 70, 6688–6696. [Google Scholar] [CrossRef] [PubMed]
- Ventura, M.; Turroni, F.; Lugli, G.A.; van Sinderen, D. Bifidobacteria and humans: Our special friends, from ecological to genomics perspectives. J. Sci. Food Agric. 2014, 94, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Fu, G.F.; Fan, Y.R.; Liu, W.H.; Liu, X.J.; Wang, J.J.; Xu, G.X. Bifidobacterium adolescentis as a delivery system of endostatin for cancer gene therapy: Selective inhibitor of angiogenesis and hypoxic tumor growth. Cancer Gene Ther. 2003, 10, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Wang, R.R.; Wang, Y.; Wang, J.J.; Xu, G.X. Preparation of selenium-enriched Bifidobacterium longum and its effect on tumor growth and immune function of tumor-bearing mice. Asian Pac. J. Cancer Prev. 2014, 15, 3681–3686. [Google Scholar] [CrossRef] [PubMed]
- Yazawa, K.; Fujimori, M.; Nakamura, T.; Sasaki, T.; Amano, J.; Kano, Y.; Taniguchi, S. Bifidobacterium longum as a delivery system for gene therapy of chemically induced rat mammary tumors. Breast Cancer Res. Treat. 2001, 66, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Lagerlöf, J.H.; Kindblom, J.; Bernhardt, P. Oxygen distribution in tumors: A qualitative analysis and modeling study providing a novel monte carlo approach. Med. Phys. 2014, 41. [Google Scholar] [CrossRef] [PubMed]
- Fukiya, S.; Hirayama, Y.; Sakanaka, M.; Kano, Y.; Yokota, A. Technological advances in bifidobacterial molecular genetics: Application to functional genomics and medical treatments. Biosci. Microbiota Food Health 2012, 31, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Tangney, M. Gene therapy for cancer: Dairy bacteria as delivery vectors. Discov. Med. 2010, 10, 195–200. [Google Scholar] [PubMed]
- Xiao, X.; Jin, R.; Li, J.; Bei, Y.; Wei, T. The antitumor effect of suicide gene therapy using Bifidobacterium infantis-mediated herpes simplex virus thymidine kinase/ganciclovir in a nude mice model of renal cell carcinoma. Urology 2014, 84, 982.e915–982.e20. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, S.; Shimatani, Y.; Fujimori, M. Tumor-targeting therapy using gene-engineered anaerobic-nonpathogenic Bifidobacterium longum. Methods Mol. Biol. 2016, 1409, 49–60. [Google Scholar] [PubMed]
- Wei, C.; Xun, A.Y.; Wei, X.X.; Yao, J.; Wang, J.Y.; Shi, R.Y.; Yang, G.H.; Li, Y.X.; Xu, Z.L.; Lai, M.G.; et al. Bifidobacteria expressing tumstatin protein for antitumor therapy in tumor-bearing mice. Technol. Cancer Res. Treat. 2015. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Xiao, X.; Ren, J.; Tang, Y.; Weng, H.; Yang, Q.; Wu, M.; Tang, W. Proteomic analysis of bladder cancer indicates Prx-I as a key molecule in BI-TK/GCV treatment system. PLoS ONE 2014, 9, e98764. [Google Scholar] [CrossRef] [PubMed]
- Raper, S.E.; Chirmule, N.; Lee, F.S.; Wivel, N.A.; Bagg, A.; Gao, G.P.; Wilson, J.M.; Batshaw, M.L. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol. Genet. Metab. 2003, 80, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.M. Lessons learned from the gene therapy trial for ornithine transcarbamylase deficiency. Mol. Genet. Metab. 2009, 96, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Falck-Pedersen, E.; Elkon, K.B. Variation in adenovirus transgene expression between BALB/c and C57BL/6 mice is associated with differences in interleukin-12 and gamma interferon production and NK cell activation. J. Virol. 2001, 75, 4540–4550. [Google Scholar] [CrossRef] [PubMed]
- Mistchenko, A.S.; Diez, R.A.; Mariani, A.L.; Robaldo, J.; Maffey, A.F.; Bayley-Bustamante, G.; Grinstein, S. Cytokines in adenoviral disease in children: Association of interleukin-6, interleukin-8, and tumor necrosis factor alpha levels with clinical outcome. J. Pediatr. 1994, 124, 714–720. [Google Scholar] [CrossRef]
- Elson, G.; Dunn-Siegrist, I.; Daubeuf, B.; Pugin, J. Contribution of toll-like receptors to the innate immune response to gram-negative and gram-positive bacteria. Blood 2007, 109, 1574–1583. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Imai, Y.; Nakayama, H.; Takahashi, K.; Takio, K.; Takahashi, R. A serine protease, HtrA2, is released from the mitochondria and interacts with XIAP, inducing cell death. Mol. Cell 2001, 8, 613–621. [Google Scholar] [CrossRef]
