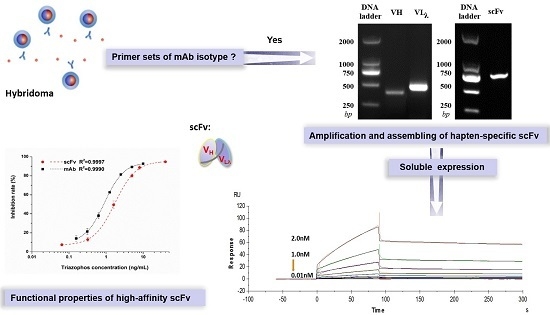
Expression and Functional Properties of an Anti-Triazophos High-Affinity Single-Chain Variable Fragment Antibody with Specific Lambda Light Chain
Abstract
:1. Introduction
2. Results
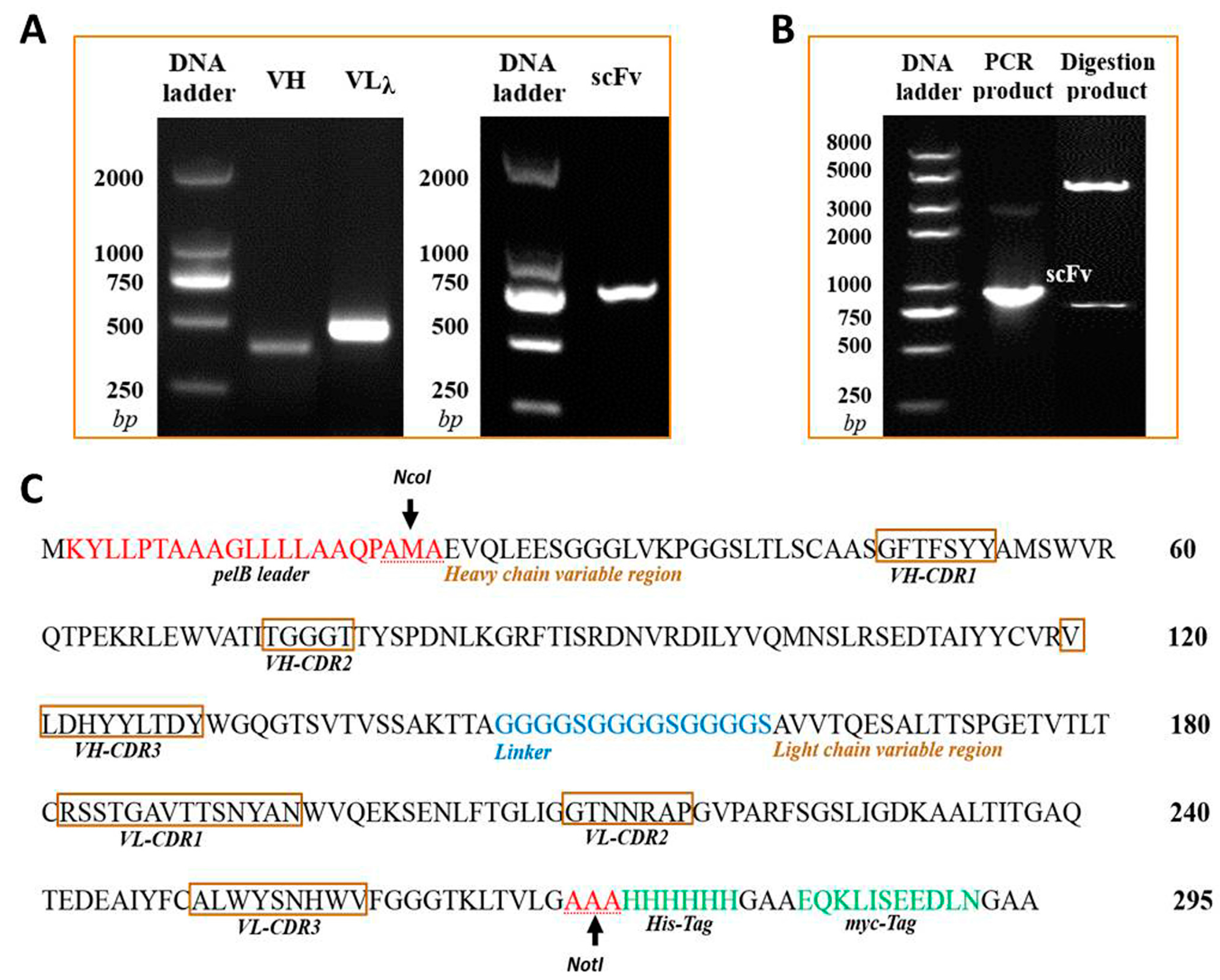
2.1. Cloning the VH and VLλ Genes of mAb-8C10 from Hybridoma
2.2. Constructing the Anti-Triazophos scFv-8C10 Fragment and the PIT2-scFv-8C10 Expression Vector
2.3. Expression and Purification of a Soluble Anti-Triazophos scFv-8C10 Antibody
2.4. Confirmation of the Anti-Triazophos scFv-8C10 Antibody
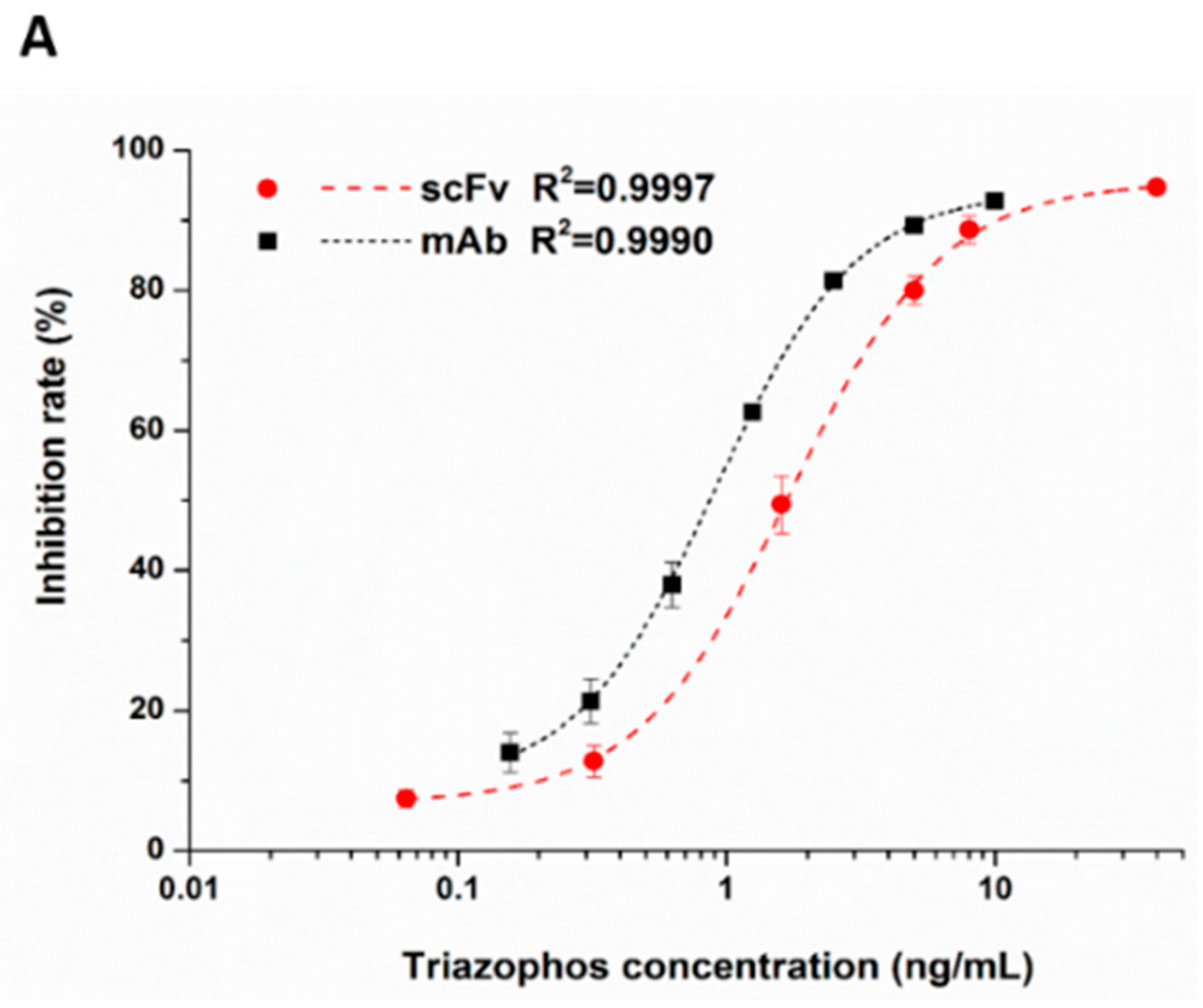
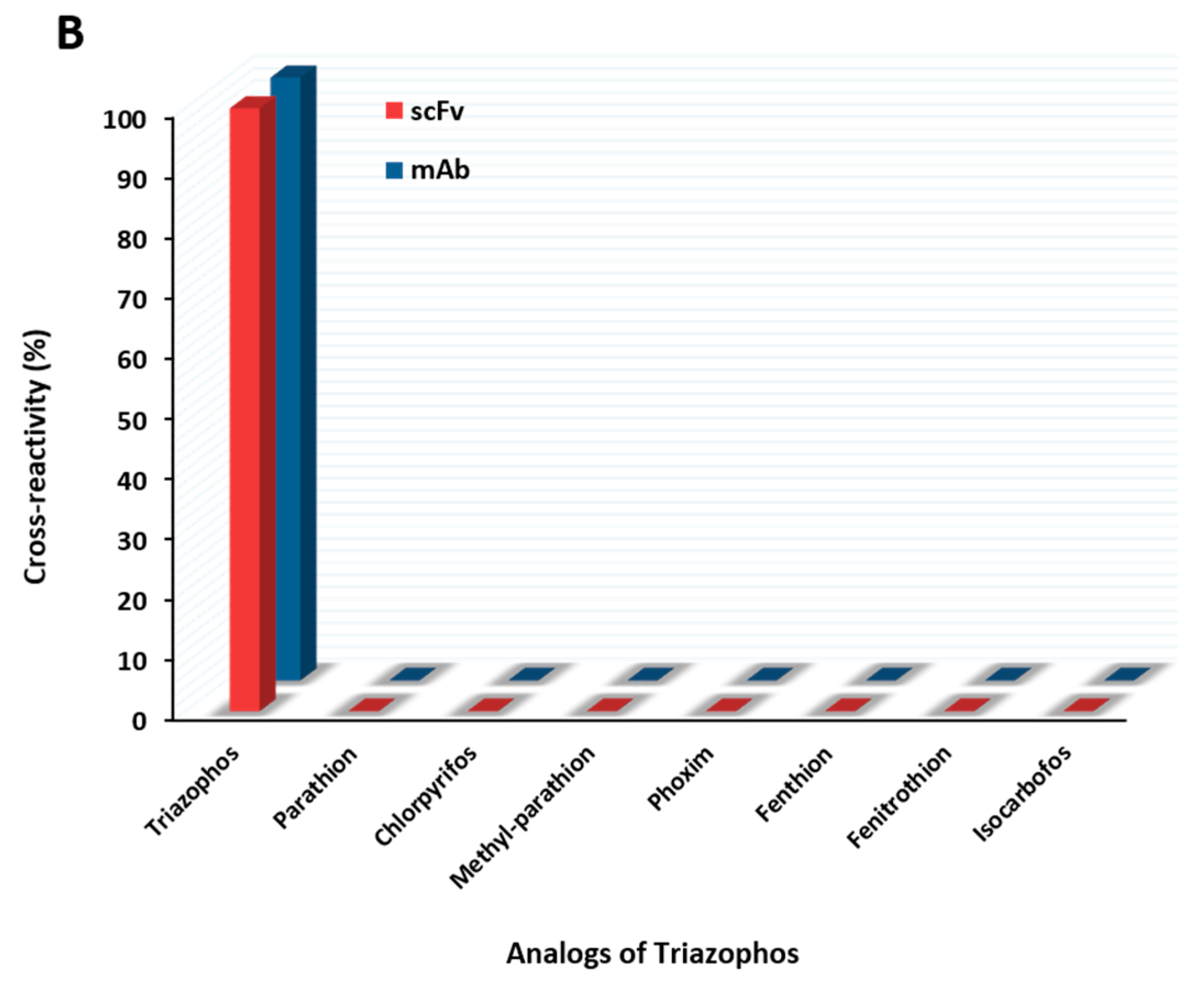
2.5. Functional Properties of the Anti-Triazophos scFv-8C10 Antibody
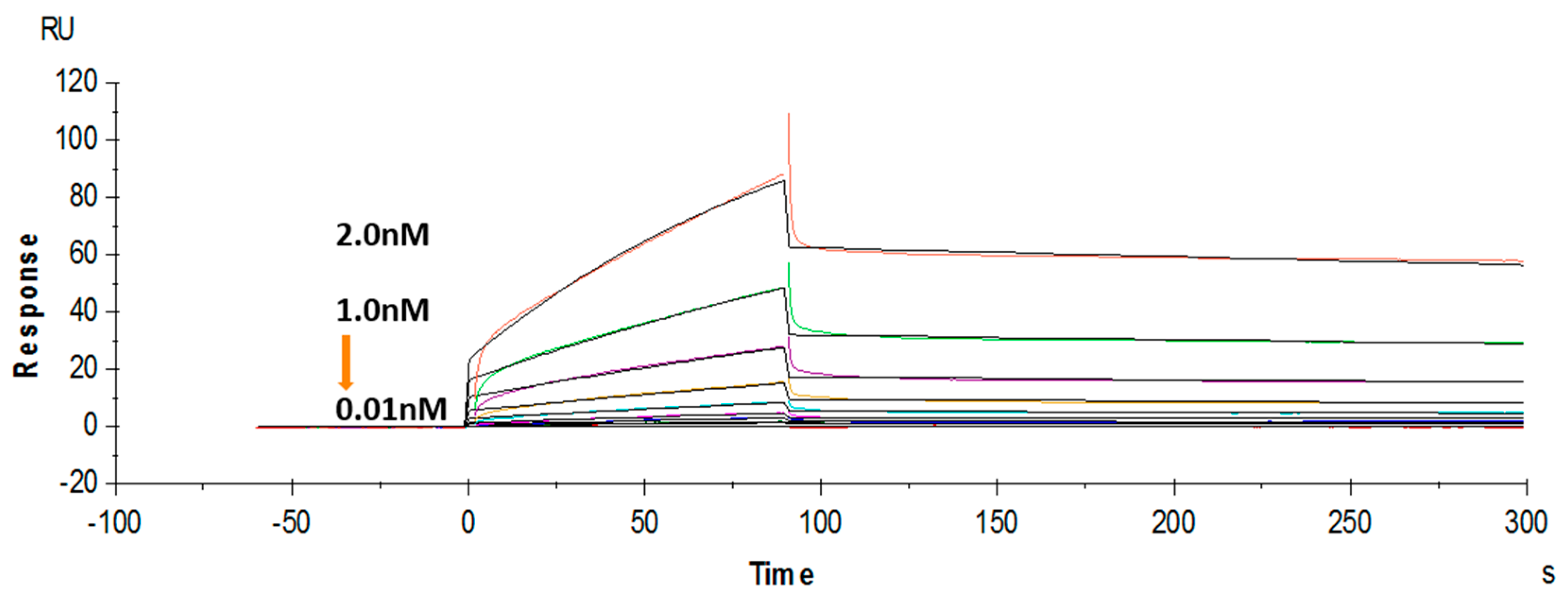
2.6. Affinity Measurement via Surface Plasmon Resonance (SPR)
2.7. Analysis of Spiked Water Samples
3. Discussion
4. Materials and Methods
4.1. Reagents and Materials
4.2. Cloning of VH and VLλ Genes from Hybridoma
4.3. Construction of the Anti-Triazophos scFv-8C10 Fragment and the PIT2-scFv-8C10 Expression Vector
4.4. Expression and Purification of the Soluble Anti-Triazophos scFv-8C10 Antibody
4.5. Protein Confirmation
4.6. Ic-ELISA
4.7. SPR Measurement
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| OPPs | Organophosphorous pesticides |
| IgG | immunoglobulin G |
| mAb | monoclonal antibody |
| scFv | single-chain variable fragment |
| VH, VL | variable heavy region, variable light region |
| CDRs | complementarity-determining regions |
| SOE-PCR | splicing by overlap extension polymerase chain reaction |
| IMAC | immobilized metal ion affinity chromatography |
| ic-ELISA | indirect competitive enzyme-linked immunosorbent assay |
| SPR | surface plasmon resonance |
| CV | coefficient of variation |
References
