1. Introduction
Uncoupling protein 3 (
UCP3) is a member of the family of uncoupling proteins (UCPs), which are members of the mitochondrial anion carrier family. UCPs consist of seven members:
UCP1,
UCP2,
UCP3,
UCP4,
BMCP1 (brainmitochondrial carrier protein 1),
StUCP (plant UCP homologs have been identified in
Solanum tuberosum) and
AtUCP (
Arabidopsis thaliana) [
1,
2].
UCP3 was first discovered and described in 1997 [
3,
4]. Because of its close homology with
UCP1, UCP3 was initially implicated in thermoregulation [
3,
4], as it has been demonstrated to uncouple in a number of experimental models. Uncoupling protein-3 (
UCP3) gene is primarily expressed in skeletal muscle and up-regulated by fatty acids [
5].
UCP3 is primarily expressed in skeletal muscle, but is also found in brown fat and heart tissue [
6].
UCP3 protein is one of the most important target proteins involved in skeletal muscle energy metabolism substance, and plays key regulatory roles in mitochondrial fatty acid oxidation [
7,
8,
9,
10,
11]. Until recently, the eukaryotic core promoter recognition complex played a critical regulatory role in driving cell specific programs of transcription during development [
12]. Several studies have shown that a transcription factor not only plays a control function in gene expression, but also inhibits the action of the complex combination [
13].
MyoD controls
UCP3 promoter activity through a noncanonical E box site located close to the transcription initiation site [
5]; in this context, it is important to note that the
MyoD response element is located at different positions in rats compared to mice/humans, which may cause species-specific differences in
UCP3 expression [
14]. The study of the molecular mechanisms of this transcription element have shown that it can synthesize muscle specific activity of the promoter, some promoters have been synthesized that have high expressing activity specificity in the muscle. With continuous development and improvement of the research, the regulation of muscle-specific promoter element values to control muscle-specific promoter elements will become a powerful tool for animal muscle development research [
