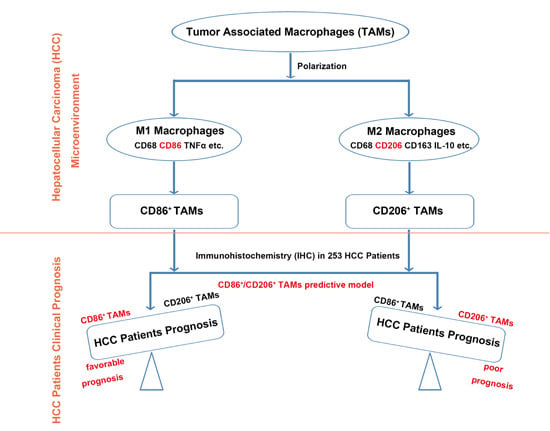
CD86+/CD206+, Diametrically Polarized Tumor-Associated Macrophages, Predict Hepatocellular Carcinoma Patient Prognosis
Abstract
:1. Introduction
2. Results
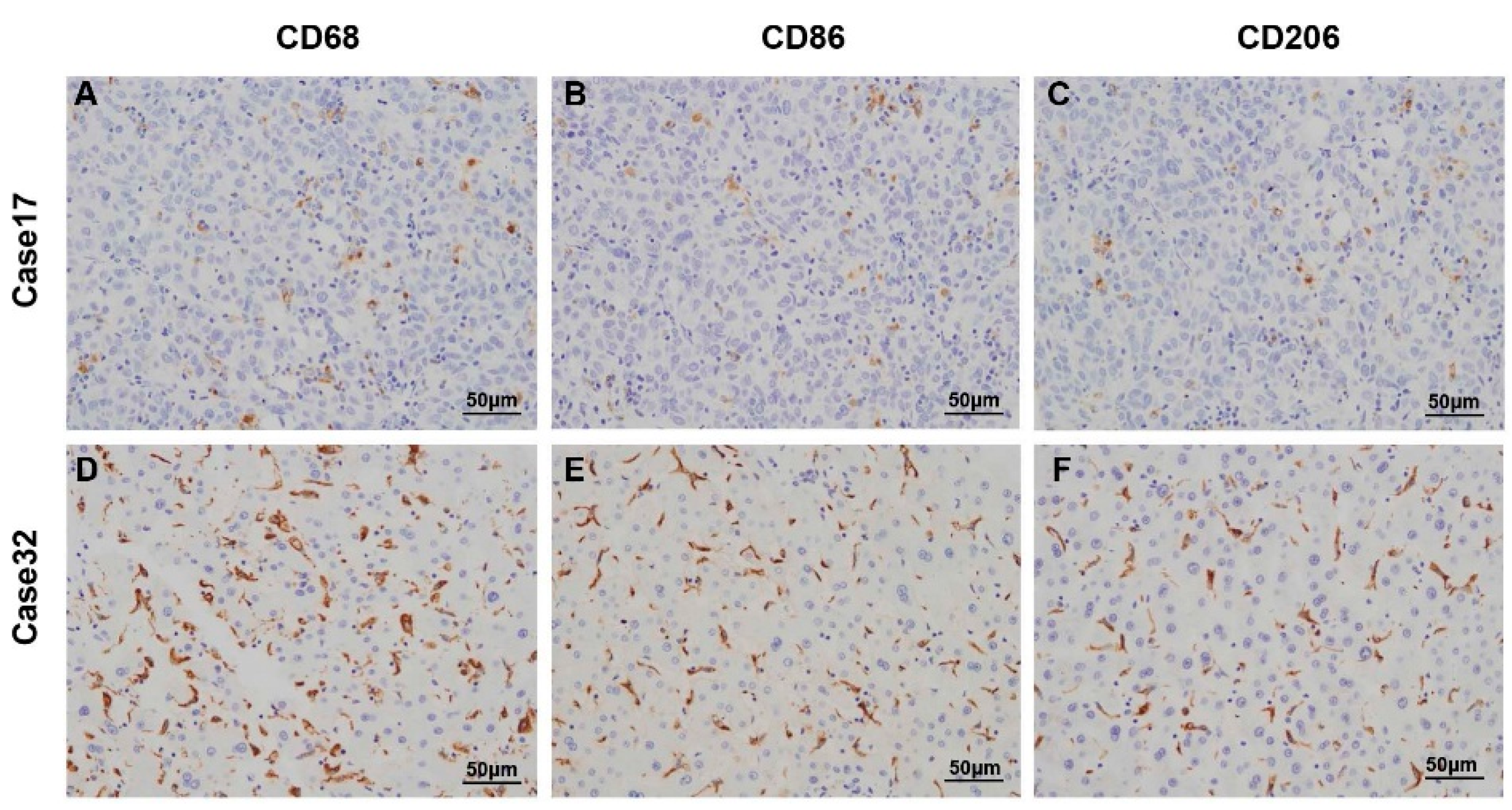
2.1. Characterization of Tumor-Associated Macrophages in Hepatocellular Carcinoma (HCC) Patients
2.2. Association between Macrophage Markers Presence (CD68, CD86 and CD206) and Clinicopathologic Characteristics in HCC Patients
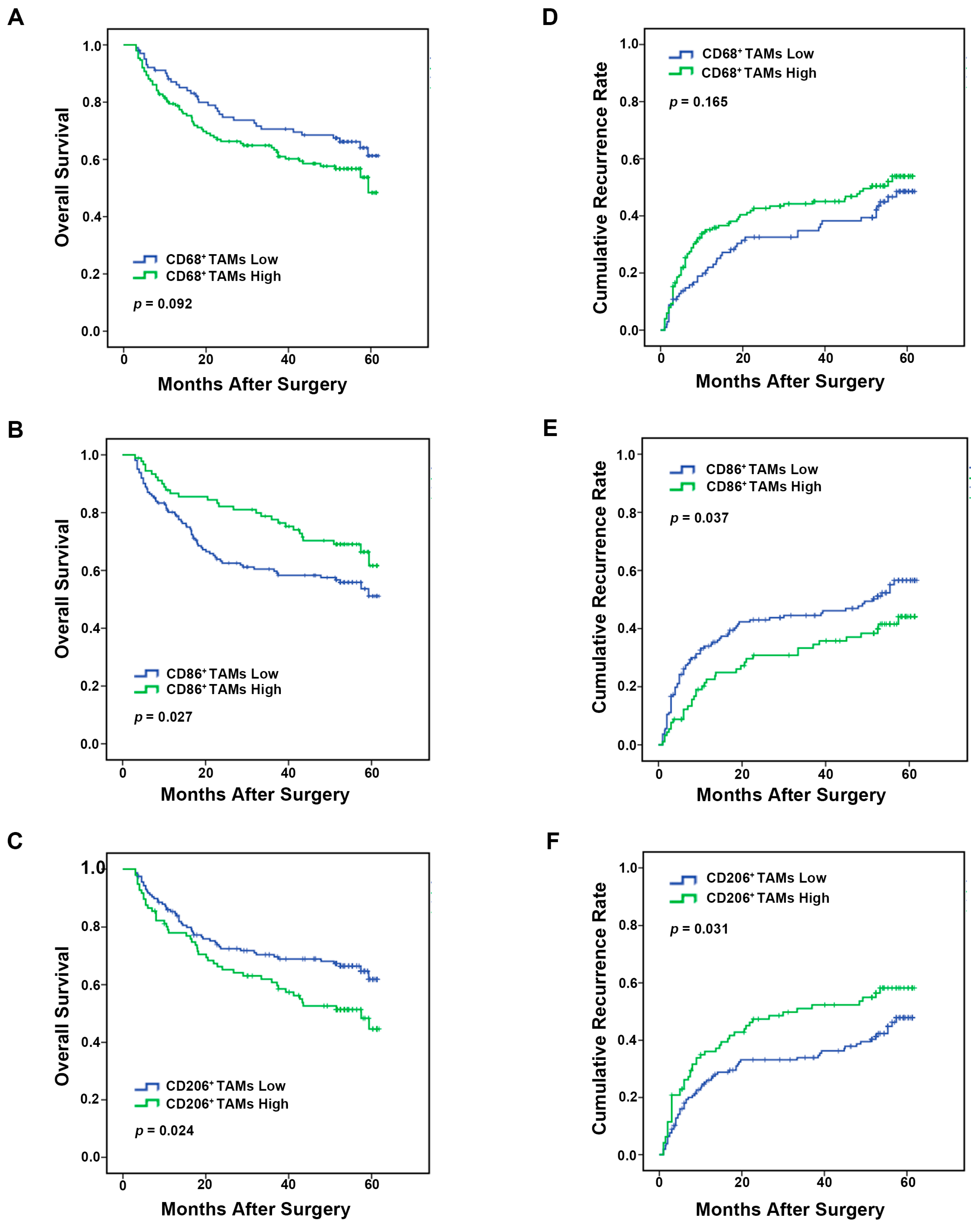
2.3. Analysis of Macrophages Immune Marker (CD68, CD86 and CD206) Prognostic Value in HCC Patients
2.4. Integrated Analysis of Immune Markers CD86 and CD206 Provides More Powerful Prognostic Value in HCC Patients
2.5. TAMs Predictive Model for α-Fetoprotein (AFP) Negative HCC Patients
3. Discussion
4. Experimental Section
4.1. Patients
4.2. Immunohistochemistry
4.3. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Maluccio, M.; Covey, A. Recent progress in understanding, diagnosing, and treating hepatocellular carcinoma. CA Cancer J. Clin. 2012, 62, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yao, W.; Yuan, Y.; Chen, P.; Li, B.; Li, J.; Chu, R.; Song, H.; Xie, D.; Jiang, X.; et al. Targeting of tumour-infiltrating macrophages via CCL2/CCR2 signalling as a therapeutic strategy against hepatocellular carcinoma. Gut 2015. [Google Scholar] [CrossRef] [PubMed]
- Xue, T.C.; Jia, Q.A.; Ge, N.L.; Zhang, B.H.; Wang, Y.H.; Ren, Z.G.; Ye, S.L. The platelet-to-lymphocyte ratio predicts poor survival in patients with huge hepatocellular carcinoma that received transarterial chemoembolization. Tumour Biol. 2015, 36, 6045–6051. [Google Scholar] [CrossRef] [PubMed]
- Capece, D.; Fischietti, M.; Verzella, D.; Gaggiano, A.; Cicciarelli, G.; Tessitore, A.; Zazzeroni, F.; Alesse, E. The inflammatory microenvironment in hepatocellular carcinoma: a pivotal role for tumor-associated macrophages. Biomed. Res. Int. 2013, 2013, 187204. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.X. Inflammatory immune responses in tumor microenvironment and metastasis of hepatocellular carcinoma. Cancer Microenviron. 