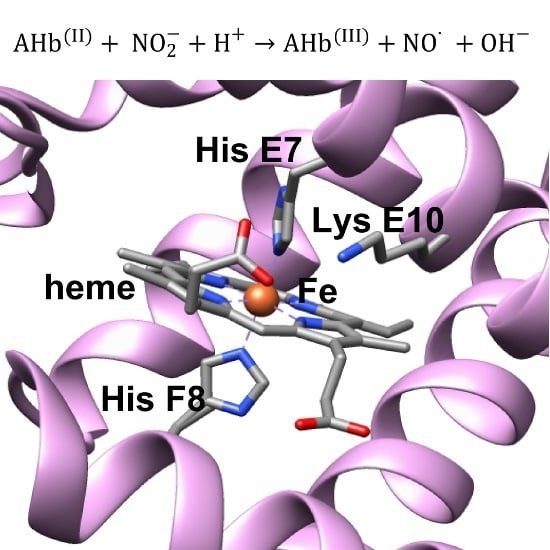
Residues in the Distal Heme Pocket of Arabidopsis Non-Symbiotic Hemoglobins: Implication for Nitrite Reductase Activity
Abstract
:1. Introduction
2. Results
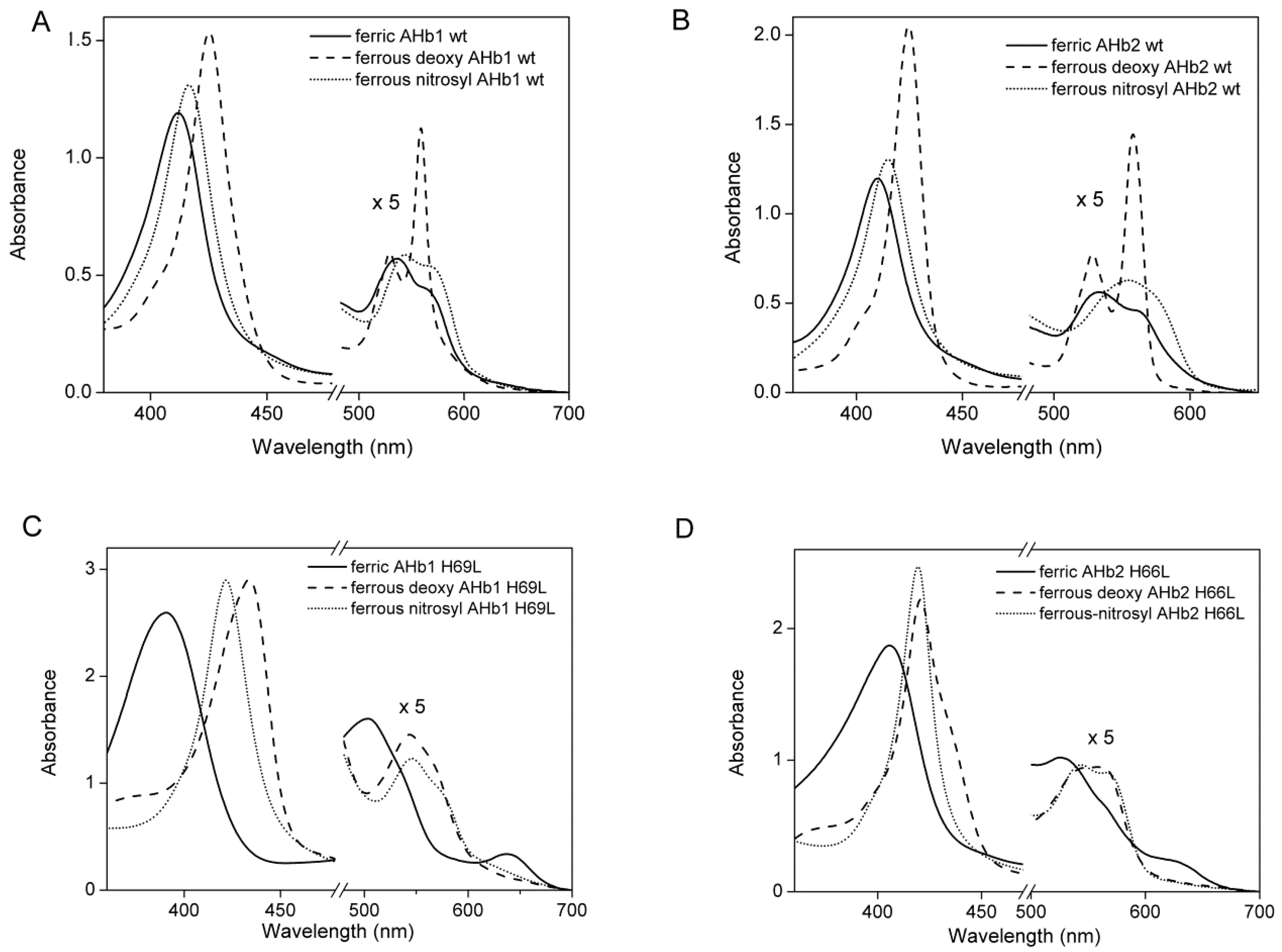
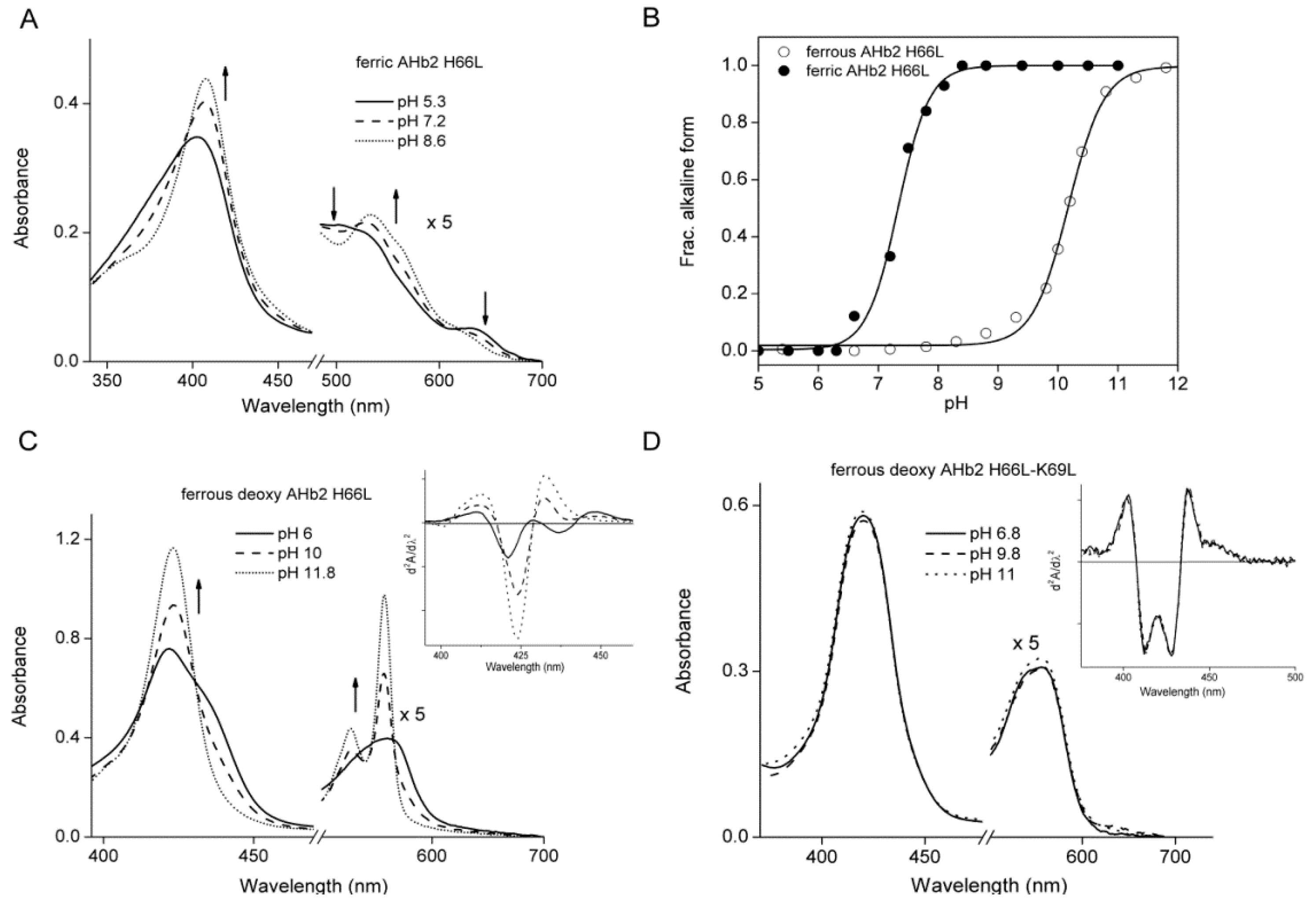
2.1. Equilibrium Conformations of Arabidopsis Hemoglobins (AHbs) in the Absence of Distal His
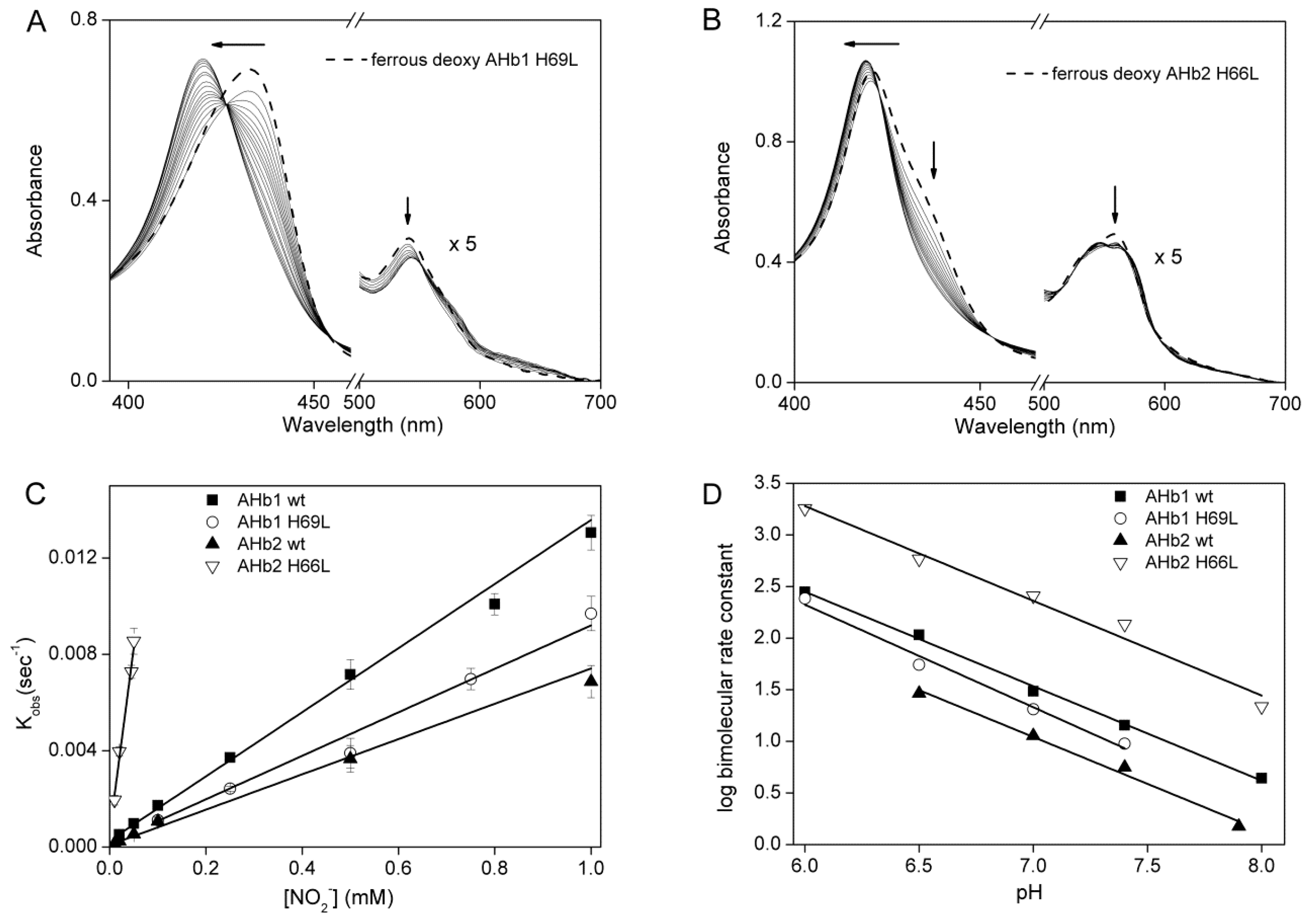
2.2. Nitrite Reductase Activity of AHbs Mutants
2.3. pH-Dependent Nitrite Reductase Activity of AHb Mutants
2.4. Detection of NO Gas by Chemiluminescence
2.5. Reaction of Nitrosyl AHbs with Peroxynitrite and H2O2
2.6. Sequence Analysis of AHbs and Homology Model of AHb2
3. Discussion
4. Materials and Methods
4.1. Reagents and AHb Preparations
4.2. Reaction of AHbs with Nitrite
4.3. NO Gas Detection by Chemiluminescence
4.4. Release of Nitric Oxide from Nitrosyl-AHb by Oxidizing Agents
4.5. Statistical Analysis
4.6. Sequence Alignment and Homology Modeling
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| Hb | Hemoglobin |
| AHb | Arabidopsis thaliana hemoglobin |
| Ngb | Neuroglobin |
| NO | Nitric oxide |
| NO2− | Nitrite |
| ROS | Reactive oxygen species |
| kobs | Observed rate constant |
| kd | Dissociation rate constant |
| E7 | Seventh amino acid at E-helix in Hb |
| Fe2+ | Ferrous |
| Fe3+ | Ferric |
References
- Stohr, C.; Ullrich, W.R. Generation and possible roles of NO in plant roots and their apoplastic space. J. Exp. Bot. 2002, 53, 2293–2303. [Google Scholar] [CrossRef] [PubMed]
- Beligni, M.V.; Lamattina, L. Nitric oxide stimulates seed germination and de-etiolation, and inhibits hypocotyl elongation, three light-inducible responses in plants. Planta 2000, 210, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Lee, U.; Wie, C.; Fernandez, B.O.; Feelisch, M.; Vierling, E. Modulation of nitrosative stress by S-nitrosoglutathione reductase is critical for thermotolerance and plant growth in Arabidopsis. Plant Cell 2008, 20, 786–802. [Google Scholar] [CrossRef] [PubMed]
- Delledonne, M.; Xia, Y.J.; Dixon, R.A.; Lamb, C. Nitric oxide functions as a signal in plant disease resistance. Nature 1998, 394, 585–588. [Google Scholar] [PubMed]
- Yu, M.; Lamattina, L.; Spoel, S.H.; Loake, G.J. Nitric oxide function in plant biology: A redox cue in deconvolution. New Phytol. 2014, 202, 1142–1156. [Google Scholar] [CrossRef] [PubMed]
