γ-Tocotrienol as a Promising Countermeasure for Acute Radiation Syndrome: Current Status
Abstract
:1. Introduction
2. Radiation Countermeasures: Radioprotectors, Mitigators and Therapeutics
3. U.S. Food and Drug Administration (U.S. FDA) Animal Efficacy Rule
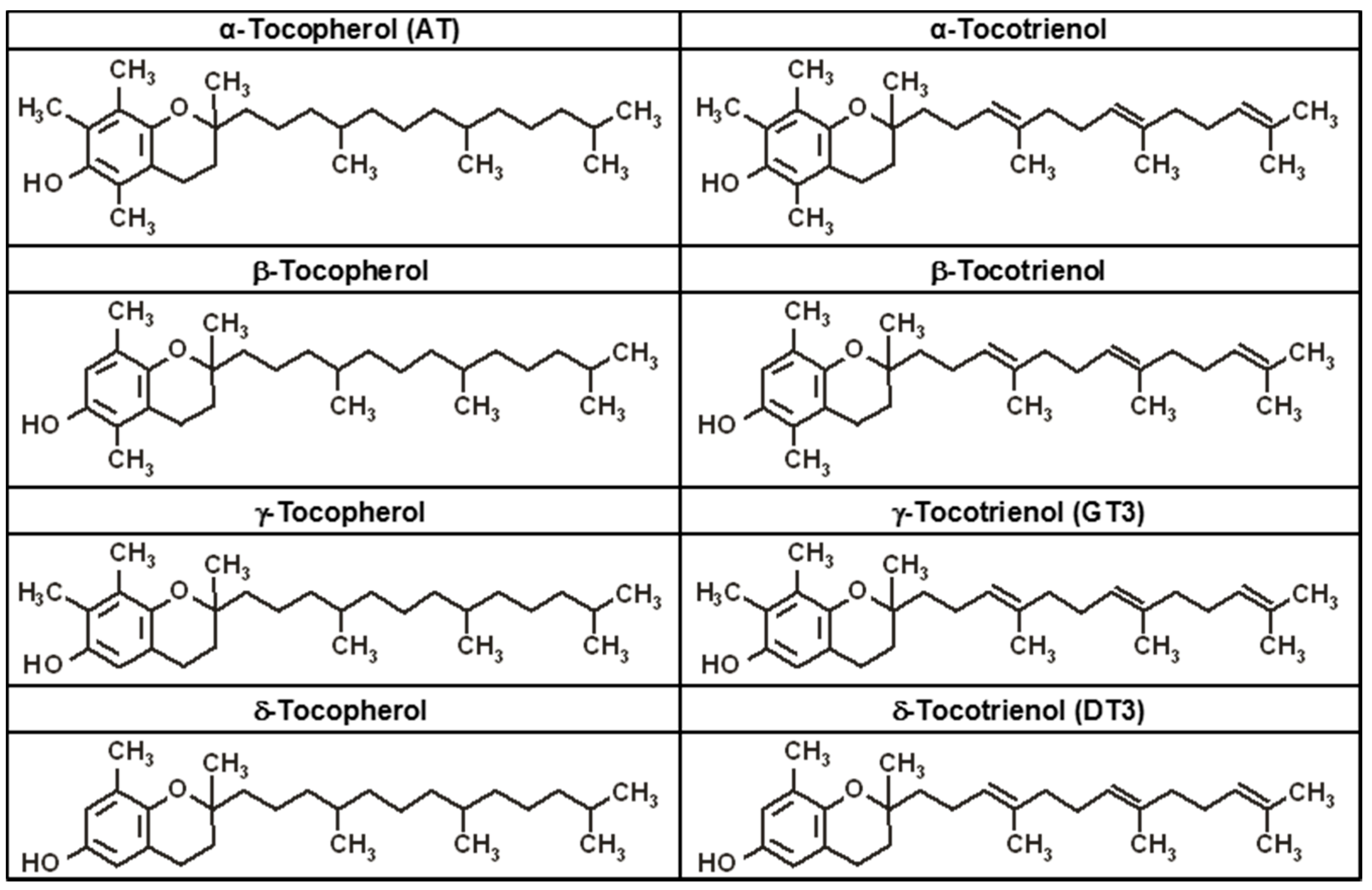
4. Tocopherols and Tocotrienols: Members of the Vitamin E Family
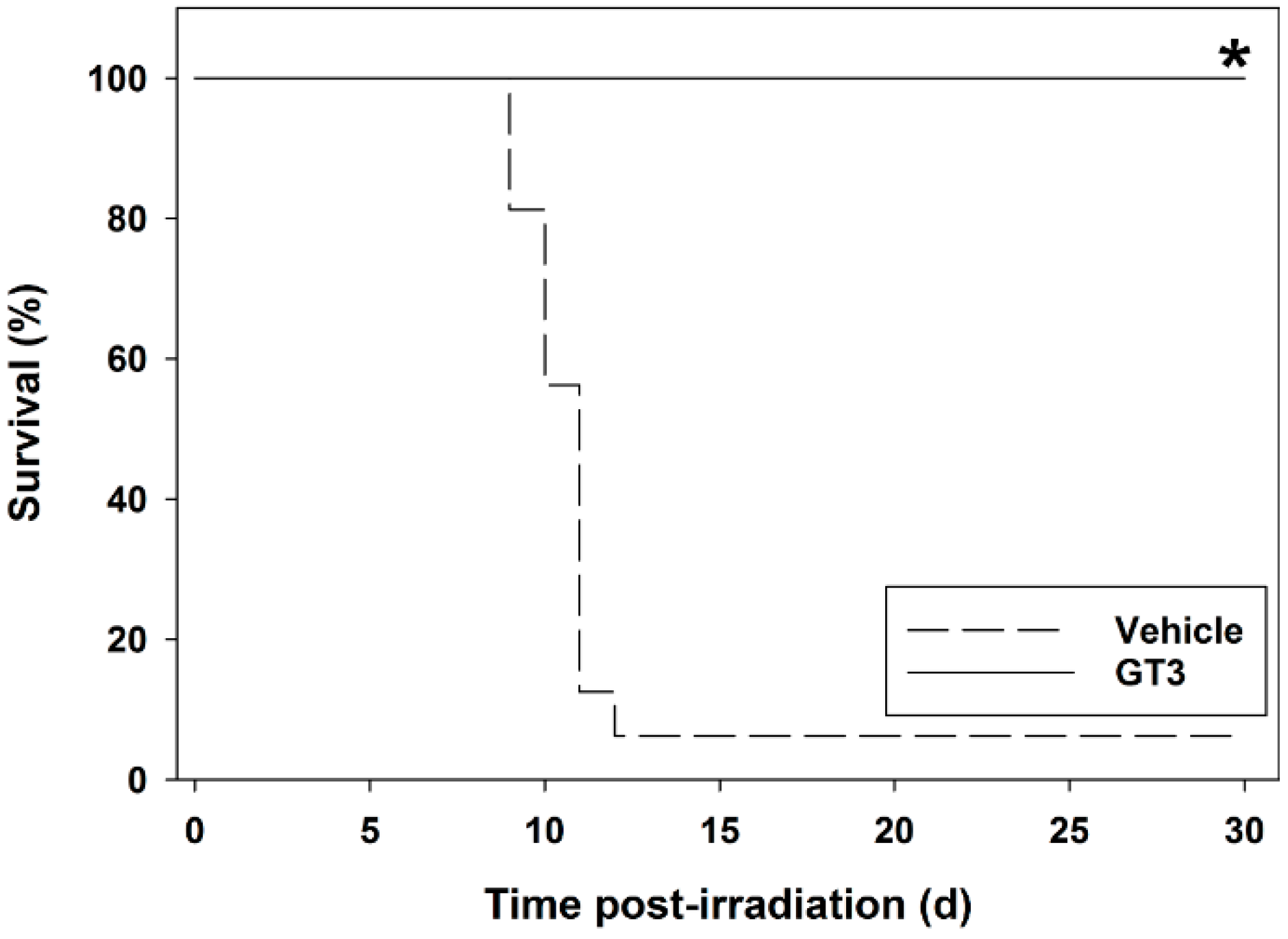
5. Radioprotective Efficacy of γ-Tocotrienol (GT3) in the Mouse Model
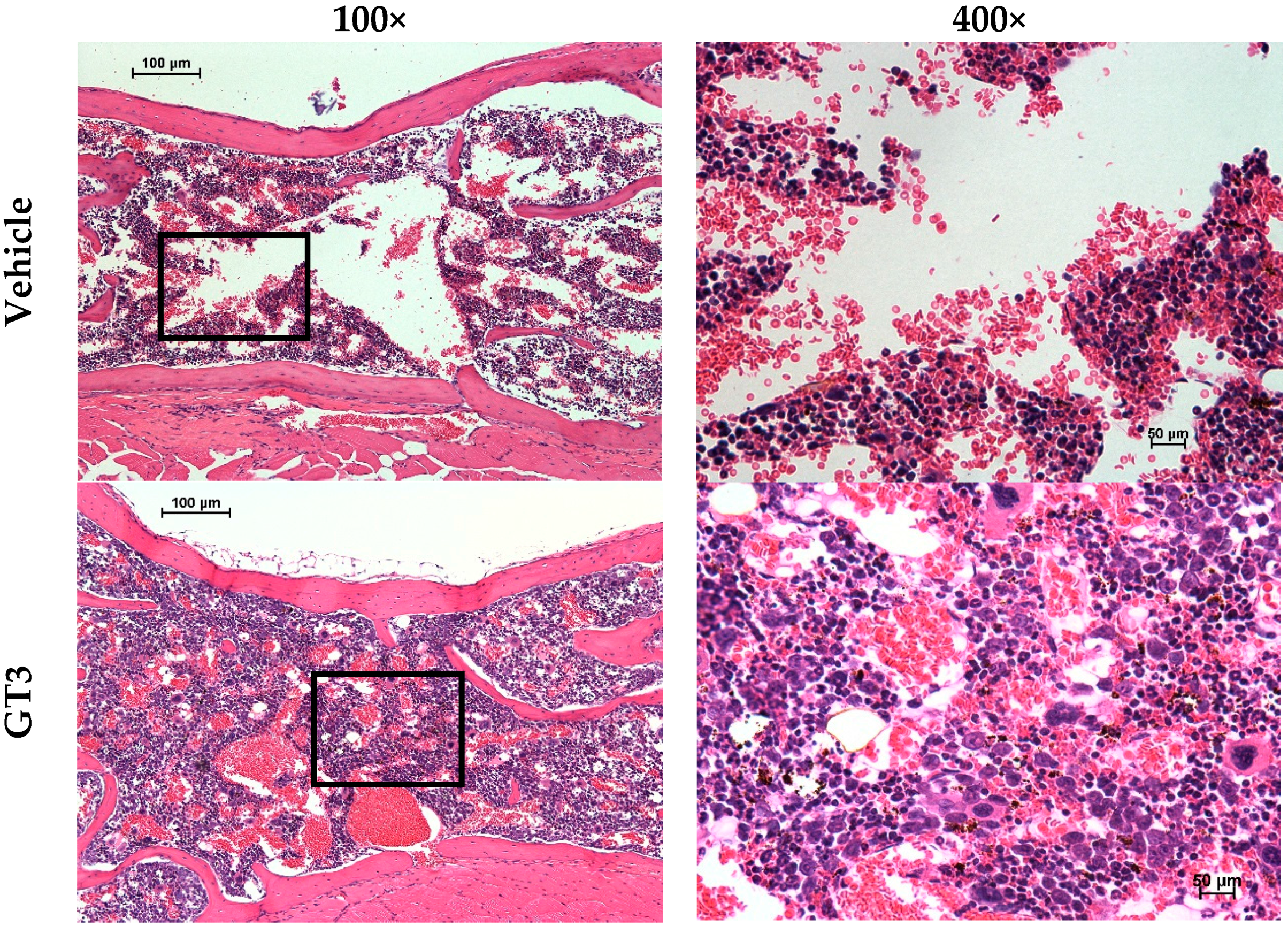
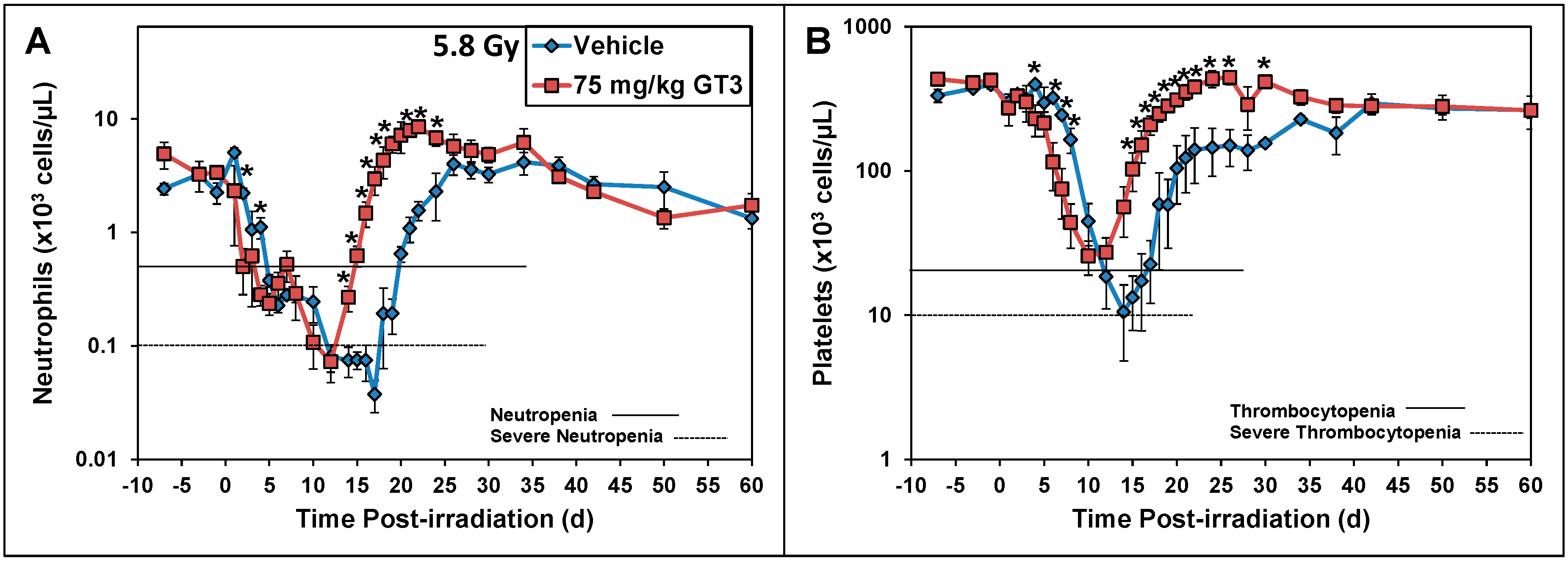
5.1. Radioprotection against Hematopoietic Injury
5.2. Radioprotection against Gastrointestinal and Vascular Injuries
6. Cytokine Induction by GT3
6.1. Efficacy of GT3 Is Mediated through Granulocyte Colony-Stimulating Factor (G-CSF) Production in the Mouse Model
6.2. Mobilization of Mouse Progenitors by GT3-Induced G-CSF
7. Combination of GT3 with Other Agents for Enhancing Its Radioprotective Efficacy
7.1. Radioprotective Efficacy of GT3 and Pentoxifylline (PTX) Combination
7.2. Radioprotective Efficacy of GT3 and Amifostine Combination
7.3. Contribution of Thrombomodulin to GT3-Mediated Lethality Protection
8. Radioprotective Efficacy of GT3 in Nonhuman Primates (NHPs)
9. Mechanisms of Action
9.1. Antioxidant Properties of GT3
9.2. Effects of GT3 on 3-Hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) Reductase
9.3. Anti-Apoptotic Activity of GT3
10. Conclusions and Future Direction
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ΔΨm | Mitochondrial membrane potential |
| ARS | Acute radiation syndrome |
| BH4 | Tetrahydrobiopterin |
| CD | Cluster of differentiation molecule |
| c-Kit+ | Stem cell factor positive |
| DRF | Dose reduction factor |
| Flk+ | Endothelial progenitor cell marker positive |
| G-CSF | Granulocyte colony-stimulating factor |
| GFRP | GTPCH feedback regulatory protein |
| GI-ARS | Gastrointestinal acute radiation syndrome |
