SAAFEC: Predicting the Effect of Single Point Mutations on Protein Folding Free Energy Using a Knowledge-Modified MM/PBSA Approach
Abstract
:1. Introduction
2. Results
2.1. Optimizing MM/PBSA Parameters
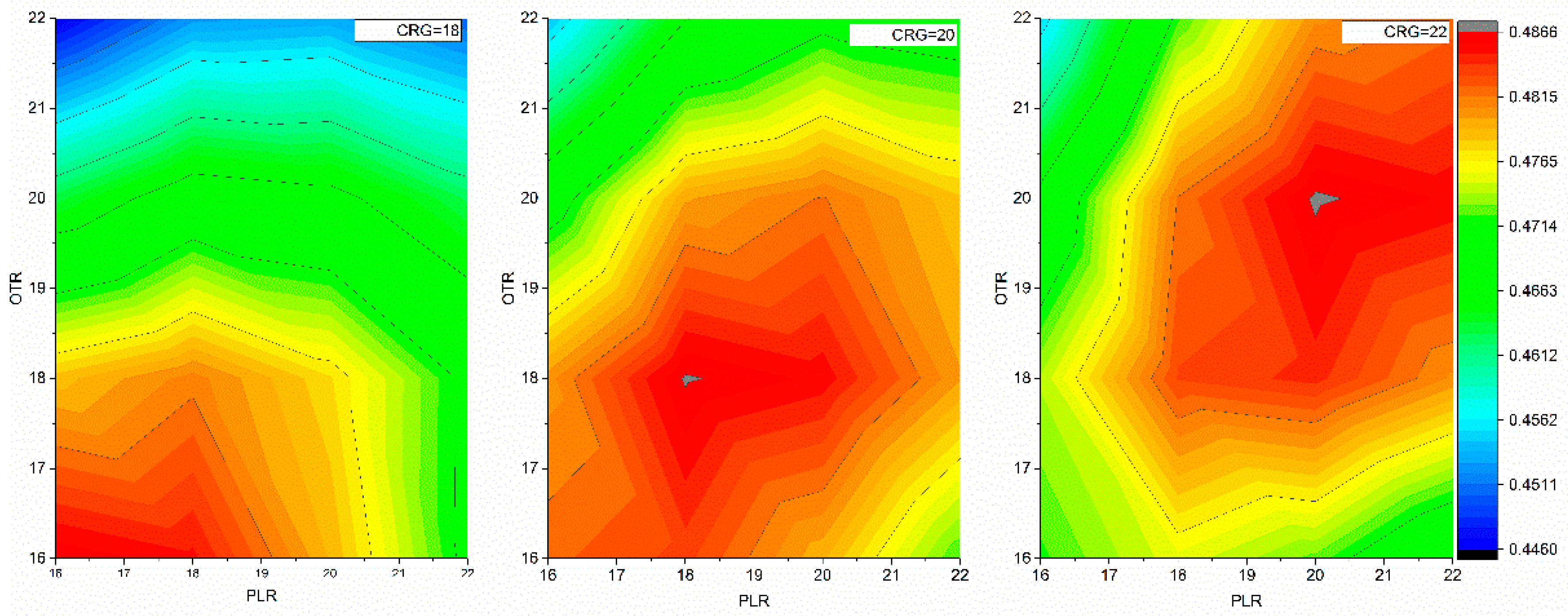
2.1.1. Determining Optimal Minimization Steps for the NAMD Protocol and for Finding the Dielectric Constants of the Generalized Born (GB) Model
2.1.2. Determining the Dielectric Constants for Various Regions in Protein Structure for the Poisson-Boltzmann (PB) Solvation Energy Calculations
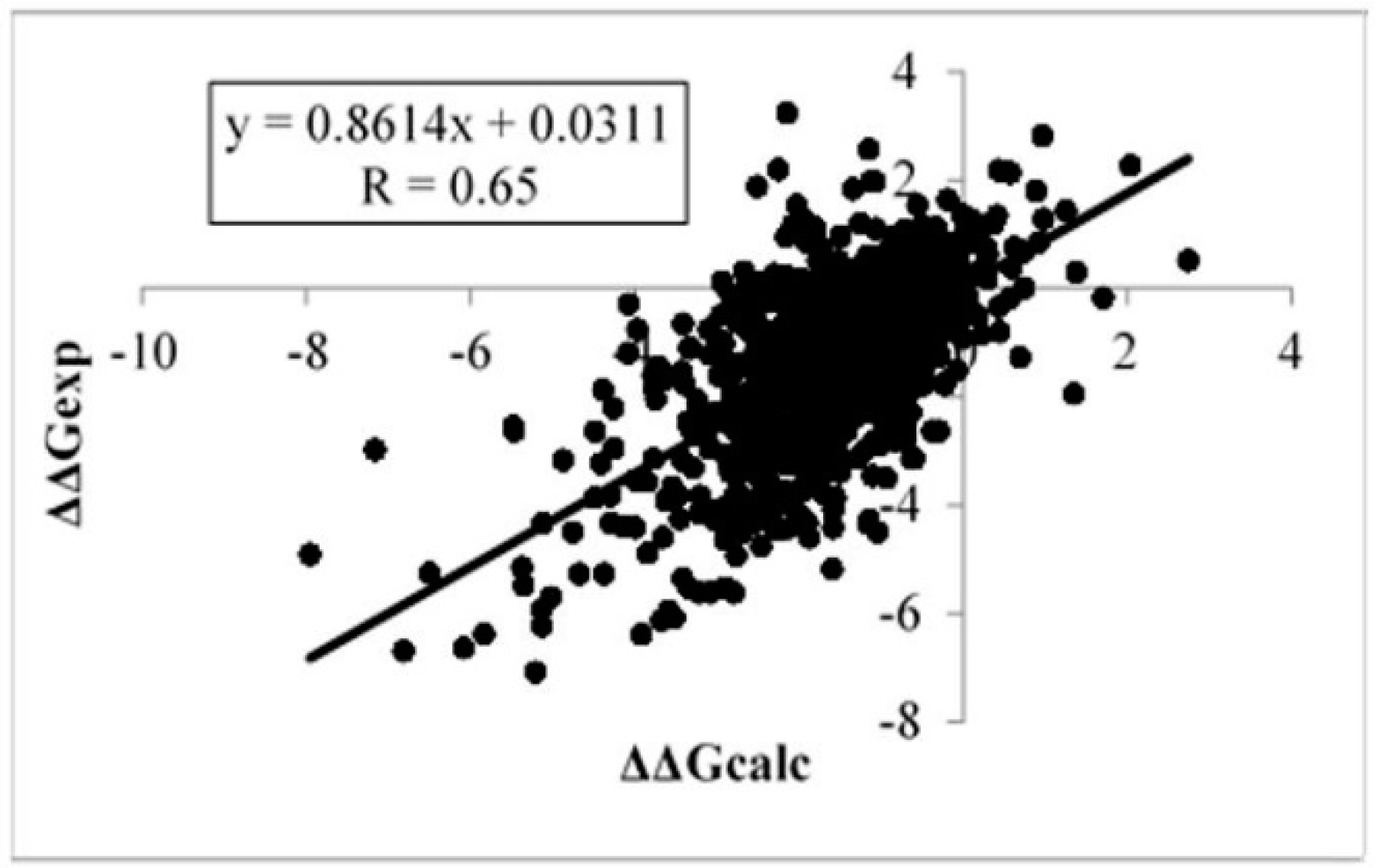
2.2. Statistical Analysis
2.3. Optimizing Weight Coeficients and Benchmarking Results
3. Discussion
4. Materials and Methods
4.1. Construction of the Experimental Dataset
4.2. Degree of Burial
4.3. Secondary Structure Element
4.4. Simulation Protocol
4.5. Free Folding Energy Calculations
4.6. The MM/PBSA-Based Components of the SAAFEC Method
4.7. The Knowledge-Based Components of the SAAFEC Method
4.8. Combining MM/PBSA-Based and Knowledge-Based Terms
5. Web Server Architecture
5.1. General Description
5.2. User Interface
5.3. Back End
5.4. Results Page
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yue, P.; Li, Z.; Moult, J. Loss of protein structure stability as a major causative factor in monogenic disease. J. Mol. Biol. 2005, 353, 459–473. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Miteva, M.A.; Wang, L.; Alexov, E. Analyzing effects of naturally occurring missense mutations. Comput. Math. Methods Med. 2012, 2012, 805827. [Google Scholar] [CrossRef] [PubMed]
- Kucukkal, T.G.; Petukh, M.; Li, L.; Alexov, E. Structural and physico-chemical effects of disease and non-disease nsSNPs on proteins. Curr. Opin. Struct. Biol. 2015, 32, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Kucukkal, T.G.; Yang, Y.; Chapman, S.C.; Cao, W.; Alexov, E. Computational and experimental approaches to reveal the effects of single nucleotide polymorphisms with respect to disease diagnostics. Int. J. Mol. Sci. 2014, 15, 9670–9717. [Google Scholar] [CrossRef] [PubMed]
- Stefl, S.; Nishi, H.; Petukh, M.; Panchenko, A.R.; Alexov, E. Molecular mechanisms of disease-causing missense mutations. J. Mol. Biol. 2013, 425, 3919–3936. [Google Scholar] [CrossRef] [PubMed]