- Igamberdiev, A.U.; Hill, R.D. Nitrate, NO and haemoglobin in plant adaptation to hypoxia: An alternative to classic fermentation pathways. J. Exp. Bot. 2004, 55, 2473–2482. [Google Scholar] [CrossRef] [PubMed]
- Shiva, S.; Huang, Z.; Grubina, R.; Sun, J.; Ringwood, L.A.; MacArthur, P.H.; Xu, X.; Murphy, E.; Darley-Usmar, V.M.; Gladwin, M.T. Deoxymyoglobin is a nitrite reductase that generates nitric oxide and regulates mitochondrial respiration. Circ. Res. 2007, 100, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Sturms, R.; DiSpirito, A.A.; Hargrove, M.S. Plant and Cyanobacterial Hemoglobins Reduce Nitrite to Nitric Oxide under Anoxic Conditions. Biochemistry 2011, 50, 3873–3878. [Google Scholar] [CrossRef] [PubMed]
- Tiso, M.; Tejero, J.; Kenney, C.; Frizzell, S.; Gladwin, M.T. Nitrite reductase activity of nonsymbiotic hemoglobins from Arabidopsis thaliana. Biochemistry 2012, 51, 5285–5292. [Google Scholar] [CrossRef] [PubMed]
- Tiso, M.; Tejero, J.; Basu, S.; Azarov, I.; Wang, X.; Simplaceanu, V.; Frizzell, S.; Jayaraman, T.; Geary, L.; Shapiro, C.; et al. Human neuroglobin functions as a redox-regulated nitrite reductase. J. Biol. Chem. 2011, 286, 18277–18289. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Jiang, M.; Zhang, J.; Ding, H.; Xu, S.; Hu, X.; Tan, M. Nitric oxide induced by hydrogen peroxide mediates abscisic acid-induced activation of the mitogen-activated protein kinase cascade involved in antioxidant defense in maize leaves. New Phytol. 2007, 175, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Sturms, R.; DiSpirito, A.A.; Fulton, D.B.; Hargrove, M.S. Hydroxylamine Reduction to Ammonium by Plant and Cyanobacterial Hemoglobins. Biochemistry 2011, 50, 10829–10835. [Google Scholar] [CrossRef] [PubMed]
- Hargrove, M.S.; Brucker, E.A.; Stec, B.; Sarath, G.; Arredondo-Peter, R.; Klucas, R.V.; Olson, J.S.; Phillips, G.N., Jr. Crystal structure of a nonsymbiotic plant hemoglobin. Structure 2000, 8, 1005–1014. [Google Scholar] [CrossRef]
- Smagghe, B.J.; Hoy, J.A.; Percifield, R.; Kundu, S.; Hargrove, M.S.; Sarath, G.; Hilbert, J.L.; Watts, R.A.; Dennis, E.S.; Peacock, W.J.; et al. Review: Correlations between oxygen affinity and sequence classifications of plant hemoglobins. Biopolymers 2009, 91, 1083–1096. [Google Scholar] [CrossRef] [PubMed]