| GRAS | Generally recognized as safe |
| Gy | Gray |
| GTPCH | Guanosine triphosphate cyclohydrolase 1 |
| H-ARS | Hematopoietic acute radiation syndrome |
| HMG-CoA | 3-Hydroxy-3-methylglutaryl-coenzyme A |
| IL | Interleukin |
| Lin− | Lineage negative |
| IκBα | Inhibitor of κBα |
| NF-κB | Nuclear factor-κB |
| PTX | Pentoxifylline |
| U.S. FDA | United States Food and Drug Administration |
References
- Baranov, A.E.; Guskova, A.K.; Nadejina, N.M.; Nugis, V.Y. Chernobyl experiences: Biological indications of exposure to ionizing radiation. Stem Cells 1995, 13 (Suppl. S1), S69–S77. [Google Scholar]
- Ohnishi, T. The disaster at Japan’s Fukushima-Daiichi nuclear power plant after the March 11, 2011 earthquake and tsunami, and the resulting spread of radioisotope contamination. Radiat. Res. 2012, 177, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Fushiki, S. Radiation hazards in children—Lessons from Chernobyl, Three Mile Island and Fukushima. Brain Dev. 2013, 35, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Seed, T.M. Radiation Effects. In Physician’s Guide to Terrorist Attack; Roy, M.J., Ed.; Humana Press: Totowa, NJ, USA, 2003; pp. 339–362. [Google Scholar]
- Pellmar, T.C.; Rockwell, S. Priority list of research areas for radiological nuclear threat countermeasures. Radiat. Res. 2005, 163, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Carter, A.B.; May, M.M.; Perry, W.J. The day after: Action following a nuclear blast in a U.S. city. Wash. Q. 2007, 30, 19–32. [Google Scholar] [CrossRef]
- Hall, E.J.; Giaccia, A.J. Radiobiology for the Radiobiologist, 6th ed.; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2006. [Google Scholar]
- Dumont, F.; le Roux, A.; Bischoff, P. Radiation countermeasure agents: An update. Expert Opin. Ther. Pat. 2010, 20, 73–101. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Ducey, E.J.; Brown, D.S.; Whitnall, M.H. A review of radiation countermeasure work ongoing at the Armed Forces Radiobiology Research Institute. Int. J. Radiat. Biol. 2012, 88, 296–310. [Google Scholar] [CrossRef] [PubMed]