- Dolzhanskaya, N.; Gonzalez, M.A.; Sperziani, F.; Stefl, S.; Messing, J.; Wen, G.Y.; Alexov, E.; Zuchner, S.; Velinov, M. A novel p.Leu(381)Phe mutation in presenilin 1 is associated with very early onset and unusually fast progressing dementia as well as lysosomal inclusions typically seen in Kufs disease. J. Alzheimers Dis. 2014, 39, 23–27. [Google Scholar] [PubMed]
- Boccuto, L.; Aoki, K.; Flanagan-Steet, H.; Chen, C.F.; Fan, X.; Bartel, F.; Petukh, M.; Pittman, A.; Saul, R.; Chaubey, A.; et al. A mutation in a ganglioside biosynthetic enzyme, ST3GAL5, results in salt & pepper syndrome, a neurocutaneous disorder with altered glycolipid and glycoprotein glycosylation. Hum. Mol. Genet. 2014, 23, 418–433. [Google Scholar] [PubMed]
- Zhang, Z.; Norris, J.; Kalscheuer, V.; Wood, T.; Wang, L.; Schwartz, C.; Alexov, E.; van Esch, H. A Y328C missense mutation in spermine synthase causes a mild form of Snyder-Robinson syndrome. Hum. Mol. Genet. 2013, 22, 3789–3797. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Teng, S.; Wang, L.; Schwartz, C.E.; Alexov, E. Computational analysis of missense mutations causing Snyder-Robinson syndrome. Hum. Mutat. 2010, 31, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Kucukkal, T.G.; Yang, Y.; Uvarov, O.; Cao, W.; Alexov, E. Impact of rett syndrome mutations on MeCP2 MBD Stability. Biochemistry 2015, 54, 6357–6368. [Google Scholar] [CrossRef] [PubMed]
- May, M.; Hwang, K.S.; Miles, J.; Williams, C.; Niranjan, T.; Kahler, S.G.; Chiurazzi, P.; Steindl, K.; van der Spek, P.J.; Swagemakers, S.; et al. ZC4H2, an XLID gene, is required for the generation of a specific subset of CNS interneurons. Hum. Mol. Genet. 2015, 24, 4848–4861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, Y.; Suryadi, J.; Yang, Y.; Kucukkal, T.G.; Cao, W.; Alexov, E. Mutations in the KDM5C ARID domain and their plausible association with syndromic Claes-Jensen-Type disease. Int. J. Mol. Sci. 2015, 16, 27270–27287. [Google Scholar] [CrossRef] [PubMed]
- Takano, K.; Liu, D.; Tarpey, P.; Gallant, E.; Lam, A.; Witham, S.; Alexov, E.; Chaubey, A.; Stevenson, R.E.; Schwartz, C.E.; et al. An X-linked channelopathy with cardiomegaly due to a CLIC2 mutation enhancing ryanodine receptor channel activity. Hum. Mol. Genet. 2012, 21, 4497–4507. [Google Scholar] [CrossRef] [PubMed]
- Alexov, E.; Sternberg, M. Understanding molecular effects of naturally occurring genetic differences. J. Mol. Biol. 2013, 425, 3911–3913. [Google Scholar] [CrossRef] [PubMed]