- Trevaskis, B.; Watts, R.A.; Andersson, C.R.; Llewellyn, D.J.; Hargrove, M.S.; Olson, J.S.; Dennis, E.S.; Peacock, W.J. Two hemoglobin genes in Arabidopsis thaliana: The evolutionary origins of leghemoglobins. Proc. Natl. Acad. Sci. USA 1997, 94, 12230–12234. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Guegler, K.; LaBrie, S.T.; Crawford, N.M. Genomic analysis of a nutrient response in Arabidopsis reveals diverse expression patterns and novel metabolic and potential regulatory genes induced by nitrate. Plant Cell 2000, 12, 1491–1509. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.D. What are hemoglobins doing in plants? Can. J. Bot. 1998, 76, 707–712. [Google Scholar]
- Kundu, S.; Premer, S.A.; Hoy, J.A.; Trent, J.T.; Hargrove, M.S. Direct measurement of equilibrium constants for high-affinity hemoglobins. Biophys. J. 2003, 84, 3931–3940. [Google Scholar] [CrossRef]
- Perazzolli, M.; Dominici, P.; Romero-Puertas, M.C.; Zago, E.; Zeier, J.; Sonoda, M.; Lamb, C.; Delledonne, M. Arabidopsis nonsymbiotic hemoglobin AHb1 modulates nitric oxide bioactivity. Plant Cell 2004, 16, 2785–2794. [Google Scholar] [CrossRef] [PubMed]
- Hunt, P.W.; Watts, R.A.; Trevaskis, B.; Llewelyn, D.J.; Burnell, J.; Dennis, E.S.; Peacock, W.J. Expression and evolution of functionally distinct haemoglobin genes in plants. Plant Mol. Biol. 2001, 47, 677–692. [Google Scholar] [CrossRef] [PubMed]
- Vigeolas, H.; Huhn, D.; Geigenberger, P. Nonsymbiotic hemoglobin-2 leads to an elevated energy state and to a combined increase in polyunsaturated fatty acids and total oil content when overexpressed in developing seeds of transgenic Arabidopsis plants. Plant Physiol. 2011, 155, 1435–1444. [Google Scholar] [CrossRef] [PubMed]
- Dordas, C. Nonsymbiotic hemoglobins and stress tolerance in plants. Plant Sci. 2009, 176, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Kakar, S.; Hoffman, F.G.; Storz, J.F.; Fabian, M.; Hargrove, M.S. Structure and reactivity of hexacoordinate hemoglobins. Biophys. Chem. 2010, 152, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Watts, R.A.; Hunt, P.W.; Hvitved, A.N.; Hargrove, M.S.; Peacock, W.J.; Dennis, E.S. A hemoglobin from plants homologous to truncated hemoglobins of microorganisms. Proc. Natl. Acad. Sci. USA 2001, 98, 10119–10124. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.J.; Hebelstrup, K.H.; Mur, L.A.J.; Igamberdiev, A.U. Plant hemoglobins: Important players at the crossroads between oxygen and nitric oxide. FEBS Lett. 2011, 585, 3843–3849. [Google Scholar] [CrossRef] [PubMed]