- Seed, T.M. Radiation protectants: Current status and future prospects. Health Phys. 2005, 89, 531–545. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.F.; Landauer, M.R. History and development of radiation-protective agents. Int. J. Radiat. Biol. 2009, 85, 539–573. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.M.; Day, R.; Singh, V.K. New approaches to radiation protection. Front. Oncol. 2015, 4, 381. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Romaine, P.L.; Seed, T.M. Medical countermeasures for radiation exposure and related injuries: Characterization of medicines, FDA-approval status and inclusion into the strategic national stockpile. Health Phys. 2015, 108, 607–630. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Romaine, P.L.; Newman, V.L. Biologics as countermeasures for acute radiation syndrome: Where are we now? Expert Opin. Biol. Ther. 2015, 15, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Newman, V.L.; Romaine, P.L.; Wise, S.Y.; Seed, T.M. Radiation countermeasure agents: An update (2011–2014). Expert Opin. Ther. Pat. 2014, 24, 1229–1255. [Google Scholar] [CrossRef] [PubMed]
- Eaton, E.B., Jr.; Varney, T.R. Mesenchymal stem cell therapy for acute radiation syndrome: Innovative medical approaches in military medicine. Mil. Med. Res. 2015, 2, 2. [Google Scholar] [CrossRef] [PubMed]
- Gaberman, E.; Pinzur, L.; Levdansky, L.; Tsirlin, M.; Netzer, N.; Aberman, Z.; Gorodetsky, R. Mitigation of Lethal Radiation Syndrome in Mice by Intramuscular Injection of 3D Cultured Adherent Human Placental Stromal Cells. PLoS ONE 2013, 8, e66549. [Google Scholar] [CrossRef] [PubMed]
- Amgen Inc. Neupogen (Filgrastim) Injection for Subcutaneous or Intravenous Use. Available online: http://pi.amgen.com/united_states/neupogen/neupogen_pi_hcp_english.pdf (accessed on 2 April 2015).