- Thusberg, J.; Olatubosun, A.; Vihinen, M. Performance of mutation pathogenicity prediction methods on missense variants. Hum. Mutat. 2011, 32, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Capriotti, E.; Fariselli, P.; Casadio, R. I-Mutant2.0: Predicting stability changes upon mutation from the protein sequence or structure. Nucleic Acids Res. 2005, 33, W306–W310. [Google Scholar] [CrossRef] [PubMed]
- Van Durme, J.; Delgado, J.; Stricher, F.; Serrano, L.; Schymkowitz, J.; Rousseau, F. A graphical interface for the FoldX forcefield. Bioinformatics 2011, 27, 1711–1712. [Google Scholar] [CrossRef] [PubMed]
- Schymkowitz, J.; Borg, J.; Stricher, F.; Nys, R.; Rousseau, F.; Serrano, L. The FoldX web server: An online force field. Nucleic Acids Res. 2005, 33, W382–W388. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Ding, F.; Dokholyan, N.V. Eris: An automated estimator of protein stability. Nat. Methods 2007, 4, 466–467. [Google Scholar] [CrossRef] [PubMed]
- Dehouck, Y.; Kwasigroch, J.M.; Gilis, D.; Rooman, M. PoPMuSiC 2.1: A web server for the estimation of protein stability changes upon mutation and sequence optimality. BMC Bioinform. 2011, 12, 151. [Google Scholar] [CrossRef] [PubMed]
- Gilis, D.; Rooman, M. PoPMuSiC, an algorithm for predicting protein mutant stability changes: Application to prion proteins. Protein Eng. 2000, 13, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, L.; Gao, Y.; Zhang, J.; Zhenirovskyy, M.; Alexov, E. Predicting folding free energy changes upon single point mutations. Bioinformatics 2012, 28, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Petukh, M.; Kucukkal, T.G.; Alexov, E. On human disease-causing amino acid variants: Statistical study of sequence and structural patterns. Hum. Mutat. 2015, 36, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Vihinen, M. Evaluation of accuracy and applicability of protein models: Retrospective analysis of biological and biomedical predictions. Silico Biol. 2009, 9, 307–331. [Google Scholar]
- Khan, S.; Vihinen, M. Performance of protein stability predictors. Hum. Mutat. 2010, 31, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Bava, K.A.; Gromiha, M.M.; Uedaira, H.; Kitajima, K.; Sarai, A. ProTherm, version 4.0: Thermodynamic database for proteins and mutants. Nucleic Acids Res. 2004, 32, D120–D121. [Google Scholar] [CrossRef] [PubMed]