- Bruno, S.; Faggiano, S.; Spyrakis, F.; Mozzarelli, A.; Abbruzzetti, S.; Grandi, E.; Viappiani, C.; Feis, A.; Mackowiak, S.; Smulevich, G.; et al. The reactivity with CO of AHb1 and AHb2 from Arabidopsis thaliana is controlled by the distal HisE7 and internal hydrophobic cavities. J. Am. Chem. Soc. 2007, 129, 2880–2889. [Google Scholar] [CrossRef] [PubMed]
- Brunori, M.; Giuffre, A.; Nienhaus, K.; Nienhaus, G.U.; Scandurra, F.M.; Vallone, B. Neuroglobin, nitric oxide, and oxygen: Functional pathways and conformational changes. Proc. Natl. Acad. Sci. USA 2005, 102, 8483–8488. [Google Scholar] [CrossRef] [PubMed]
- Bruno, S.; Faggiano, S.; Spyrakis, F.; Mozzarelli, A.; Cacciatori, E.; Dominici, P.; Grandi, E.; Abbruzzetti, S.; Viappiani, C. Different roles of protein dynamics and ligand migration in non-symbiotic hemoglobins AHb1 and AHb2 from Arabidopsis thaliana. Gene 2007, 398, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Abbruzzetti, S.; Faggiano, S.; Spyrakis, F.; Bruno, S.; Mozzarelli, A.; Astegno, A.; Dominici, P.; Viappiani, C. Oxygen and nitric oxide rebinding kinetics in nonsymbiotic hemoglobin AHb1 from Arabidopsis thaliana. IUBMB Life 2011, 63, 1094–1100. [Google Scholar] [CrossRef] [PubMed]
- Nienhaus, K.; Dominici, P.; Astegno, A.; Abbruzzetti, S.; Viappiani, C.; Nienhaus, G.U. Ligand migration and binding in nonsymbiotic hemoglobins of Arabidopsis thaliana. Biochemistry 2010, 49, 7448–7458. [Google Scholar] [CrossRef] [PubMed]
- Gladwin, M.T.; Grubina, R.; Doyle, M.P. The new chemical biology of nitrite reactions with hemoglobin: R-state catalysis, oxidative denitrosylation, and nitrite reductase/anhydrase. Acc. Chem. Res. 2009, 42, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Quillin, M.L.; Arduini, R.M.; Olson, J.S.; Phillips, G.N., Jr. High-resolution crystal structures of distal histidine mutants of sperm whale myoglobin. J. Mol. Biol. 1993, 234, 140–155. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, T.; Yonetani, T. Spin changes in hemoproteins. Adv. Biophys. 1970, 1, 157–182. [Google Scholar] [PubMed]
- Uno, T.; Ryu, D.; Tsutsumi, H.; Tomisugi, Y.; Ishikawa, Y.; Wilkinson, A.J.; Sato, H.; Hayashi, T. Residues in the distal heme pocket of neuroglobin. Implications for the multiple ligand binding steps. J. Biol. Chem. 2004, 279, 5886–5893. [Google Scholar] [CrossRef] [PubMed]