15].
Guanling cattle are a yellow-coated breed that was developed in China [
16]. In this study, 5′ upstream regions of
UCP3 of Guanling cattle were analyzed, and PCR amplified segments of different length
UCP3 gene promoter sequences, different length fragments of
UCP3 promoter activity and the core of
UCP3 promoter were tested. Transcription factor binding sites of cattle
UCP3 promoter were screened using Promoter-Binding transcription factors (TF) Profiling assay combined with bioinformatics analysis, and we identified several transcription factor binding sites for
UCP3. We investigated whether the
UCP3 promoter activity could be increased by myogenic regulatory factors (MRFs) family and myocyte-specific enhancer factor 2A (
MEF2A) transcriptional factors. These results of cattle
UCP3 promoter provide a platform for further researche on promoter activity and expression regulation. The results are meaningful in the research of muscle-specific promoters and the mechanism of cattle’s muscle development and growth.
3. Discussion
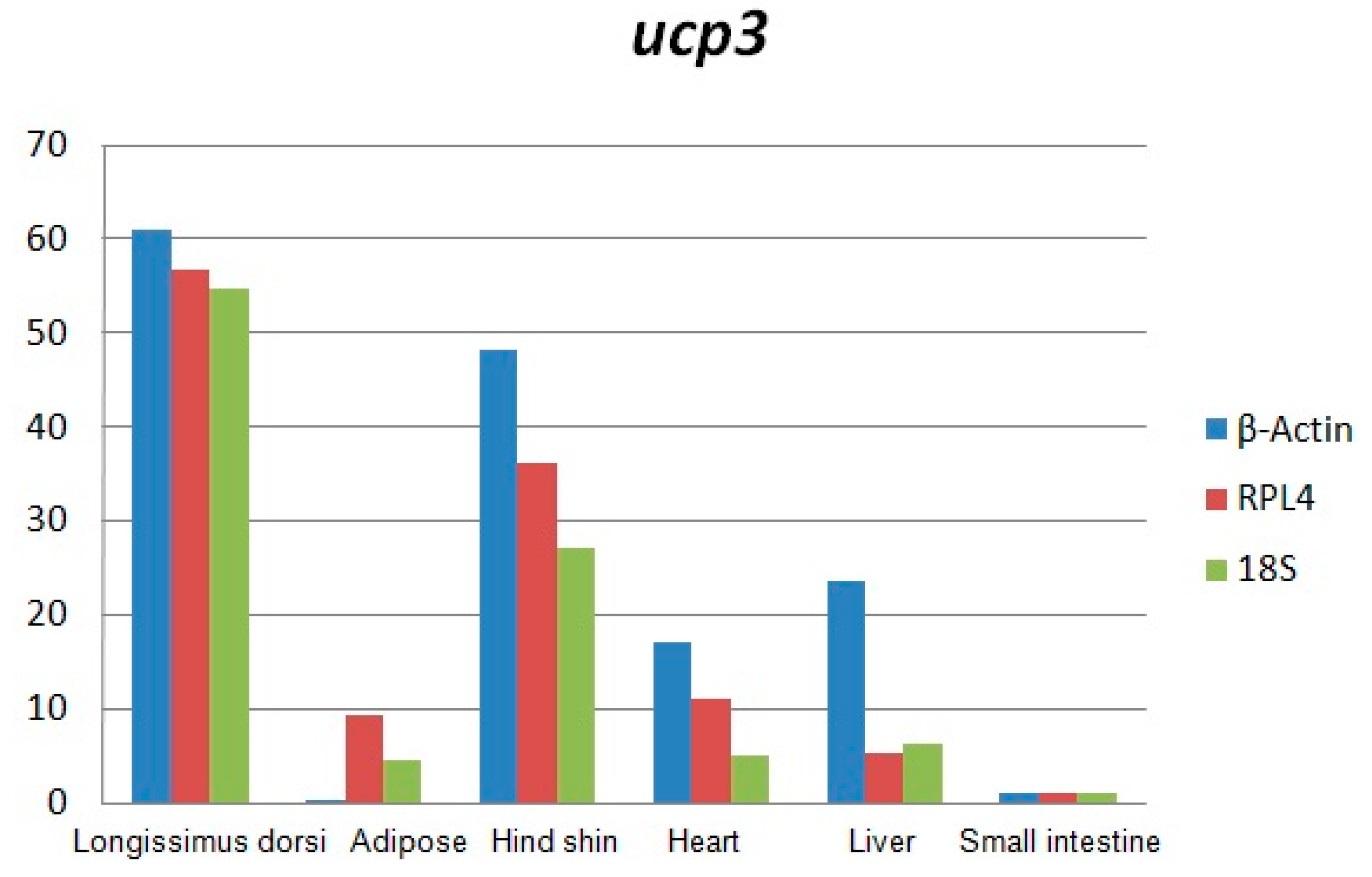
The results of this study showed that the expression of the
UCP3 was almost undetectable in small intestine and adipose. However, the expression of the
UCP3 in longissimus dorsi and hind shin tissue were about 60-fold and 40-fold greater than the expression of
UCP3 in adipose and the small intestine, and about 6-fold and 4-fold greater than the in liver and heart, suggesting it has characteristics of high efficiency, and specificity expression in skeletal muscle, which was consistent with the observation that the preferential expression of
UCP3 in skeletal muscle and brown fat [
3,
17,
18,
19,
20] and the
UCP3 promoter being activated in the muscle cell differentiation process [
21].