- Cabello, G.; Juarranz, A.; Botella, L.M.; Calaf, G.M. Organophosphorous pesticides in breast cancer progression. J. Submicr. Cytol. Pathol. 2003, 35, 1–9. [Google Scholar]
- Mostafalou, S.; Abdollahi, M. Pesticides and human chronic diseases: Evidences, mechanisms, and perspectives. Toxicol. Appl. Pharm. 2013, 268, 157–177. [Google Scholar] [CrossRef] [PubMed]
- Mahboob, S.; Niazi, F.; Alghanim, K.; Sultana, S.; Al-Misned, F.; Ahmed, Z. Health risks associated with pesticide residues in water, sediments and the muscle tissues of Catla catla at Head Balloki on the River Ravi. Environ. Monit. Assess. 2015, 187, 81. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Gong, Y.X.; Liu, L.; Li, D.L.; Wang, Y.; Ling, F.; Wang, G.X. Toxic effects of triazophos on rare minnow (Gobiocypris rarus) embryos and larvae. Chemosphere 2014, 108, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Sangha, G.K.; Khera, K.S. Triazophos-induced oxidative stress and histomorphological changes in ovary of female Wistar rats. Pestic. Biochem. Phys. 2015, 117, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Banerjee, B.D.; Ahmed, R.S.; Arora, V.K.; Mediratta, P.K. Possible role of oxidative stress and brain derived neurotrophic factor in triazophos induced cognitive impairment in rats. Neurochem. Res. 2013, 38, 2136–2147. [Google Scholar] [CrossRef] [PubMed]
- Hayward, D.G.; Wong, J.W.; Park, H.Y. Determinations for pesticides on Black, Green, Oolong, and White Teas by gas chromatography triple-quadrupole mass spectrometry. J. Agric. Food Chem. 2015, 63, 8116–8124. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Liu, X.; Hu, J.; Zhao, X.; Wang, H.; Wang, X. Application of dispersive liquid-liquid microextraction for the analysis of triazophos and carbaryl pesticides in water and fruit juice samples. Anal. Chim. Acta 2009, 632, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Du, D.; Cai, J.; Song, D.D.; Zhang, A.D. Rapid determination of triazophos using acetylcholinesterase biosensor based on sol–gel interface assembling muldwall carbon nanotubes. J. Appl. Electrochem. 2007, 37, 893–898. [Google Scholar] [CrossRef]