- Bray, F.; Jemal, A.; Grey, N.; Ferlay, J.; Forman, D. Global cancer transitions according to the human development index (2008–2030): A population-based study. Lancet Oncol. 2012, 13, 790–801. [Google Scholar] [CrossRef]
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer statistics, 2013. CA Cancer J. Clin. 2013, 63, 11–30. [Google Scholar] [CrossRef] [PubMed]
- Sekine, K.; Ohta, J.; Onishi, M.; Tatsuki, T.; Shimokawa, Y.; Toida, T.; Kawashima, T.; Hashimoto, Y. Analysis of antitumor properties of effector cells stimulated with a cell wall preparation (WPG) of Bifidobacterium infantis. Biol. Pharm. Bull. 1995, 18, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Cronin, M.; Akin, A.R.; Collins, S.A.; Meganck, J.; Kim, J.B.; Baban, C.K.; Joyce, S.A.; van Dam, G.M.; Zhang, N.; van Sinderen, D.; et al. High resolution in vivo bioluminescent imaging for the study of bacterial tumour targeting. PLoS ONE 2012, 7, e30940. [Google Scholar] [CrossRef] [PubMed]
- Somia, N.; Verma, I.M. Gene therapy: Trials and tribulations. Nat. Rev. Genet. 2000, 1, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Calcedo, R.; Vandenberghe, L.H.; Roy, S.; Somanathan, S.; Wang, L.; Wilson, J.M. Host immune responses to chronic adenovirus infections in human and nonhuman primates. J. Virol. 2009, 83, 2623–2631. [Google Scholar] [CrossRef] [PubMed]
- Bermudez-Brito, M.; Munoz-Quezada, S.; Gomez-Llorente, C.; Matencio, E.; Bernal, M.J.; Romero, F.; Gil, A. Cell-free culture supernatant of Bifidobacterium breve CNCM I-4035 decreases pro-inflammatory cytokines in human dendritic cells challenged with Salmonella typhi through TLR activation. PLoS ONE 2013, 8, e59370. [Google Scholar]
- Bai, A.P.; Ouyang, Q.; Xiao, X.R.; Li, S.F. Probiotics modulate inflammatory cytokine secretion from inflamed mucosa in active ulcerative colitis. Int. J. Clin. Pract. 2006, 60, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Saharinen, P.; Eklund, L.; Pulkki, K.; Bono, P.; Alitalo, K. VEGF and angiopoietin signaling in tumor angiogenesis and metastasis. Trends Mol. Med. 2011, 17, 347–362. [Google Scholar] [CrossRef] [PubMed]
- Pinter, M.; Sieghart, W.; Schmid, M.; Dauser, B.; Prager, G.; Dienes, H.P.; Trauner, M.; Peck-Radosavljevic, M. Hedgehog inhibition reduces angiogenesis by downregulation of tumoral VEGF-A expression in hepatocellular carcinoma. United Eur. Gastroenterol. J. 2013, 1, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Gollmer, J.C.; Ladoux, A.; Gioanni, J.; Paquis, P.; Dubreuil, A.; Chatel, M.; Frelin, C. Expression of vascular endothelial growth factor-b in human astrocytoma. Neuro Oncol. 2000, 2, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Luo, Y.; Huang, X.; Song, F.; Liu, G. Construction of Bifidobacterium infantis as a live oral vaccine that expresses antigens of the major fimbrial subunit (CfaB) and the B subunit of heat-labile enterotoxin (LTB) from enterotoxigenic Escherichia coli. Microbiology 2012, 158, 498–504. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, H.; He, Z.; Wang, C.; Xie, T.; Liu, L.; Liu, C.; Song, F.; Ma, Y. Intravenous Administration Is an Effective and Safe Route for Cancer Gene Therapy Using the Bifidobacterium-Mediated Recombinant HSV-1 Thymidine Kinase and Ganciclovir. Int. J. Mol. Sci. 2016, 17, 891. https://doi.org/10.3390/ijms17060891
Zhou H, He Z, Wang C, Xie T, Liu L, Liu C, Song F, Ma Y. Intravenous Administration Is an Effective and Safe Route for Cancer Gene Therapy Using the Bifidobacterium-Mediated Recombinant HSV-1 Thymidine Kinase and Ganciclovir. International Journal of Molecular Sciences. 2016; 17(6):891. https://doi.org/10.3390/ijms17060891
Chicago/Turabian StyleZhou, Huicong, Zhiliang He, Changdong Wang, Tingting Xie, Lin Liu, Chuanyang Liu, Fangzhou Song, and Yongping Ma. 2016. "Intravenous Administration Is an Effective and Safe Route for Cancer Gene Therapy Using the Bifidobacterium-Mediated Recombinant HSV-1 Thymidine Kinase and Ganciclovir" International Journal of Molecular Sciences 17, no. 6: 891. https://doi.org/10.3390/ijms17060891
APA StyleZhou, H., He, Z., Wang, C., Xie, T., Liu, L., Liu, C., Song, F., & Ma, Y. (2016). Intravenous Administration Is an Effective and Safe Route for Cancer Gene Therapy Using the Bifidobacterium-Mediated Recombinant HSV-1 Thymidine Kinase and Ganciclovir. International Journal of Molecular Sciences, 17(6), 891. https://doi.org/10.3390/ijms17060891