- U.S. Food and Drug Administration. FDA Approves Neupogen for Treatment of Patients with Radiation-Induced Myelosuppression Following a Radiological/Nuclear Incident. Available online: http://www.fda.gov/EmergencyPreparedness/Counterterrorism/MedicalCountermeasures/AboutMCMi/ucm443245.htm (accessed on 17 April 2015).
- Amgen Inc. Neulasta (Pegfilgrastim) Injection for Subcutaneous Use. Available online: http://pi.amgen.com/united_states/neulasta/neulasta_pi_hcp_english.pdf (accessed on 19 November 2015).
- Rasey, J.S.; Spence, A.M.; Badger, C.C.; Krohn, K.A.; Vera, D.M.; Livesey, J.C. Specific protection of different normal tissues. Pharmacol. Ther. 1988, 39, 33–43. [Google Scholar] [CrossRef]
- Glover, D.; Fox, K.R.; Weiler, C.; Kligerman, M.M.; Turrisi, A.; Glick, J.H. Clinical trials of WR-2721 prior to alkylating agent chemotherapy and radiotherapy. Pharmacol. Ther. 1988, 39, 3–7. [Google Scholar] [CrossRef]
- Weiss, J.F. Pharmacologic approaches to protection against radiation-induced lethality and other damage. Environ. Health Perspect. 1997, 105 (Suppl. S6), 1473–1478. [Google Scholar] [CrossRef] [PubMed]
- MedImmune. Available online: http://www.medimmune.com/docs/default-source/pdfs/prescribing-information-for-amifostine.pdf (accessed on 30 September 2013).
- Culy, C.R.; Spencer, C.M. Amifostine: An update on its clinical status as a cytoprotectant in patients with cancer receiving chemotherapy or radiotherapy and its potential therapeutic application in myelodysplastic syndrome. Drugs 2001, 61, 641–684. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.S.; Singh, V.K. Immunomodulators: A review of studies on Indian medicinal plants and synthetic peptides. Part I: Medicinal plants. Proc. Ind. Natl. Sci. Acad. B 1999, 65, 179–204. [Google Scholar]
- Papas, A. The Vitamin Factor; Harper Perennial, Harper-Collins Publishers Inc.: New York, NY, USA, 1999. [Google Scholar]
- Singh, V.K.; Beattie, L.A.; Seed, T.M. Vitamin E: Tocopherols and tocotrienols as potential radiation countermeasures. J. Radiat. Res. 2013, 54, 973–988. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K.; Khanna, S.; Roy, S.; Packer, L. Molecular basis of vitamin E action. Tocotrienol potently inhibits glutamate-induced pp60(c-Src) kinase activation and death of HT4 neuronal cells. J. Biol. Chem. 2000, 275, 13049–13055. [Google Scholar] [CrossRef] [PubMed]
- Palozza, P.; Simone, R.; Picci, N.; Buzzoni, L.; Ciliberti, N.; Natangelo, A.; Manfredini, S.; Vertuani, S. Design, synthesis, and antioxidant potency of novel α-tocopherol analogues in isolated membranes and intact cells. Free Radic. Biol. Med. 2008, 44, 1452–1464. [Google Scholar] [CrossRef] [PubMed]
- Palozza, P.; Verdecchia, S.; Avanzi, L.; Vertuani, S.; Serini, S.; Iannone, A.; Manfredini, S. Comparative antioxidant activity of tocotrienols and the novel chromanyl-polyisoprenyl molecule FeAox-6 in isolated membranes and intact cells. Mol. Cell. Biochem. 2006, 287, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Packer, L. Protective role of vitamin E in biological systems. Am. J. Clin. Nutr. 1991, 53, 1050S–1055S. [Google Scholar] [PubMed]
- Nesaretnam, K. Multitargeted therapy of cancer by tocotrienols. Cancer Lett. 2008, 269, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K.; Khanna, S.; Roy, S. Tocotrienols: Vitamin E beyond tocopherols. Life Sci. 2006, 78, 2088–2098. [Google Scholar] [CrossRef] [PubMed]
- Pearce, B.C.; Parker, R.A.; Deason, M.E.; Qureshi, A.A.; Wright, J.J. Hypocholesterolemic activity of synthetic and natural tocotrienols. J. Med. Chem. 1992, 35, 3595–3606. [Google Scholar] [CrossRef] [PubMed]
- Kamal-Eldin, A.; Appelqvist, L.A. The chemistry and antioxidant properties of tocopherols and tocotrienols. Lipids 1996, 31, 671–701. [Google Scholar] [CrossRef] [PubMed]
- Pearce, B.C.; Parker, R.A.; Deason, M.E.; Dischino, D.D.; Gillespie, E.; Qureshi, A.A.; Volk, K.; Wright, J.J. Inhibitors of cholesterol biosynthesis. 2. Hypocholesterolemic and antioxidant activities of benzopyran and tetrahydronaphthalene analogues of the tocotrienols. J. Med. Chem. 1994, 37, 526–541. [Google Scholar] [CrossRef] [PubMed]
- Serbinova, E.; Kagan, V.; Han, D.; Packer, L. Free radical recycling and intramembrane mobility in the antioxidant properties of α-tocopherol and α-tocotrienol. Free Radic. Biol. Med. 1991, 10, 263–275. [Google Scholar] [CrossRef]
- Peh, H.Y.; Tan, W.S.; Liao, W.; Wong, W.S. Vitamin E therapy beyond cancer: Tocopherol versus tocotrienol. Pharmacol. Ther. 2015, in press. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.P.; Kulkarni, S.; Hieber, K.; Toles, R.; Romanyukha, L.; Kao, T.C.; Hauer-Jensen, M.; Kumar, K.S. Gamma-tocotrienol, a tocol antioxidant as a potent radioprotector. Int. J. Radiat. Biol. 2009, 85, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Brown, D.S.; Kao, T.C. α-Tocopherol succinate protects mice from γ-radiation by induction of granulocyte-colony stimulating factor. Int. J. Radiat. Biol. 2010, 86, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Li, X.H.; Ghosh, S.P.; Ha, C.T.; Fu, D.; Elliott, T.B.; Bolduc, D.L.; Villa, V.; Whitnall, M.H.; Landauer, M.R.; Xiao, M. δ-Tocotrienol protects mice from radiation-induced gastrointestinal injury. Radiat. Res. 2013, 180, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Stone, H.B.; Moulder, J.E.; Coleman, C.N.; Ang, K.K.; Anscher, M.S.; Barcellos-Hoff, M.H.; Dynan, W.S.; Fike, J.R.; Grdina, D.J.; Greenberger, J.S.; et al. Models for evaluating agents intended for the prophylaxis, mitigation and treatment of radiation injuries. Report of an NCI Workshop, December 3–4, 2003. Radiat. Res. 2004, 162, 711–728. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Christensen, J.; Fatanmi, O.O.; Gille, D.; Ducey, E.J.; Wise, S.Y.; Karsunky, H.; Sedello, A.K. Myeloid Progenitors: A radiation countermeasure that is effective when initiated days after irradiation. Radiat. Res. 2012, 177, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Boerma, M.; Roberto, K.A.; Hauer-Jensen, M. Prevention and treatment of functional and structural radiation injury in the rat heart by pentoxifylline and α-tocopherol. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Delanian, S.; Porcher, R.; Balla-Mekias, S.; Lefaix, J.L. Randomized, placebo-controlled trial of combined pentoxifylline and tocopherol for regression of superficial radiation-induced fibrosis. J. Clin. Oncol. 2003, 21, 2545–2550. [Google Scholar] [CrossRef] [PubMed]