- Jin, M.; Shao, H.; Jin, F.; Gui, W.; Shi, X.; Wang, J.; Zhu, G. Enhanced competitive chemiluminescent enzyme immunoassay for the trace detection of insecticide triazophos. J. Food Sci. 2012, 77, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Gui, W.J.; Jin, R.Y.; Chen, Z.L.; Cheng, J.L.; Zhu, G.N. Hapten synthesis for enzyme-linked immunoassay of the insecticide triazophos. Anal. Biochem. 2006, 357, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Du, P.; Jin, M.; Yang, L.; Du, X.; Chen, G.; Zhang, C.; Jin, F.; Shao, H.; She, Y.; Wang, S.; et al. A rapid immunomagnetic-bead-based immunoassay for triazophos analysis. RSC Adv. 2015, 5, 81046–81051. [Google Scholar] [CrossRef]
- Guo, Y.; Tian, J.; Liang, C.; Zhu, G.; Gui, W. Multiplex bead-array competitive immunoassay for simultaneous detection of three pesticides in vegetables. Microchim. Acta 2013, 180, 387–395. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, S.; Gui, W.; Zhu, G. Gold immunochromatographic assay for simultaneous detection of carbofuran and triazophos in water samples. Anal. Biochem. 2009, 389, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Gai, L.; Ye, Z.; Wang, J. Piezoelectric immunosensor for rapid determination of triazophos pesticide. Chin. J. Anal. Chem. 2010, 38, 1483–1486. [Google Scholar]
- Emanuel, P.A.; Dang, J.; Gebhardt, J.S.; Aldrich, J.; Garber, E.A.E.; Kulaga, H.; Stopa, P.; Valdes, J.J.; Dion-Schultz, A. Recombinant antibodies: A new reagent for biological agent detection. Biosens. Bioelectron. 2000, 14, 751–759. [Google Scholar] [CrossRef]