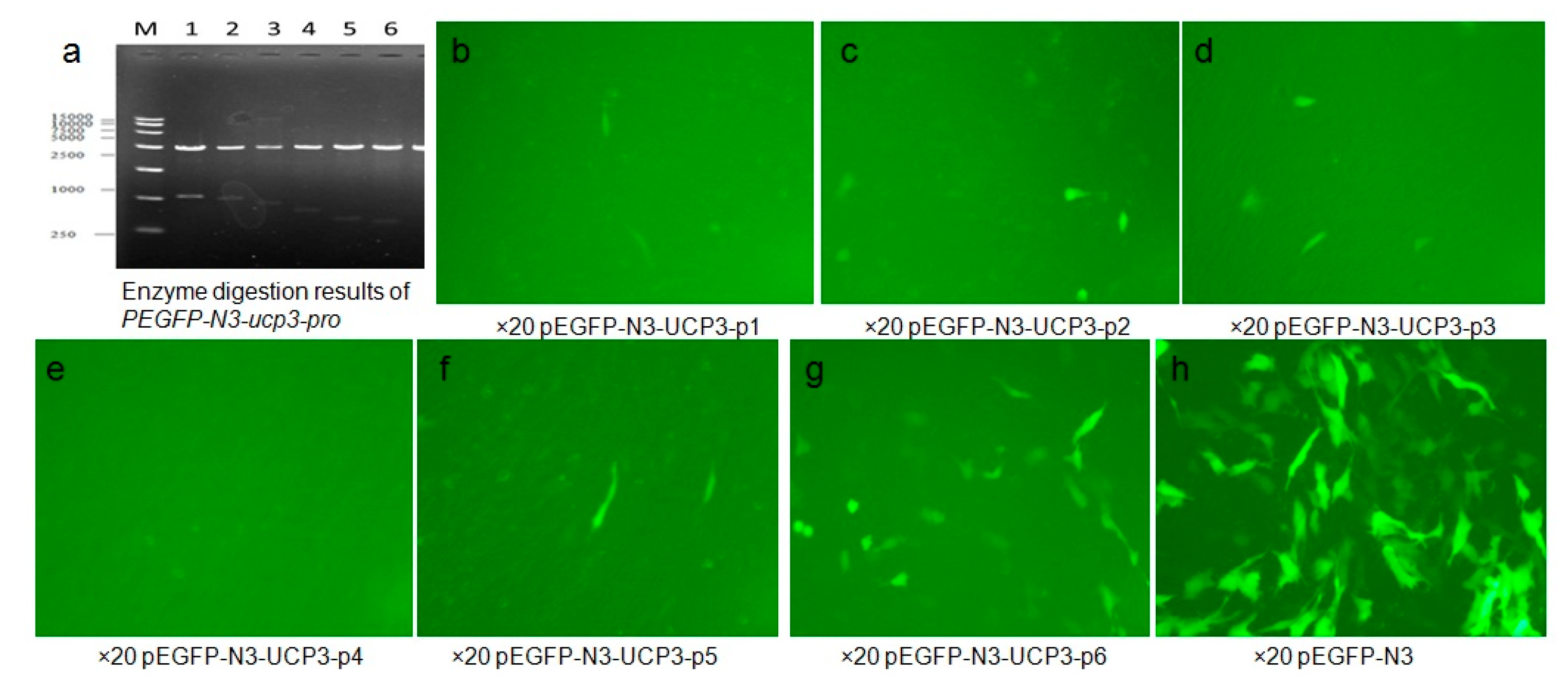
The cloned 5′-upstream regions of the Guanling cattle
UCP3 genes showed varying degrees of promoter activity transfection into C2C12 cells. The result showed that the sequence of
UCP3 promoter from −385 to +3 bp has the minimal promoter region containing the transcription start site necessary for
UCP3 promoter activity, and several studies have reported activity of the
UCP3 promoter in C2C12 [
22,
23]. Deletion of
UCP3 promoter expression showed that it has some negative-regulatory elements from −620 to −433 bp, and there exist some positive-regulatory elements between −433 and −385 bp. Therefore, this difference in the promoter activities between different segments of
UCP3 promoter suggests that additional factors were needed to activate the
UCP3 promoter. Note that pGL3-basic-ucp3-P5 had much lower promoter activity, but pGL3-promoter-ucp3-p5 and pGL3-promoter-ucp3-p6 exhibited significantly higher promoter activity (96.54 ± 3.96 and 95.25 ± 3.91 RLU, relative to pGL3-basic). In a word, the results showed that additional SV40 promoter can strengthen the activity of
UCP3 promoter, thereby optimizing the question about low activity of
UCP3 promoter in skeletal muscles. The result of PEGFP-N3-ucp3 recombinant vector transfection into C2C12 cells was in agreement with the result of pGL3-promoter-ucp3 transfection into C2C12 cells.