- Nienhaus, K.; Kriegl, J.M.; Nienhaus, G.U. Structural dynamics in the active site of murine neuroglobin and its effects on ligand binding. J. Biol. Chem. 2004, 279, 22944–22952. [Google Scholar] [CrossRef] [PubMed]
- Hvitved, A.N.; Trent, J.T.; Premer, S.A.; Hargrove, M.S. Ligand binding and hexacoordination in synechocystis hemoglobin. J. Biol. Chem. 2001, 276, 34714–34721. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, A.; Suzuki, T.; Shin, M. An enzymic reduction system for metmyoglobin and methemoglobin, and its application to functional studies of oxygen carriers. Biochim. Biophys. Acta 1973, 310, 309–316. [Google Scholar] [CrossRef]
- Herold, S. The outer-sphere oxidation of nitrosyliron(II)hemoglobin by peroxynitrite leads to the release of nitrogen monoxide. Inorg. Chem. 2004, 43, 3783–3785. [Google Scholar] [CrossRef] [PubMed]
- Gladwin, M.T.; Ognibene, F.P.; Pannell, L.K.; Nichols, J.S.; Pease-Fye, M.E.; Shelhamer, J.H.; Schechter, A.N. Relative role of heme nitrosylation and β-cysteine 93 nitrosation in the transport and metabolism of nitric oxide by hemoglobin in the human circulation. Proc. Natl. Acad. Sci. USA 2000, 97, 9943–9948. [Google Scholar] [CrossRef] [PubMed]
- Gladwin, M.T.; Wang, X.; Reiter, C.D.; Yang, B.K.; Vivas, E.X.; Bonaventura, C.; Schechter, A.N. S-Nitrosohemoglobin is unstable in the reductive erythrocyte environment and lacks O2/NO-linked allosteric function. J. Biol. Chem. 2002, 277, 27818–27828. [Google Scholar] [CrossRef] [PubMed]
- Giuffre, A.; Moschetti, T.; Vallone, B.; Brunori, M. Neuroglobin: Enzymatic reduction and oxygen affinity. Biochem. Biophys. Res. Commun. 2008, 367, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Vallone, B.; Nienhaus, K.; Brunori, M.; Nienhaus, G.U. The structure of murine neuroglobin: Novel pathways for ligand migration and binding. Proteins 2004, 56, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Weiland, T.R.; Kundu, S.; Trent, J.T.; Hoy, J.A.; Hargrove, M.S. Bis-histidyl hexacoordination in hemoglobins facilitates heme reduction kinetics. J. Am. Chem. Soc. 2004, 126, 11930–11935. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Heinecke, J.; Tan, H.; Ford, P.C.; Richter-Addo, G.B. The distal pocket histidine residue in horse heart myoglobin directs the O-binding mode of nitrite to the heme iron. J. Am. Chem. Soc. 2009, 131, 18119–18128. [Google Scholar] [CrossRef] [PubMed]