2012, 5, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Schmieder, A.; Michel, J.; Schonhaar, K.; Goerdt, S.; Schledzewski, K. Differentiation and gene expression profile of tumor-associated macrophages. Semin. Cancer Biol. 2012, 22, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J.; Wynn, T.A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 2011, 11, 723–737. [Google Scholar] [CrossRef] [PubMed]
- Falini, B.; Flenghi, L.; Pileri, S.; Gambacorta, M.; Bigerna, B.; Durkop, H.; Eitelbach, F.; Thiele, J.; Pacini, R.; Cavaliere, A.; et al. PG-M1: A new monoclonal antibody directed against a fixative-resistant epitope on the macrophage-restricted form of the CD68 molecule. Am. J. Pathol. 1993, 142, 1359–1372. [Google Scholar] [PubMed]
- Biswas, S.K.; Mantovani, A. Macrophage plasticity and interaction with lymphocyte subsets: Cancer as a paradigm. Nat. Immunol. 2010, 11, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Olsson, A.; Nakhle, J.; Sundstedt, A.; Plas, P.; Bauchet, A.L.; Pierron, V.; Bruetschy, L.; Deronic, A.; Torngren, M.; Liberg, D.; et al. Tasquinimod triggers an early change in the polarization of tumor associated macrophages in the tumor microenvironment. J. Immunother. Cancer 2015, 3, 53. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.Y.; Wang, N.; Man, K.; Tsao, S.W.; Che, C.M.; Feng, Y. Autophagy-induced RelB/p52 activation mediates tumour-associated macrophage repolarisation and suppression of hepatocellular carcinoma by natural compound baicalin. Cell Death Dis. 2015, 6, e1942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shirabe, K.; Mano, Y.; Muto, J.; Matono, R.; Motomura, T.; Toshima, T.; Takeishi, K.; Uchiyama, H.; Yoshizumi, T.; Taketomi, A.; et al. Role of tumor-associated macrophages in the progression of hepatocellular carcinoma. Surg. Today 2012, 42, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ding, T.; Xu, J.; Wang, F.; Shi, M.; Zhang, Y.; Li, S.P.; Zheng, L. High tumor-infiltrating macrophage density predicts poor prognosis in patients with primary hepatocellular carcinoma after resection. Hum. Pathol. 