Previous studies had shown that transcription factors combined with these special sequences can open or close a set of specific gene expressions. The interaction of regulatory sequence and transcription factors plays a key role in gene expression [
13]. Bioinformatic analysis revealed the presence of some potential transcription factor binding sites of
UCP3 promoters such as
MyoD,
MZF,
sox-5,
TATA,
Nkx2. The result of the software also includes
MyoD,
MZF1 and
Nkx-2. We have shown that the
UCP3 promoter of Guanling cattle have
MyoD,
TFIID,
Pax3,
MEF1 and
MEF2 transcription factor binding sites by promoter-binding TF brofiling Assay II study. Our experimental and bioinformatics analysis revealed that the Guanling cattle
UCP3 promoter region had
MyoD,
TFIID,
Pax3,
MEF1,
MEF2 transcription factor binding sites. Several studies have reported that the muscle-specific promoter contained Trex/MEF-3 components,
MEF-1,
Pax3/7,
SRE,
MEF-2,
CACCC boxes and other transcriptional regulatory components [
14]. Multiple lines of evidence have shown that PPARα and PPARδ are mediators of the fatty acid-dependent control of
UCP3 transcription in skeletal muscle [
24].
Pax3 is a key regulator of
MyoG during development [
25,
26]. The embryonic progenitors that express
Pax3, and its close homolog
Pax7, give rise to a population of adult muscle stem cells [
27,
28].
MEF2 was considered to be a conservative member of the vast majority of muscle-specific genes [
29,
30,
31,
32,
33].
MEF2 and the transcription factor containing the basic helix-loop-helix (bHLH) could coordinate muscle genes expression and regulation of the initial myogenic differentiation [
34]. In addition, promoter activity was induced by overexpression of
MyoD1, which bound to this canonical E-box during C2C12 differentiation [
35].
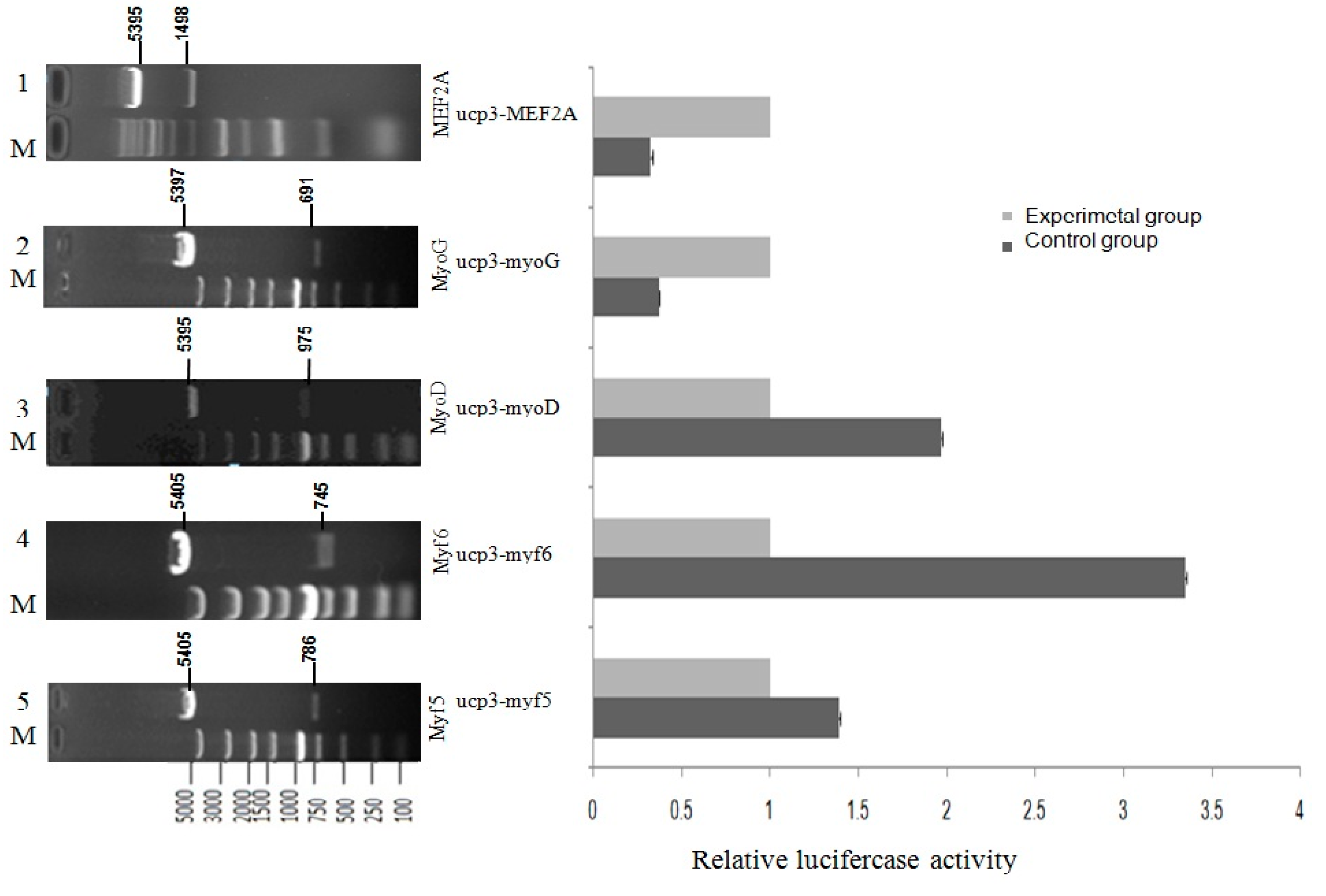
To explore the role of
UCP3 promoter for a possible regulation of MRFs family and
MEF2A transcription factor,
Myf5,
Myf6,
MyoD,
MyoG and
MEF2A were selected as the most likely transcription factors to be involved in this study. C2C12 myoblasts were co-transfected with the MRFs family transcription factor or