- Milani, M.; Pesce, A.; Ouellet, Y.; Dewilde, S.; Friedman, J.; Ascenzi, P.; Guertin, M.; Bolognesi, M. Heme-ligand tunneling in group I truncated hemoglobins. J. Biol. Chem. 2004, 279, 21520–21525. [Google Scholar] [CrossRef] [PubMed]
- Bidon-Chanal, A.; Marti, M.A.; Crespo, A.; Milani, M.; Orozco, M.; Bolognesi, M.; Luque, F.J.; Estrin, D.A. Ligand-induced dynamical regulation of NO conversion in Mycobacterium tuberculosis truncated hemoglobin-N. Proteins 2006, 64, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Tejero, J.; Sparacino-Watkins, C.E.; Ragireddy, V.; Frizzell, S.; Gladwin, M.T. Exploring the mechanisms of the reductase activity of neuroglobin by site-directed mutagenesis of the heme distal pocket. Biochemistry 2015, 54, 722–733. [Google Scholar] [CrossRef] [PubMed]
- Smagghe, B.J.; Kundu, S.; Hoy, J.A.; Halder, P.; Weiland, T.R.; Savage, A.; Venugopal, A.; Goodman, M.; Premer, S.; Hargrove, M.S. Role of phenylalanine B10 in plant nonsymbiotic hemoglobins. Biochemistry 2006, 45, 9735–9745. [Google Scholar] [CrossRef] [PubMed]
- Faggiano, S.; Abbruzzetti, S.; Spyrakis, F.; Grandi, E.; Viappiani, C.; Bruno, S.; Mozzarelli, A.; Cozzini, P.; Astegno, A.; Dominici, P.; et al. Structural plasticity and functional implications of internal cavities in distal mutants of type 1 non-symbiotic hemoglobin AHb1 from Arabidopsis thaliana. J. Phys. Chem. B 2009, 113, 16028–16038. [Google Scholar] [CrossRef] [PubMed]
- Bisht, N.K.; Abbruzzetti, S.; Uppal, S.; Bruno, S.; Spyrakis, F.; Mozzarelli, A.; Viappiani, C.; Kundu, S. Ligand migration and hexacoordination in type 1 non-symbiotic rice hemoglobin. Biochim. Biophys. Acta 2011, 1814, 1042–1053. [Google Scholar] [CrossRef] [PubMed]
- Spyrakis, F.; Faggiano, S.; Abbruzzetti, S.; Dominici, P.; Cacciatori, E.; Astegno, A.; Droghetti, E.; Feis, A.; Smulevich, G.; Bruno, S.; et al. Histidine E7 dynamics modulates ligand exchange between distal pocket and solvent in AHb1 from Arabidopsis thaliana. J. Phys. Chem. B 2011, 115, 4138–4146. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.J.; Igamberdiev, A.U. The anoxic plant mitochondrion as a nitrite: NO reductase. Mitochondrion 2011, 11, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Gladwin, M.T.; Kim-Shapiro, D.B. The functional nitrite reductase activity of the heme-globins. Blood 2008, 112, 2636–2647. [Google Scholar] [CrossRef] [PubMed]