- Gromiha, M.M.; Uedaira, H.; An, J.; Selvaraj, S.; Prabakaran, P.; Sarai, A. ProTherm, Thermodynamic database for proteins and mutants: developments in version 3.0. Nucleic Acids Res. 2002, 30, 301–302. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.D.; Bava, K.A.; Gromiha, M.M.; Prabakaran, P.; Kitajima, K.; Uedaira, H.; Sarai, A. ProTherm and ProNIT: Thermodynamic databases for proteins and protein-nucleic acid interactions. Nucleic Acids Res. 2006, 34, D204–D206. [Google Scholar] [CrossRef] [PubMed]
- Petukh, M.; Li, M.; Alexov, E. Predicting binding free energy change caused by point mutations with knowledge-modified MM/PBSA method. PLoS Comput. Biol. 2015, 11, e1004276. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, Z.; Rocchia, W.; Alexov, E. Using DelPhi capabilities to mimic protein’s conformational reorganization with amino acid specific dielectric constants. Commun. Comput. Phys. 2013, 13, 13–30. [Google Scholar] [PubMed]
- Yang, Y.; Chen, B.; Tan, G.; Vihinen, M.; Shen, B. Structure-based prediction of the effects of a missense variant on protein stability. Amino Acids 2013, 44, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Tokuriki, N.; Tawfik, D.S. Stability effects of mutations and protein evolvability. Curr. Opin. Struct. Biol. 2009, 19, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Heinig, M.; Frishman, D. STRIDE: A web server for secondary structure assignment from known atomic coordinates of proteins. Nucleic Acids Res. 2004, 32, W500–W502. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Steinbach, P.J.; Jacobson, M.P.; Friesner, R.A.; Honig, B. Prediction of side-chain conformations on protein surfaces. Proteins 2007, 66, 814–823. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Honig, B. Extending the accuracy limits of prediction for side-chain conformations. J. Mol. Biol. 2001, 311, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Yahyavi, M.; Falsafi-Zadeh, S.; Karimi, Z.; Kalatarian, G.; Galehdari, H. VMD-SS: A graphical user interface plug-in to calculate the protein secondary structure in VMD program. Bioinformation 2014, 10, 548–550. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Jiang, W.; Hardy, D.J.; Phillips, J.C.; Mackerell, A.D., Jr.; Schulten, K.; Roux, B. High-performance scalable molecular dynamics simulations of a polarizable force field based on classical Drude oscillators in NAMD. J. Phys. Chem. Lett. 2011, 2, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.C.; Braun, R.; Wang, W.; Gumbart, J.; Tajkhorshid, E.; Villa, E.; Chipot, C.; Skeel, R.D.; Kale, L.; Schulten, K. Scalable molecular dynamics with NAMD. J. Comput. Chem. 2005, 26, 1781–1802. [Google Scholar] [CrossRef] [PubMed]