- Koenig, K.L.; Goans, R.E.; Hatchett, R.J.; Mettler, F.A., Jr.; Schumacher, T.A.; Noji, E.K.; Jarrett, D.G. Medical treatment of radiological casualties: Current concepts. Ann. Emerg. Med. 2005, 45, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Gronvall, G.K.; Trent, D.; Borio, L.; Brey, R.; Nagao, L. The FDA animal efficacy rule and biodefense. Nat. Biotechnol. 2007, 25, 1084–1087. [Google Scholar] [CrossRef] [PubMed]
- Nightengale, S.L.; Prasher, J.M.; Simonson, S. Emergency use authorization (EUA) to enable use of needed products in civilian and military emergencies, United States. Emerg. Infect. Dis. 2002, 7, 1046–1055. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. Guidance for Industry: Product Developoment under the Animal Rule. Available online: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM399217.pdf (accessed on 18 July 2014).
- Singh, V.K.; Newman, V.L.; Berg, A.N.; MacVittie, T.J. Animal models for acute radiation syndrome drug discovery. Expert Opin. Drug Discov. 2015, 10, 497–517. [Google Scholar] [CrossRef] [PubMed]
- Hall, E.J.; Giaccia, A.J. Radiobiology for the Radiobiologist, 7th ed.; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2012. [Google Scholar]
- Singh, V.K.; Newman, V.L.; Romaine, P.L.; Hauer-Jensen, M.; Pollard, H.B. Use of biomarkers for assessing radiation injury and efficacy of countermeasures. Expert Rev. Mol. Diagn. 2016, 16, 65–81. [Google Scholar] [CrossRef] [PubMed]
- Aebersold, P. FDA Experience with Medical Countermeasures under the Animal Rule. Adv. Prev. Med. 2012, 2012, 507571. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, E.X.; Pereira, E.F.; Aracava, Y.; Fawcett, W.P.; Oliveira, M.; Randall, W.R.; Hamilton, T.A.; Kan, R.K.; Romano, J.A., Jr.; Adler, M. Effective countermeasure against poisoning by organophosphorus insecticides and nerve agents. Proc. Natl. Acad. Sci. USA 2006, 103, 13220–13225. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. FDA Approves New Antibacterial Treatment for Plague. Available online: http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm302220.htm (accessed on 10 February 2014).
- U.S. Food and Drug Administration. FDA Approves Raxibacumab to Treat Inhalational Anthrax. Available online: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm332341.htm (accessed on 17 January 2016).
- U.S. Food and Drug Administration. FDA Approves First Botulism Antitoxin for Use in Neutralizing All Seven Known Botulinum Nerve Toxin Serotypes. Available online: http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm345128.htm (accessed on 13 February 2014).
- Aggarwal, B.B.; Sundaram, C.; Prasad, S.; Kannappan, R. Tocotrienols, the vitamin E of the 21st century: Its potential against cancer and other chronic diseases. Biochem. Pharmacol. 2010, 80, 1613–1631. [Google Scholar] [CrossRef] [PubMed]
- Luk, S.U.; Yap, W.N.; Chiu, Y.T.; Lee, D.T.; Ma, S.; Lee, T.K.; Vasireddy, R.S.; Wong, Y.C.; Ching, Y.P.; Nelson, C.; et al. γ-Tocotrienol as an effective agent in targeting prostate cancer stem cell-like population. Int. J. Cancer 2011, 128, 2182–2191. [Google Scholar] [CrossRef] [PubMed]
- Hussein, D.; Mo, H. d-δ-Tocotrienol-mediated suppression of the proliferation of human PANC-1, MIA PaCa-2, and BxPC-3 pancreatic carcinoma cells. Pancreas 2009, 38, e124–e136. [Google Scholar] [CrossRef] [PubMed]
- Davis-Yadley, A.H.; Malafa, M.P. Vitamins in pancreatic cancer: A review of underlying mechanisms and future applications. Adv. Nutr. 2015, 6, 774–802. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q. Natural forms of vitamin E: Metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free Radic. Biol. Med. 2014, 72, 76–90. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, H.; Ahad, A.; Iqbal, J.; Siddiqui, W.A. Pharmacological potential of tocotrienols: A review. Nutr. Metab. (Lond.) 2014, 11, 52. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.K.; Wise, S.Y.; Ducey, E.J.; Fatanmi, O.O.; Elliott, T.B.; Singh, V.K. α-Tocopherol succinate protects mice against radiation-induced gastrointestinal injury. Radiat. Res. 2012, 177, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Li, X.H.; Fu, D.; Latif, N.H.; Mullaney, C.P.; Ney, P.H.; Mog, S.R.; Whitnall, M.H.; Srinivasan, V.; Xiao, M. δ-Tocotrienol protects mouse and human hematopoietic progenitors from γ-irradiation through extracellular signal-regulated kinase/mammalian target of rapamycin signaling. Haematologica 2010, 95, 1996–2004. [Google Scholar] [CrossRef] [PubMed]
- Satyamitra, M.M.; Kulkarni, S.; Ghosh, S.P.; Mullaney, C.P.; Condliffe, D.; Srinivasan, V. Hematopoietic recovery and amelioration of radiation-induced lethality by the vitamin E isoform δ-tocotrienol. Radiat. Res. 2011, 175, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.A.; Burger, W.C.; Peterson, D.M.; Elson, C.E. The structure of an inhibitor of cholesterol biosynthesis isolated from barley. J. Biol. Chem. 1986, 261, 10544–10550. [Google Scholar] [PubMed]