2009, 40, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.J.; Wang, H.Y.; Teng, L.S. Correlation analysis of preoperative serum α-fetoprotein (AFP) level and prognosis of hepatocellular carcinoma (HCC) after hepatectomy. World J. Surg. Oncol. 2013, 11. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Park, J.Y. Clinical significance of AFP and PIVKA-II responses for monitoring treatment outcomes and predicting prognosis in patients with hepatocellular carcinoma. Biomed. Res. Int. 2013, 2013, 310427. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.H.; Kim, D.Y.; Jeon, S.M.; Ahn, S.H.; Park, J.Y.; Kim, S.U.; Kim, J.K.; Lee, K.S.; Chon, C.Y.; Han, K.H. Clinical characteristics and prognosis of hepatocellular carcinoma with different sets of serum AFP and PIVKA-II levels. Eur. J. Gastroenterol. Hepatol. 2012, 24, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, H.; Kumada, T.; Tada, T.; Sone, Y.; Kaneoka, Y.; Maeda, A. Tumor Markers for Hepatocellular Carcinoma: Simple and Significant Predictors of Outcome in Patients with HCC. Liver Cancer 2015, 4, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, H.; Kumada, T.; Kiriyama, S.; Sone, Y.; Tanikawa, M.; Hisanaga, Y.; Yamaguchi, A.; Isogai, M.; Kaneoka, Y.; Washizu, J. Prognostic significance of simultaneous measurement of three tumor markers in patients with hepatocellular carcinoma. Clin. Gastroenterol. Hepatol. 2006, 4, 111–117. [Google Scholar] [CrossRef]
- Fukuda, K.; Kobayashi, A.; Watabe, K. The role of tumor-associated macrophage in tumor progression. Front. Biosci. Sch. Ed. 2012, 4, 787–798. [Google Scholar] [CrossRef]
- Locatelli, L.; Cadamuro, M.; Spirli, C.; Fiorotto, R.; Lecchi, S.; Maria, M.C.; Popov, Y.; Scirpo, R.; de Matteis, M.; Amenduni, M.; et al. Macrophage recruitment by fibrocystin-defective biliary epithelial cells promotes portal fibrosis in congenital hepatic fibrosis. Hepatology 2015. [Google Scholar] [CrossRef] [PubMed]