- Benedix, A.; Becker, C.M.; de Groot, B.L.; Caflisch, A.; Bockmann, R.A. Predicting free energy changes using structural ensembles. Nat. Methods 2009, 6, 3–4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shapovalov, M.V.; Dunbrack, R.L., Jr. A smoothed backbone-dependent rotamer library for proteins derived from adaptive kernel density estimates and regressions. Structure 2011, 19, 844–858. [Google Scholar] [CrossRef] [PubMed]

| WT | MT | |||||
|---|---|---|---|---|---|---|
| AA | Total Cases | Total Cases | ||||
| A | 91 | 0.60 | 0.56 | 374 | 0.54 | 0.53 |
| C | 7 | 0.69 | 0.55 | 36 | 0.44 | 0.51 |
| D | 52 | 0.48 | 0.50 | 34 | 0.58 | 0.63 |
| E | 93 | 0.44 | 0.48 | 43 | 0.62 | 0.64 |
| F | 52 | 0.72 | 0.69 | 85 | 0.45 | 0.41 |
| G | 79 | 0.52 | 0.57 | 167 | 0.68 | 0.69 |
| H | 41 | 0.36 | 0.37 | 10 | 0.78 | 0.79 |
| I | 103 | 0.66 | 0.63 | 65 | 0.40 | 0.32 |
| K | 114 | 0.22 | 0.20 | 32 | 0.47 | 0.45 |
| L | 92 | 0.83 | 0.80 | 69 | 0.33 | 0.25 |
| M | 23 | 0.58 | 0.59 | 15 | 0.43 | 0.45 |
| N | 40 | 0.62 | 0.60 | 19 | 0.65 | 0.67 |
| P | 42 | 0.35 | 0.36 | 16 | 0.40 | 0.36 |
| Q | 25 | 0.25 | 0.30 | 41 | 0.26 | 0.30 |
| R | 37 | 0.51 | 0.49 | 20 | 0.62 | 0.53 |
| S | 40 | 0.30 | 0.21 | 55 | 0.60 | 0.53 |
| T | 86 | 0.50 | 0.46 | 39 | 0.74 | 0.70 |
| V | 141 | 0.61 | 0.59 | 107 | 0.58 | 0.52 |
| W | 23 | 0.81 | 0.86 | 17 | 0.44 | 0.45 |
| Y | 81 | 0.67 | 0.66 | 18 | 0.58 | 0.65 |
| Location | SSE | ||||||
|---|---|---|---|---|---|---|---|
| Location Type | Total Cases | P(loc) | P′(loc) | SSE Type | Total Cases | P(SSE) | P′(SSE) |
| B-B | 102 | 0.74 | 0.70 | BB | 14 | 0.26 | 0.27 |
| B-PE | 132 | 0.78 | 0.76 | CC | 182 | 0.47 | 0.45 |
| E-E | 457 | 0.31 | 0.29 | CH | 6 | 0.81 | 0.65 |
| E-PE | 130 | 0.56 | 0.55 | CS | 8 | 0.59 | 0.61 |
| PE-PE | 441 | 0.65 | 0.65 | CT | 6 | 0.15 | 0.16 |
| ‒ | ‒ | ‒ | ‒ | HH | 378 | 0.55 | 0.53 |
| ‒ | ‒ | ‒ | ‒ | HS | 1 | 0.50 | 0.50 |
| ‒ | ‒ | ‒ | ‒ | HT | 2 | 0.50 | 0.50 |
| ‒ | ‒ | ‒ | ‒ | SS | 455 | 0.63 | 0.61 |
| ‒ | ‒ | ‒ | ‒ | ST | 2 | 0.50 | 0.50 |
| ‒ | ‒ | ‒ | ‒ | TT | 208 | 0.39 | 0.38 |
| Weight, Small | p, Small | Weight, Large | p, Large | Weight, All | p, All | |