- Li, X.; Li, P.; Zhang, Q.; Li, Y.; Zhang, W.; Ding, X. Molecular characterization of monoclonal antibodies against aflatoxins: A possible explanation for the highest sensitivity. Anal. Chem. 2012, 84, 5229–5235. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, G.M.; Garrett, S.D.; Lee, H.A.; Morgan, M.R.A. Alteration of the binding characteristics of a recombinant scFv anti-parathion antibody—1. Mutagenesis targeted at the V(H) CDR3 domain. Food Agric. Immunol. 1999, 11, 207–218. [Google Scholar] [CrossRef]
- Karsunke, X.Z.; Wang, H.; Weber, E.; Mclean, M.; Niessner, R.; Hall, J.C.; Knopp, D. Development of single-chain variable fragment (scFv) antibodies against hapten benzo[a]pyrene: A binding study. Anal. Bioanal. Chem. 2012, 402, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Xu, Y.; Li, Y.; He, Q.; Chen, B.; Wang, D. Development of a single-chain variable fragment antibody-based enzyme-linked immunosorbent assay for determination of fumonisin B1 in corn samples. J. Sci. Food Agric. 2014, 94, 1865–1871. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, Y.; Zhang, C.; Wang, Y.; Xu, C.; Liu, X. Rapid isolation of single-chain antibodies from a human synthetic phage display library for detection of Bacillus thuringiensis (Bt) Cry1B toxin. Ecotoxicol. Environ. Saf. 2012, 81, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, P.; Lei, J.; Zhang, Q.; Zhang, W.; Li, C. A simple strategy to obtain ultra-sensitive single-chain fragment variable antibodies for aflatoxin detection. RSC Adv. 2013, 3, 22367–22372. [Google Scholar] [CrossRef]
- Garrett, S.D.; Appleford, D.J.A.; Wyatt, G.M.; Lee, H.A.; Morgan, M.R.A. Production of a recombinant anti-parathion antibody (scFv); Stability in methanolic food extracts and comparison to an anti-parathion monoclonal antibody. J. Agric. Food Chem. 1997, 45, 4183–4189. [Google Scholar] [CrossRef]