- Singh, V.K.; Kulkarni, S.; Fatanmi, O.O.; Wise, S.Y.; Newman, V.L.; Romaine, P.L.; Hendrickson, H.; Gulani, J.; Ghosh, S.P.; Kumar, K.S.; et al. Radioprotective efficacy of γ-tocotrienol in nonhuman primates. Radiat. Res. 2016, 185, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.; Singh, P.K.; Ghosh, S.P.; Posarac, A.; Singh, V.K. Granulocyte colony-stimulating factor antibody abrogates radioprotective efficacy of γ-tocotrienol, a promising radiation countermeasure. Cytokine 2013, 62, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Berbee, M.; Fu, Q.; Boerma, M.; Pathak, R.; Zhou, D.; Kumar, K.S.; Hauer-Jensen, M. Reduction of radiation-induced vascular nitrosative stress by the vitamin E analog γ-tocotrienol: Evidence of a role for tetrahydrobiopterin. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 884–891. [Google Scholar] [CrossRef] [PubMed]
- Berbee, M.; Fu, Q.; Boerma, M.; Wang, J.; Kumar, K.S.; Hauer-Jensen, M. γ-Tocotrienol ameliorates intestinal radiation injury and reduces vascular oxidative stress after total-body irradiation by an HMG-CoA reductase-dependent mechanism. Radiat. Res. 2009, 171, 596–605. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.; Ghosh, S.P.; Satyamitra, M.; Mog, S.; Hieber, K.; Romanyukha, L.; Gambles, K.; Toles, R.; Kao, T.C.; Hauer-Jensen, M.; et al. γ-Tocotrienol protects hematopoietic stem and progenitor cells in mice after total-body irradiation. Radiat. Res. 2010, 173, 738–747. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Lee, M.J.; Zhao, Y.; Yang, C.S. Metabolism of tocotrienols in animals and synergistic inhibitory actions of tocotrienols with atorvastatin in cancer cells. Genes Nutr. 2012, 7, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Naito, Y.; Shimozawa, M.; Kuroda, M.; Nakabe, N.; Manabe, H.; Katada, K.; Kokura, S.; Ichikawa, H.; Yoshida, N.; Noguchi, N.; et al. Tocotrienols reduce 25-hydroxycholesterol-induced monocyte-endothelial cell interaction by inhibiting the surface expression of adhesion molecules. Atherosclerosis 2005, 180, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.; Ma, X.; Li, X.; Wang, X.; Mei, Q.; Li, X.; Wu, Z.; Han, W. The Accomplices of NF-κB Lead to Radioresistance. Curr. Protein Pept. Sci. 2015, 16, 279–294. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Park, N.Y.; Jang, Y.; Ma, A.; Jiang, Q. Vitamin E γ-tocotrienol inhibits cytokine-stimulated NF-κB activation by induction of anti-inflammatory A20 via stress adaptive response due to modulation of sphingolipids. J. Immunol. 2015, 195, 126–133. [Google Scholar] [CrossRef]
- Kulkarni, S.S.; Cary, L.H.; Gambles, K.; Hauer-Jensen, M.; Kumar, K.S.; Ghosh, S.P. γ-Tocotrienol, a radiation prophylaxis agent, induces high levels of granulocyte colony-stimulating factor. Int. Immunopharmacol. 2012, 14, 495–503. [Google Scholar] [CrossRef]
- Singh, V.K.; Newman, V.L.; Seed, T.M. Colony-stimulating factors for the treatment of the hematopoietic component of the acute radiation syndrome (H-ARS): A review. Cytokine 2015, 71, 22–37. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Yadav, V.S. Role of cytokines and growth factors in radioprotection. Exp. Mol. Pathol. 2005, 78, 156–169. [Google Scholar] [CrossRef]
- Singh, V.K.; Fatanmi, O.O.; Singh, P.K.; Whitnall, M.H. Role of radiation-induced granulocyte colony-stimulating factor in recovery from whole body γ-irradiation. Cytokine 2012, 58, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Grace, M.B.; Singh, V.K.; Rhee, J.G.; Jackson, W.E., 3rd; Kao, T.C.; Whitnall, M.H. 5-AED enhances survival of irradiated mice in a G-CSF-dependent manner, stimulates innate immune cell function, reduces radiation-induced DNA damage and induces genes that modulate cell cycle progression and apoptosis. J. Radiat. Res. 2012, 53, 840–853. [Google Scholar] [CrossRef] [PubMed]
- Krivokrysenko, V.I.; Shakhov, A.N.; Singh, V.K.; Bone, F.; Kononov, Y.; Shyshynova, I.; Cheney, A.; Maitra, R.K.; Purmal, A.; Whitnall, M.H.; et al. Identification of granulocyte colony-stimulating factor and interleukin-6 as candidate biomarkers of CBLB502 efficacy as a medical radiation countermeasure. J. Pharmacol. Exp. Ther. 2012, 343, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Wise, S.Y.; Scott, J.R.; Romaine, P.L.; Newman, V.L.; Fatanmi, O.O. Radioprotective efficacy of delta-tocotrienol, a vitamin E isoform, is mediated through granulocyte colony-stimulating factor. Life Sci. 2014, 98, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Brown, D.S.; Kao, T.C.; Seed, T.M. Preclinical development of a bridging therapy for radiation casualties. Exp. Hematol. 2010, 38, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Kulkarni, S.S.; Chakraborty, K.; Pessu, R.; Hauer-Jensen, M.; Kumar, K.S.; Ghosh, S.P. Mobilization of progenitor cells into peripheral blood by γ-tocotrienol: A promising radiation countermeasure. Int. Immunopharmacol. 2013, 15, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Wise, S.Y.; Fatanmi, O.O.; Scott, J.; Romaine, P.L.; Newman, V.L.; Verma, A.; Elliott, T.B.; Seed, T.M. Progenitors mobilized by γ-tocotrienol as an effective radiation countermeasure. PLoS ONE 2014, 9, e114078. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Wise, S.Y.; Singh, P.K.; Ducey, E.J.; Fatanmi, O.O.; Seed, T.M. α-Tocopherol succinate- and AMD3100-mobilized progenitors mitigate radiation-induced gastrointestinal injury in mice. Exp. Hematol. 2012, 40, 407–417. [Google Scholar] [CrossRef]