MEF2A transcription factors expression vectors and pGL3-basic-ucp3-pro reporter vectors. The results showed that
Myf5,
Myf6 and
MyoD contributed to the up-regulation of the
UCP3 expression in C2C12 myoblasts, which was consistent with the present results that
MyoD was required to activate the promoter of human
UCP3 [
5]. Several transcription factor binding sites in the
UCP3 promoter from cattle have been identified including
MyoD, which is also present in mouse [
5] and rat [
14]. Conversely, the
MyoG and
MEF2A had down-regulation effects on the
UCP3 promoter region. Interestingly, the effects of MRFs family and
MEF2A were different in C2C12 cell line,
Myf5,
My6 and
MyoD increased the dual luciferase activity, but myogenin and
MEF2A had no effect. These results indicated that both the MRFs family and
MEF2A are necessary, but, together, they were not sufficient enough transcription factors to induce
UCP3 promoter activity. The result is consistent with previous findings [
21].
In summary, the results indicated that this difference was most likely conferred by different ways in which
UCP3 promoter regions respond to the
Myf5,
Myf6 and
MyoD transcription factors, and the promoter-binding TF profiling assay shows that these transcription factors bind directly to the
UCP3 promoter. We only focus the
UCP3 promoter in this study, so we can not exclude the possibility that the intoronic region (e.g., intron 1) is associated with the regulation of
UCP3 gene [
36,
37].
4. Experimental Section
4.1. Experimental Animals and Tissue Sampling
Guanling cattle (castrated steers, n = 3) with similar genetic backgrounds in Guanling county of Guizhou province were reared under the same experimental conditions. At a mean age of 18 months, the animals were slaughtered using standard commercial procedures. We collected longissimus dorsi, adipose tissue, hind shin, heart, liver, and small intestine tissue samples. The tissue samples were placed in the RNAlater RNA stabilization solution (Qiagen, Hilden, Germany) immediately after collection. The samples were frozen in liquid nitrogen, and stored at −80 °C.