- Alcocer, M.J.C.; Doyen, C.; Lee, H.A.; Morgan, M.R.A. Properties of polyclonal, monoclonal, and recombinant antibodies recognizing the organophosphorus pesticide chlorpyrifos-ethyl. J. Agric. Food Chem. 2000, 48, 4053–4059. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Xia, Y. Selection of single-chain variable fragment antibodies against fenitrothion by ribosome display. Anal. Biochem. 2012, 421, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Huang, Z.; Wang, L.; Liu, X. Construction of a single chain variable fragment antibody (scFv) against carbaryl and its interaction with carbaryl. Biochem.-Moscow. 2015, 80, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Moreno, M.; Plana, E.; Manclús, J.; Montoya, A. Comparative study of monoclonal and recombinant antibody-based immunoassays for fungicide analysis in fruit juices. Food Anal. Method. 2014, 7, 481–489. [Google Scholar] [CrossRef]
- Xu, Z.L.; Dong, J.X.; Wang, H.; Li, Z.F.; Beier, R.C.; Jiang, Y.M.; Lei, H.T.; Shen, Y.D.; Yang, J.Y.; Sun, Y.M. Production and characterization of a single-chain variable fragment linked alkaline phosphatase fusion protein for detection of O,O-diethyl organophosphorus pesticides in a one-step enzyme-linked immunosorbent assay. J. Agric. Food Chem. 2012, 60, 5076–5083. [Google Scholar] [CrossRef] [PubMed]
- Krebber, A.; Bornhauser, S.; Burmester, J.; Honegger, A.; Willuda, J.; Bosshard, H.R.; Plückthun, A. Reliable cloning of functional antibody variable domains from hybridomas and spleen cell repertoires employing a reengineered phage display system. J. Immunol. Methods 1997, 201, 35–55. [Google Scholar] [CrossRef]
- Juste, M.; Muzard, J.; Billiald, P. Cloning of the antibody κ light chain V-gene from murine hybridomas by bypassing the aberrant MOPC21-derived transcript. Anal. Biochem. 2006, 349, 159–161. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Liu, Y.; Lan, M.; Taheri, N.; Cheng, J.L.; Guo, Y.; Zhu, G. Evaluation of a water-soluble adjuvant for the development of monoclonal antibodies against small-molecule compounds. J. Zhejiang Univ. SCI B 2016, 17, 282–293. [Google Scholar] [CrossRef]