- Singh, V.K.; Wise, S.Y.; Singh, P.K.; Posarac, A.; Fatanmi, O.O.; Ducey, E.J.; Bolduc, D.L.; Elliott, T.B.; Seed, T.M. α-Tocopherol succinate-mobilized progenitors improve intestinal integrity after whole body irradiation. Int. J. Radiat. Biol. 2013, 89, 334–345. [Google Scholar] [CrossRef]
- Bockorny, B.; Dasanu, C.A. HMG-CoA reductase inhibitors as adjuvant treatment for hematologic malignancies: What is the current evidence? Ann. Hematol. 2015, 94, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Berbee, M.; Fu, Q.; Garg, S.; Kulkarni, S.; Kumar, K.S.; Hauer-Jensen, M. Pentoxifylline enhances the radioprotective properties of γ-tocotrienol: Differential effects on the hematopoietic, gastrointestinal and vascular systems. Radiat. Res. 2011, 175, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.; Chakraborty, K.; Kumar, K.S.; Kao, T.C.; Hauer-Jensen, M.; Ghosh, S.P. Synergistic radioprotection by γ-tocotrienol and pentoxifylline: Role of cAMP signaling. ISRN Radiol. 2013, 2013, 390379. [Google Scholar] [CrossRef] [PubMed]
- Seed, T.M.; Inal, C.E.; Singh, V.K. Radioprotection of hematopoietic progenitors by low dose amifostine prophylaxis. Int. J. Radiat. Biol. 2014, 90, 594–604. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Singh, V.K.; Fatanmi, O.O.; Wise, S.Y.; Newman, V.L.; Romaine, L.P.; Seed, T.M. The potentiation of the radioprotective efficacy of two medical countermeasures, γ-tocotrienol and amifostine, by a combination prophylactic modality. Radiat. Prot. Dosimet. 2016. Communicated. [Google Scholar]
- Pathak, R.; Shao, L.; Ghosh, S.P.; Zhou, D.; Boerma, M.; Weiler, H.; Hauer-Jensen, M. Thrombomodulin contributes to gamma tocotrienol-mediated lethality protection and hematopoietic cell recovery in irradiated mice. PLoS ONE 2015, 10, e0122511. [Google Scholar] [CrossRef]
- Fliedner, T.M.; Friesecke, I.; Beyrer, K. Medical Management of Radiation Accidents—Manual on the Acute Radiation Syndrome; British Institute of Radiology: Oxford, UK, 2001; pp. 18–27. [Google Scholar]
- Farese, A.M.; Cohen, M.V.; Katz, B.P.; Smith, C.P.; Gibbs, A.; Cohen, D.M.; MacVittie, T.J. Filgrastim improves survival in lethally irradiated nonhuman primates. Radiat. Res. 2013, 179, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Farese, A.M.; Cohen, M.V.; Stead, R.B.; Jackson, W., 3rd; Macvittie, T.J. Pegfilgrastim administered in an abbreviated schedule, significantly improved neutrophil recovery after high-dose radiation-induced myelosuppression in rhesus macaques. Radiat. Res. 2012, 178, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Monroy, R.L.; Skelly, R.R.; Taylor, P.; Dubois, A.; Donahue, R.E.; MacVittie, T.J. Recovery from severe hematopoietic suppression using recombinant human granulocyte-macrophage colony-stimulating factor. Exp. Hematol. 1988, 16, 344–348. [Google Scholar] [PubMed]
- Basile, L.A.; Ellefson, D.; Gluzman-Poltorak, Z.; Junes-Gill, K.; Mar, V.; Mendonca, S.; Miller, J.D.; Tom, J.; Trinh, A.; Gallaher, T.K. HemaMax, a recombinant human interleukin-12, is a potent mitigator of acute radiation injury in mice and non-human primates. PLoS ONE 2012, 7, e30434. [Google Scholar] [CrossRef]
- Gluzman-Poltorak, Z.; Mendonca, S.R.; Vainstein, V.; Kha, H.; Basile, L.A. Randomized comparison of single dose of recombinant human IL-12 versus placebo for restoration of hematopoiesis and improved survival in rhesus monkeys exposed to lethal radiation. J. Hematol. Oncol. 2014, 7, 31. [Google Scholar] [CrossRef] [PubMed]
- Burdelya, L.G.; Krivokrysenko, V.I.; Tallant, T.C.; Strom, E.; Gleiberman, A.S.; Gupta, D.; Kurnasov, O.V.; Fort, F.L.; Osterman, A.L.; Didonato, J.A.; et al. An agonist of Toll-like receptor 5 has radioprotective activity in mouse and primate models. Science 2008, 320, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Stickney, D.R.; Dowding, C.; Authier, S.; Garsd, A.; Onizuka-Handa, N.; Reading, C.; Frincke, J.M. 5-Androstenediol improves survival in clinically unsupported rhesus monkeys with radiation-induced myelosuppression. Int. Immunopharmacol. 2007, 7, 500–505. [Google Scholar] [CrossRef]
- Hankey, K.G.; Farese, A.M.; Blaauw, E.C.; Gibbs, A.M.; Smith, C.P.; Katz, B.P.; Tong, Y.; Prado, K.L.; MacVittie, T.J. Pegfilgrastim Improves Survival of Lethally Irradiated Nonhuman Primates. Radiat. Res. 2015, 183, 643–655. [Google Scholar] [CrossRef] [PubMed]
- Gluzman-Poltorak, Z.; Vainstein, V.; Basile, L.A. Recombinant interleukin-12, but not granulocyte-colony stimulating factor, improves survival in lethally irradiated nonhuman primates in the absence of supportive care: Evidence for the development of a frontline radiation medical countermeasure. Am. J. Hematol. 2014, 89, 868–873. [Google Scholar] [CrossRef] [PubMed]
- Krivokrysenko, V.I.; Toshkov, I.A.; Gleiberman, A.S.; Krasnov, P.; Shyshynova, I.; Bespalov, I.; Maitra, R.K.; Narizhneva, N.V.; Singh, V.K.; Whitnall, M.H.; et al. The Toll-like receptor 5 agonist Entolimod mitigates lethal acute radiation syndrome in non-human primates. PLoS ONE 2015, 10, e0135388. [Google Scholar] [CrossRef] [PubMed]
- Stickney, D.R.; Dowding, C.; Garsd, A.; Ahlem, C.; Whitnall, M.; McKeon, M.; Reading, C.; Frincke, J. 5-Androstenediol stimulates multilineage hematopoiesis in rhesus monkeys with radiation-induced myelosuppression. Int. Immunopharmacol. 2006, 6, 1706–1713. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.W.; Tudor, G.; Bennett, A.; Farese, A.M.; Moroni, M.; Booth, C.; MacVittie, T.J.; Kane, M.A. Development and validation of a LC–MS/MS assay for quantitation of plasma citrulline for application to animal models of the acute radiation syndrome across multiple species. Anal. Bioanal. Chem. 2014, 406, 4663–4675. [Google Scholar] [CrossRef] [PubMed]