|---|---|---|---|---|---|---|
| Y-intercept | −7.44 × 10−1 | 0.00 × 100 | −2.27 × 100 | 0.00 × 100 | −1.58 × 100 | 0.00 × 100 |
| IE | 9.28 × 10−2 | 1.36 × 10−2 | ‒ | ‒ | ‒ | ‒ |
| EE | 5.93 × 10−1 | 3.37 × 10−7 | 8.54 × 10−1 | 0.00 × 100 | 8.93 × 10−1 | 0.00 × 100 |
| VE | 7.51 × 10−2 | 2.03 × 10−4 | 1.63 × 10−1 | 0.00 × 100 | 1.69 × 10−1 | 0.00 × 100 |
| SP | 4.53 × 10−1 | 5.14 × 10−8 | 6.32 × 10−1 | 0.00 × 100 | 6.68 × 10−1 | 0.00 × 100 |
| S | ‒ | ‒ | 4.07 × 10−1 | 4.18 × 10−2 | 4.85 × 10−1 | 1.03 × 10−3 |
| HYDR | ‒ | ‒ | ‒ | ‒ | −1.57 × 100 | 9.63 × 10−3 |
| Ssum | −1.26 × 10−1 | 1.99 × 10−5 | −6.55 × 10−1 | 4.05 × 10−4 | −6.67 × 10−1 | 2.24 × 10−6 |
| SASMT | NA | NA | 9.36 × 10−5 | 1.10 × 10−4 | −5.46 × 101 | 2.88 × 10−3 |
| SN/SASMT | NA | NA | −7.71 × 10−1 | 6.84 × 10−3 | −2.78 × 101 | 4.77 × 10−2 |
| R | 0.36 | ‒ | 0.62 | ‒ | ‒ | ‒ |
| #Poins | 426 | ‒ | 558 | ‒ | 984 | ‒ |
| R final | 0.65 (0.61) | ‒ | ‒ | ‒ | 0.62 | ‒ |
| Cases | R | Slope | Y-Intercept | Min | Max | ||
|---|---|---|---|---|---|---|---|
| SSE | HS, HH, SS | 652 | 0.67 | 0.92 | 0.07 | 0.00 | 7.09 |
| CC, CT, TT | 310 | 0.58 | 0.67 | −0.08 | 0.00 | 6.13 | |
| Location | B-B | 83 | 0.60 | 0.86 | −0.02 | 0.02 | 7.09 |
| B-PE | 99 | 0.62 | 0.93 | 0.08 | 0.00 | 6.71 | |
| PE-PE | 308 | 0.64 | 0.84 | 0.04 | 0.00 | 6.39 | |
| E-PE | 102 | 0.52 | 0.80 | 0.01 | 0.03 | 6.39 | |
| E-E | 396 | 0.37 | 0.64 | −0.12 | 0.00 | 4.51 | |
| Residues | Any→A | 301 | 0.69 | 0.89 | 0.18 | 0.00 | 5.54 |
| Large (RFWY)→Small (AGSV) | 67 | 0.67 | 0.86 | 0.06 | 0.04 | 6.39 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Getov, I.; Petukh, M.; Alexov, E. SAAFEC: Predicting the Effect of Single Point Mutations on Protein Folding Free Energy Using a Knowledge-Modified MM/PBSA Approach. Int. J. Mol. Sci. 2016, 17, 512. https://doi.org/10.3390/ijms17040512
Getov I, Petukh M, Alexov E. SAAFEC: Predicting the Effect of Single Point Mutations on Protein Folding Free Energy Using a Knowledge-Modified MM/PBSA Approach. International Journal of Molecular Sciences. 2016; 17(4):512. https://doi.org/10.3390/ijms17040512
Chicago/Turabian StyleGetov, Ivan, Marharyta Petukh, and Emil Alexov. 2016. "SAAFEC: Predicting the Effect of Single Point Mutations on Protein Folding Free Energy Using a Knowledge-Modified MM/PBSA Approach" International Journal of Molecular Sciences 17, no. 4: 512. https://doi.org/10.3390/ijms17040512