- Brady, J.L.; Corbett, A.J.; Mckenzie, B.S.; Lew, A.M. Rapid specific amplification of rat antibody cDNA from nine hybridomas in the presence of myeloma light chains. J. Immunol. Methods 2006, 315, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Cochet, O.; Kenigsberg, M.; Delumeau, I.; Duchesne, M.; Schweighoffer, F.; Tocqué, B.; Teillaud, J.L. Intracellular expression and functional properties of an anti-p21Ras scFv derived from a rat hybridoma containing specific λ and irrelevant κ light chains. Mol. Immunol. 1998, 35, 1097–1110. [Google Scholar] [CrossRef]
- Le, Y.; Peng, J.; Wu, H.; Sun, J.; Shao, W. An approach to the production of soluble protein from a fungal gene encoding an aggregation-prone xylanase in Escherichia coli. PLoS ONE 2011, 6, e18489. [Google Scholar] [CrossRef] [PubMed]
- Sahdev, S.; Khattar, S.K.; Saini, K.S. Production of active eukaryotic proteins through bacterial expression systems: A review of the existing biotechnology strategies. Mol. Cell. Biochem. 2008, 307, 249–264. [Google Scholar] [CrossRef] [PubMed]
- Studier, F.W. Protein production by auto-induction in high-density shaking cultures. Protein Exp. Purif. 2005, 41, 207–234. [Google Scholar] [CrossRef]
- Sharma, S.K.; Suresh, M.R.; Wuest, F.R. Improved soluble expression of a single-chain antibody fragment in E. coli for targeting CA125 in epithelial ovarian cancer. Protein Exp. Purif. 2014, 102, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.; Sankhyan, A.; Khanna, N.; Sinha, S. Enhanced periplasmic expression of high affinity humanized scFv against hepatitis B surface antigen by codon optimization. Protein Exp. Purif. 