- Den Breems, N.Y.; Eftimie, R. The re-polarisation of M2 and M1 macrophages and its role on cancer outcomes. J. Theor. Biol. 2015, 390, 23–39. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.O.; Gordon, S. The M1 and M2 paradigm of macrophage activation: Time for reassessment. F1000Prime Rep. 2014, 6. [Google Scholar] [CrossRef] [PubMed]
- Solinas, G.; Germano, G.; Mantovani, A.; Allavena, P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J. Leukoc. Biol. 2009, 86, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.Q.; Zhu, X.D.; Xu, H.X.; Zhang, J.B.; Lu, L.; Wang, W.Q.; Zhang, Q.B.; Wu, W.Z.; Wang, L.; Fan, J.; et al. The clinical significance of the CD163+ and CD68+ macrophages in patients with hepatocellular carcinoma. PLoS ONE 2013, 8, e59771. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, C.; Barrachina, M.D.; Cosin-Roger, J.; Ortiz-Masia, D.; Alvarez, A.; Terradez, L.; Nicolau, M.J.; Alos, R.; Esplugues, J.V.; Calatayud, S. Progastrin represses the alternative activation of human macrophages and modulates their influence on colon cancer epithelial cells. PLoS ONE 2014, 9, e98458. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, X.; Shen, Z.; Xu, J.; Qin, J.; Sun, Y. Infiltration of diametrically polarized macrophages predicts overall survival of patients with gastric cancer after surgical resection. Gastric Cancer 2015, 18, 740–750. [Google Scholar] [CrossRef] [PubMed]
- Medrek, C.; Ponten, F.; Jirstrom, K.; Leandersson, K. The presence of tumor associated macrophages in tumor stroma as a prognostic marker for breast cancer patients. BMC Cancer 2012, 12. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, G.; Hau, H.M.; Dietel, C.; Benzing, C.; Krenzien, F.; Brandl, A.; Wiltberger, G.; Matia, I.; Prager, I.; Schierle, K.; et al. Prognostic significance of macrophage invasion in hilar cholangiocarcinoma. BMC Cancer 2015, 15. [Google Scholar] [CrossRef] [PubMed]