- Vandelle, E.; Delledonne, M. Peroxynitrite formation and function in plants. Plant Sci. 2011, 181, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Exner, M.; Herold, S. Kinetic and mechanistic studies of the peroxynitrite-mediated oxidation of oxymyoglobin and oxyhemoglobin. Chem. Res. Toxicol. 2000, 13, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, T.E.; Varner, J.E. Intact tissue assay for nitrite reductase in barley aleurone layers. Plant Physiol. 1971, 47, 790–794. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.J.; Fernie, A.R.; Kaiser, W.M.; van Dongen, J.T. On the origins of nitric oxide. Trends Plant. Sci. 2011, 16, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.K.; Callis, J.; Jardetzky, O.; Walbot, V.; Freeling, M. Cytoplasmic acidosis as a determinant of flooding intolerance in plants. Proc. Natl. Acad. Sci. USA 1984, 81, 6029–6033. [Google Scholar] [CrossRef] [PubMed]
- Scott, N.L.; Xu, Y.; Shen, G.; Vuletich, D.A.; Falzone, C.J.; Li, Z.; Ludwig, M.; Pond, M.P.; Preimesberger, M.R.; Bryant, D.A.; et al. Functional and structural characterization of the 2/2 hemoglobin from Synechococcus sp. PCC 7002. Biochemistry 2010, 49, 7000–7011. [Google Scholar] [CrossRef] [PubMed]
- Hebelstrup, K.H.; van Zanten, M.; Mandon, J.; Voesenek, L.A.; Harren, F.J.; Cristescu, S.M.; Moller, I.M.; Mur, L.A. Haemoglobin modulates NO emission and hyponasty under hypoxia-related stress in Arabidopsis thaliana. J. Exp. Bot. 2012, 63, 5581–5591. [Google Scholar] [CrossRef] [PubMed]
- Hebelstrup, K.H.; Shah, J.K.; Simpson, C.; Schjoerring, J.K.; Mandon, J.; Cristescu, S.M.; Harren, F.J.; Christiansen, M.W.; Mur, L.A.; Igamberdiev, A.U. An assessment of the biotechnological use of hemoglobin modulation in cereals. Physiol. Plant. 2014, 150, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Riggs, A. Preparation of blood hemoglobins of vertebrates. Methods Enzym. 1981, 76, 5–29. [Google Scholar]
- Salhany, J.M. Kinetics of reaction of nitrite with deoxy hemoglobin after rapid deoxygenation or predeoxygenation by dithionite measured in solution and bound to the cytoplasmic domain of band 3 (SLC4A1). Biochemistry 2008, 47, 6059–6072. [Google Scholar] [CrossRef] [PubMed]