2010, 74, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Kramer, K.; Hock, B. Recombinant antibodies for environmental analysis. Anal. Bioanal. Chem. 2003, 377, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Maragos, C.M.; Li, L.; Chen, D. Production and characterization of a single chain variable fragment (scFv) against the mycotoxin deoxynivalenol. Food Agric. Immunol. 2012, 23, 51–67. [Google Scholar] [CrossRef]
- Munoz, E.M.; Lorenzo-Abalde, S.; González-Fernández, Á.; Quintela, O.; Lopez-Rivadulla, M.; Riguera, R. Direct surface plasmon resonance immunosensor for in situ detection of benzoylecgonine, the major cocaine metabolite. Biosens. Bioelectron. 2011, 26, 4423–4428. [Google Scholar] [CrossRef] [PubMed]
- Kou, G.; Shi, S.; Wang, H.; Tan, M.; Xue, J.; Zhang, D.; Hou, S.; Qian, W.; Wang, S.; Dai, J.; et al. Preparation and characterization of recombinant protein ScFv(CD11c)-TRP2 for tumor therapy from inclusion bodies in Escherichia coli. Protein Exp. Purif. 2007, 52, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Raifu, M.; Howard, M.; Smith, L.; Hansen, D.; Goldsby, R.; Ratner, D. Universal PCR amplification of mouse immunoglobulin gene variable regions: The design of degenerate primers and an assessment of the effect of DNA polymerase 3′ to 5′ exonuclease activity. J. Immunol. Methods 2000, 233, 167–177. [Google Scholar] [CrossRef]

| Peptide No. | Medium Fraction | Periplasm Fraction | Regions |
|---|---|---|---|
| 1 | LEWVATITGGGTTYSPDNLKGR | LEWVATITGGGTTYSPDNLKGR | VH-FR2-CDR2 |
| 2 | DILYVQMNSLR | DILYVQMNSLR | VH-FR3 |
| 3 | VLDHYYLTDYWGQGTSVTVSSAK | – 1 | VH-CDR3-FR4 |
| 4 | SSTGAVTTSNYANWVQEK | SSTGAVTTSNYANWVQEK | VLλ-CDR1-FR2 |
| 5 | SENLFTGLIGGTNNR | SENLFTGLIGGTNNR | VLλ-FR2-CDR2 |
| 6 | FSGSLIGDK | FSGSLIGDK | VLλ-FR3 |
| 7 | – 1 | LTVLGAAAHHHHHHGAAEQK | VLλ-FR4-His-tag |
| 8 | LISEEDLNGAA | LISEEDLNGAA | myc-tag |
| Sample | Triazophos Added (ng/mL) | scFv-Based ic-ELISA | mAb-Based ic-ELISA | ||