4.2. RNA Isolation and Synthesis of cDNA
Total RNA was isolated from each tissue sample using the TRIzol reagent (Invitrogen, Waltham, MA, USA), according to the manufacturer’s instructions. An aliquot containing 1 μg of total RNA was used for the synthesis of complementary DNA (cDNA) by reverse transcription using TransStart® One-Step gDNA Removal and cDNA Removal and cDNA Synthesis Super Mix (TransGen Biotech, Beijing, China).
4.3. Confirmation of Gene Expression Using qRT-PCR
The primer pairs of
UCP3 were designed to have matching melting temperatures. The primer sequences are listed in
Table 3. The same RNA used for
UCP3 analysis of the longissimus dorsi, adipose, hind shin, heart, liver, and small intestine tissue samples were used in the quantitative reverse transcription and polymerase chain reaction (qRT-PCR) experiments. The first strand of the cDNA was synthesized from 1 µg of total RNA using the TransScript II One-Step gDNA Removal and cDNA Synthesis SuperMixkits (TransGen Biotech, Beijing, China) with the oligo (dT) 20 primer (TransGen Biotech). The real-time PCR reactions were performed in a final volume of 20 µL using the Trans Start Green qPCR SuperMix UDG kit (TransGen Biotech), according to the manufacturer’s instructions. The mRNA expression were normalized to that of the β-actin, RPL4 and 18SRNA gene. The real-time PCR assays were performed using a CFX96 real-time PCR system (Bio-Rad, Hercules, CA, USA). Thermal cycling was performed using an initial denaturation step of 95 °C for 1 min, followed by 40 cycles of 95 °C for 30 s, 58 °C for 30 s, and 72 °C for 1 min. A melting curve was constructed using annealing temperatures from 58 to 95 °C to verify the specificity of the amplified product. The data were analyzed using the 2
−ΔΔCt method.
4.4. Amplify UCP3 Promoter of Guanling Cattle
Genomic DNA of Guanling cattle was extracted from blood samples using DNA extraction kit (TransGen Biotech, Beijing, China) according to the manufacturer’s instructions. Based on the sequence of UCP3 gene of cattle (UCSC NM_174210), a pair of PCR primers were designed using Primer Premier 5.0 (version 5.00, PREMIER Biosoft international, Palo Alto, CA, USA, 2000) to amplify 1080, 980, 814, 624, 437 and 389 bp sequence of
UCP3 promoter (
Table 4). Six fragments of
UCP3 promoter were amplified by PCR form DNA genome of Guanling cattle (
Figure 9). The PCR system consisted of 1 µL templates, 1 µL of each antiprimer, 1 µL primer, 10 µL 2× PCR Master Mix (Takara, Dalian, China) and 7 µL ddH2O. Thermal cycling was performed using an initial denaturation step of 94 °C for 3 min, followed by 30 cycles of 94 °C for 30 s, 56–60 °C for 30 s, and 72 °C for 1 min. After the cycles, a final extension was performed at 72 °C to 7 min. The amplified fragments were digested with Nhe I and xho I, and ligated together into pGL3-basic, pGL3-promoter and PEGFP-N3 at NheI and xhoI site. Double enzyme fragment lengths were 1080, 980, 814, 624, 437 and 389 bp, respectively (
Figure 5,
Figure 6 and
Figure 7). The PCR product was ligated into pUCm-T easy vector, pGL3-basic, PGL3-promoter, PEGFP-N3 and sequenced.
4.5. Transcription Factor Profiling of UCP3 Promoter by Filter Assay
For monitoring the activation of multiple TFs of the
UCP3 promoter simultaneously, Promoter-Binding TF Profiling Assay II was used according to the protocol provided by Signosis Inc. (Signosis Inc., Santa Clara, CA, USA). Nuclear proteins were isolated from longissimus dorsi using the reagents and protocol provided by a Nuclear Extraction Kit (Signosis Inc., Santa Clara, CA, USA). Androgen receptor (AR) was used to normalize the readings for as a blank control (
Table 5). Luminescence was reported as relative light units (RLUs) using a multidetection microplate reader (Bio Tek, Vermont, VT, USA). TFSEARCH (
http://diyhpl.us/~bryan/irc/protocol-online/protocol-cache/TFSEARCH.html) and ALGGEN ROMO (
http://alggen.lsi.upc.es/) were used to predict the transcription factor binding sites of
UCP3 promoter region.