- Burnett, A.F.; Biju, P.G.; Lui, H.; Hauer-Jensen, M. Oral interleukin 11 as a countermeasure to lethal total-body irradiation in a murine model. Radiat. Res. 2013, 180, 595–602. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jones, J.W.; Scott, A.J.; Tudor, G.; Xu, P.T.; Jackson, I.L.; Vujaskovic, Z.; Booth, C.; MacVittie, T.J.; Ernst, R.K.; Kane, M.A. Identification and quantitation of biomarkers for radiation-induced injury via mass spectrometry. Health Phys. 2014, 106, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shao, L.; Hendrickson, H.P.; Liu, L.; Chang, J.; Luo, Y.; Seng, J.; Pouliot, M.; Authier, S.; Zhou, D.; et al. Total Body Irradiation in the “Hematopoietic” Dose Range Induces Substantial Intestinal Injury in Non-Human Primates. Radiat. Res. 2015, 184, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Berbee, M.; Fu, Q.; Boerma, M.; Sree Kumar, K.; Loose, D.S.; Hauer-Jensen, M. Mechanisms underlying the radioprotective properties of γ-tocotrienol: Comparative gene expression profiling in tocol-treated endothelial cells. Genes Nutr. 2012, 7, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Serbinova, E.A.; Packer, L. Antioxidant properties of α-tocopherol and α-tocotrienol. Methods Enzymol. 1994, 234, 354–366. [Google Scholar] [PubMed]
- Muller, L.; Theile, K.; Bohm, V. In vitro antioxidant activity of tocopherols and tocotrienols and comparison of vitamin E concentration and lipophilic antioxidant capacity in human plasma. Mol. Nutr. Food Res. 2010, 54, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Nowak, G.; Bakajsova, D.; Hayes, C.; Hauer-Jensen, M.; Compadre, C.M. γ-Tocotrienol protects against mitochondrial dysfunction and renal cell death. J. Pharmacol. Exp. Ther. 2012, 340, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Khor, H.T.; Ng, T.T. Effects of administration of α-tocopherol and tocotrienols on serum lipids and liver HMG CoA reductase activity. Int. J. Food Sci. Nutr. 2000, 51, S3–S11. [Google Scholar] [CrossRef] [PubMed]
- Song, B.L.; DeBose-Boyd, R.A. Insig-dependent ubiquitination and degradation of 3-hydroxy-3-methylglutaryl coenzyme a reductase stimulated by δ- and γ-tocotrienols. J. Biol. Chem. 2006, 281, 25054–25061. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, J.L.; Brown, M.S. Regulation of the mevalonate pathway. Nature 1990, 343, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Tsuzuki, W.; Yunoki, R.; Yoshimura, H. Intestinal epithelial cells absorb γ-tocotrienol faster than α-tocopherol. Lipids 2007, 42, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Suman, S.; Datta, K.; Chakraborty, K.; Kulkarni, S.S.; Doiron, K.; Fornace, A.J., Jr.; Sree Kumar, K.; Hauer-Jensen, M.; Ghosh, S.P. Gamma tocotrienol, a potent radioprotector, preferentially upregulates expression of anti-apoptotic genes to promote intestinal cell survival. Food Chem. Toxicol. 2013, 60, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.S.; Raghavan, M.; Hieber, K.; Ege, C.; Mog, S.; Parra, N.; Hildabrand, A.; Singh, V.; Srinivasan, V.; Toles, R.; et al. Preferential radiation sensitization of prostate cancer in nude mice by nutraceutical antioxidant γ-tocotrienol. Life Sci. 2006, 78, 2099–2104. [Google Scholar] [CrossRef] [PubMed]
- Kannappan, R.; Yadav, V.R.; Aggarwal, B.B. γ-Tocotrienol but not γ-tocopherol blocks STAT3 cell signaling pathway through induction of protein-tyrosine phosphatase SHP-1 and sensitizes tumor cells to chemotherapeutic agents. J. Biol. Chem. 2010, 285, 33520–33528. [Google Scholar] [CrossRef] [PubMed]
- Kunnumakkara, A.B.; Sung, B.; Ravindran, J.; Diagaradjane, P.; Deorukhkar, A.; Dey, S.; Koca, C.; Yadav, V.R.; Tong, Z.; Gelovani, J.G.; et al. γ-Tocotrienol inhibits pancreatic tumors and sensitizes them to gemcitabine treatment by modulating the inflammatory microenvironment. Cancer Res. 2010, 70, 8695–8705. [Google Scholar] [CrossRef] [PubMed]
- Rasool, A.H.; Yuen, K.H.; Yusoff, K.; Wong, A.R.; Rahman, A.R. Dose dependent elevation of plasma tocotrienol levels and its effect on arterial compliance, plasma total antioxidant status, and lipid profile in healthy humans supplemented with tocotrienol rich vitamin E. J. Nutr. Sci. Vitaminol. (Tokyo) 2006, 52, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Mahalingam, D.; Radhakrishnan, A.K.; Amom, Z.; Ibrahim, N.; Nesaretnam, K. Effects of supplementation with tocotrienol-rich fraction on immune response to tetanus toxoid immunization in normal healthy volunteers. Eur. J. Clin. Nutr. 2011, 65, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Compadre, C.M.; Singh, A.; Thakkar, S.; Zheng, G.; Breen, P.J.; Ghosh, S.; Kiaei, M.; Boerma, M.; Varughese, K.I.; Hauer-Jensen, M. Molecular dynamics guided design of tocoflexol: A new radioprotectant tocotrienol with enhanced bioavailability. Drug Dev. Res. 2014, 75, 10–22. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, V.K.; Hauer-Jensen, M. γ-Tocotrienol as a Promising Countermeasure for Acute Radiation Syndrome: Current Status. Int. J. Mol. Sci. 2016, 17, 663. https://doi.org/10.3390/ijms17050663
Singh VK, Hauer-Jensen M. γ-Tocotrienol as a Promising Countermeasure for Acute Radiation Syndrome: Current Status. International Journal of Molecular Sciences. 2016; 17(5):663. https://doi.org/10.3390/ijms17050663
Chicago/Turabian StyleSingh, Vijay K., and Martin Hauer-Jensen. 2016. "γ-Tocotrienol as a Promising Countermeasure for Acute Radiation Syndrome: Current Status" International Journal of Molecular Sciences 17, no. 5: 663. https://doi.org/10.3390/ijms17050663
APA StyleSingh, V. K., & Hauer-Jensen, M. (2016). γ-Tocotrienol as a Promising Countermeasure for Acute Radiation Syndrome: Current Status. International Journal of Molecular Sciences, 17(5), 663. https://doi.org/10.3390/ijms17050663