- Jensen, F.B. Nitric oxide formation from nitrite in zebrafish. J. Exp. Biol. 2007, 210 Pt 19, 3387–3394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smagghe, B.J.; Trent, J.T., III; Hargrove, M.S. NO Dioxygenase Activity in Hemoglobins Is Ubiquitous in Vitro, but Limited by Reduction in Vivo. PLoS ONE 2008, 3, e2039. [Google Scholar] [CrossRef] [PubMed]
- Sievers, F.; Wilm, A.; Dineen, D.; Gibson, T.J.; Karplus, K.; Li, W.; Lopez, R.; McWilliam, H.; Remmert, M.; Soding, J.; Thompson, J.D.; et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011, 7, 539. [Google Scholar] [CrossRef] [PubMed]
- Goujon, M.; McWilliam, H.; Li, W.; Valentin, F.; Squizzato, S.; Paern, J.; Lopez, R. A new bioinformatics analysis tools framework at EMBL-EBI. Nucleic Acids Res. 2010, 38 (Suppl. S2), W695–W699. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]



| AHb Proteins | Ligation State | pH | pK | Soret | Visible Bands |
|---|---|---|---|---|---|
| nm | nm | ||||
| AHb1 H69L | |||||
| Ferric | Five-coordinate | 6–9 | 390 | 504/637 | |
| Ferrous-deoxy | Five-coordinate | 6–9 | 435 | 540/569 | |
| AHb2 H66L | |||||
| Ferric | Five/six-coordinate | 6 | 7.2 | 404 | 500/631 |
| 8 | 408 | 532/562 | |||
| 11 | 10 | 409 | 535/568 | ||
| Ferrous-deoxy | Five/six-coordinate | 6 | 422/436 | 560 | |
| 11 | 10 | 422 | 527/557 | ||
| AHb2 H66L-K69L | |||||
| Ferric | Five-coordinate | 6–11 | 397 | 498/624 | |
| Ferrous-deoxy | Five-coordinate | 6–11 | 420/436 | 538/560 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, N.; Astegno, A.; Chen, J.; Giorgetti, A.; Dominici, P. Residues in the Distal Heme Pocket of Arabidopsis Non-Symbiotic Hemoglobins: Implication for Nitrite Reductase Activity. Int. J. Mol. Sci. 2016, 17, 640. https://doi.org/10.3390/ijms17050640
Kumar N, Astegno A, Chen J, Giorgetti A, Dominici P. Residues in the Distal Heme Pocket of Arabidopsis Non-Symbiotic Hemoglobins: Implication for Nitrite Reductase Activity. International Journal of Molecular Sciences. 2016; 17(5):640. https://doi.org/10.3390/ijms17050640
Chicago/Turabian StyleKumar, Nitin, Alessandra Astegno, Jian Chen, Alejandro Giorgetti, and Paola Dominici. 2016. "Residues in the Distal Heme Pocket of Arabidopsis Non-Symbiotic Hemoglobins: Implication for Nitrite Reductase Activity" International Journal of Molecular Sciences 17, no. 5: 640. https://doi.org/10.3390/ijms17050640