4.6. Construction of Myogenic Regulatory Factors (MRFs) Family and MEF2A Transcription Factors Expression Vectors
RNA was isolated from longissimus muscle tissue of Guanling cattle using Trizol (Invitrogen). First strand cDNA was synthesized by the Reverse transcription kit (TransGen Biotech, Beijing, China). The primers of
MyoD,
Myf5,
Myf6,
MyoG and
MEF2A were designed according to the sequence of NCBI were amplified from the cDNA of longissimus dorsi (
Table 6). The MRFs family and
MEF2A fragments were excised with restriction enzyme, which were ligated into the pcDNA3.1 (Promega, Madison, WI, USA). Then they were confirmed by dual-enzyme digestion and sequencing. Recombinant expression vectors were pcDNA3.1(+)-MyoD, pcDNA3.1(+)-Myf5, pcDNA3.1(+)-Myf6, pcDNA3.1(+)-Myogenin and pcDNA3.1(+)-MEF2A respectively.
4.7. Analyse Activity of UCP3 Promoter
Purification of UCP3 promoter fragments was excised with NheI and XhoI restriction enzyme, and ligated into the pGL3-Basic, pGL3-promoter and PEGFP-N3 (Promega, Madison, WI, USA), respectively. Then they were confirmed by dual-enzyme digestion and sequencing.
The C2C12 myoblasts were obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). C2C12 myoblasts cells were cultured in DMEM (Hyclone, logan, UT, USA) medium with 10% fetal bovine serum (FBS) (Gibco, Aucland, New Zealand) under normal culture conditions (5% CO2 at 37 °C). C2C12 myoblasts were seeded into 24-well plates at a density of 1 × 105/well. C2C12 myoblasts reached 80% confluence after 18–24 h. Subsequently, C2C12 myoblasts were cultured in OPTI-MEM (500 µL/well) containing of 2 µL of lipofectamine 2000, 0.8 µg of the pGL3-Basic-ucp3-pro and pGL3-promoter-ucp3-pro recombinant vcectors and 0.06 µg of the internal control vector pRL-TK/luciferase reporter plasmid, C2C12 myoblasts was cultured in OPTI-MEM (500 µL/well), after 5 h, the OPTI-MEM medium was removed and replaced by DMEM medium containing 10% FBS. After 24 h transfection, cells were harvested, luciferase activity was measured using the Dual-Luciferase® Reporter Assay System (Promega, Madison, WI, USA), and then the activities of firefly luciferase in pGL3 and Renilla luciferase in pRL-TK were analyzed.
Cotransfection of eukaryotic expression vectors of MyoD, Myf5, Myf6, MyoG and MEF2A with UCP3 promoter, C2C12 cells was seeded at the density of 1 × 105/well into 24-well plates using DMEM containing 10% FBS medium. After 18–24 h, the plated cells were transfected with 0.6 µg of pGL3-basic-ucp3-P1 vector, 0.06 µg of the internal control vector pRL-TK, 0.2 µg of the expression vectors of pcDNA3.1(+)-MyoD, pcDNA3.1(+)-Myf5, pcDNA3.1(+)-Myf6, pcDNA3.1(+)-Myogenin and pcDNA3.1(+)-MEF2A, 2 µL of Lipofectamine 2000 using Lipofectamine 2000 (Invitrogen Corporation, Carlsbad, NM, USA) according to the manufacturer’s protocol, and the pcDNA3.1 vectors were cotransfected with pGL3-basic-ucp3-P1, which was the control. After 5 h, the OPTI-MEM medium was replaced by DMEM medium containing 10% FBS. After 24 h transfection, luciferase activity was measured using the same techniques as those described for C2C12 myoblasts cells.
4.8. The Data Analysis
Statistical analyses were performed using SPSS17.0. Data (SPSS statistics 17.0, WinWrap Basic, New York, NY, USA, 2008) were presented as the mean ± standard error of the mean. Statistical differences between conditions were assessed using one-way ANOVA, p < 0.05 was considered statistically significant.