|---|---|---|---|---|---|
| Recovery (%) | CV (%) | Recovery (%) | CV (%) | ||
| Tap water | 1.0 | 90 | 8.8 | 96 | 6.8 |
| 2.5 | 84 | 5.2 | 94 | 8.4 | |
| 5.0 | 90 | 4.4 | 86 | 8.0 | |
| Lake water | 1.0 | 110 | 4.5 | 98 | 7.2 |
| 2.5 | 104 | 3.1 | 105 | 5.3 | |
| 5.0 | 100 | 1.2 | 92 | 6.9 | |
| Paddy water | 1.0 | 90 | 10.0 | 94 | 10.6 |
| 2.5 | 88 | 11.8 | 91 | 7.5 | |
| 5.0 | 104 | 8.3 | 85 | 10.2 | |
| Mean | 95.6 | 6.4 | 93.4 | 7.9 | |
| Name | Sequence (5′-3′) | Annotation |
|---|---|---|
| VHF | SARGTNMAGCTGSAGSAGTC | IgG1 [43] |
| VHR | TGGGGSTGTYGTTTTGGCTGMRGAGACRGTGA | |
| VLλF | GGGAATTCATGGCCTGGAYTYCWCTYWTMYTCT | Lambda (Novagen Ig-primer sets) |
| VLλR | CCCAAGCTTAGCTCYTCWGWGGAIGGYGGRAA | |
| NcoI-cc-VHF | CCATGGCCGAAGTGCAGCTGGAGGAGTC | Anti-triazophos scFv-8C10 fragment |
| VHR-Linker | GGCTGTTGTTTTGGCTGAAG | |
| Linker-VLλF | GCTGTTGTGACTCAGGAATC | |
| VLλR-NotI | GCGGCCGCGCCTAGGACAGTCAGTTTG |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, R.; Liang, X.; Xiang, D.; Guo, Y.; Liu, Y.; Zhu, G. Expression and Functional Properties of an Anti-Triazophos High-Affinity Single-Chain Variable Fragment Antibody with Specific Lambda Light Chain. Int. J. Mol. Sci. 2016, 17, 823. https://doi.org/10.3390/ijms17060823
Liu R, Liang X, Xiang D, Guo Y, Liu Y, Zhu G. Expression and Functional Properties of an Anti-Triazophos High-Affinity Single-Chain Variable Fragment Antibody with Specific Lambda Light Chain. International Journal of Molecular Sciences. 2016; 17(6):823. https://doi.org/10.3390/ijms17060823
Chicago/Turabian StyleLiu, Rui, Xiao Liang, Dandan Xiang, Yirong Guo, Yihua Liu, and Guonian Zhu. 2016. "Expression and Functional Properties of an Anti-Triazophos High-Affinity Single-Chain Variable Fragment Antibody with Specific Lambda Light Chain" International Journal of Molecular Sciences 17, no. 6: 823. https://doi.org/10.3390/ijms17060823