- Tzankov, A.; Matter, M.S.; Dirnhofer, S. Refined prognostic role of CD68-positive tumor macrophages in the context of the cellular micromilieu of classical Hodgkin lymphoma. Pathobiology 2010, 77, 301–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinouchi, M.; Miura, K.; Mizoi, T.; Ishida, K.; Fujibuchi, W.; Ando, T.; Yazaki, N.; Saito, K.; Shiiba, K.; Sasaki, I. Infiltration of CD14-positive macrophages at the invasive front indicates a favorable prognosis in colorectal cancer patients with lymph node metastasis. Hepatogastroenterology 2011, 58, 352–358. [Google Scholar] [PubMed]
- Beider, K.; Bitner, H.; Leiba, M.; Gutwein, O.; Koren-Michowitz, M.; Ostrovsky, O.; Abraham, M.; Wald, H.; Galun, E.; Peled, A.; et al. Multiple myeloma cells recruit tumor-supportive macrophages through the CXCR4/CXCL12 axis and promote their polarization toward the M2 phenotype. Oncotarget 2014, 5, 11283–11296. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Qian, Y.; Yu, F.; Liu, W.; Wu, Y.; Fang, X.; Hao, W. Alternatively activated macrophages are associated with metastasis and poor prognosis in prostate adenocarcinoma. Oncol. Lett. 2015, 10, 1390–1396. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhu, Y.; Chen, L.; An, H.; Zhang, W.; Wang, G.; Lin, Z.; Xu, J. Prognostic value of diametrically polarized tumor-associated macrophages in renal cell carcinoma. Ann. Surg. Oncol. 2014, 21, 3142–3150. [Google Scholar] [CrossRef] [PubMed]
- Bosca, L.; Gonzalez-Ramos, S.; Prieto, P.; Fernandez-Velasco, M.; Mojena, M.; Martin-Sanz, P.; Alemany, S. Metabolic signatures linked to macrophage polarization: from glucose metabolism to oxidative phosphorylation. Biochem. Soc. Trans. 2015, 43, 740–744. [Google Scholar] [CrossRef] [PubMed]
- Galvan-Pena, S.; O’Neill, L.A. Metabolic reprograming in macrophage polarization. Front. Immunol. 2014, 5, 420. [Google Scholar] [PubMed]
- He, X.; Wang, Y.; Zhang, W.; Li, H.; Luo, R.; Zhou, Y.; Liao, C.L.; Huang, H.; Lv, X.; Xie, Z.; et al. Screening differential expression of serum proteins in AFP-negative HBV-related hepatocellular carcinoma using iTRAQ -MALDI-MS/MS. Neoplasma 2014, 61, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Beppu, T.; Okabe, H.; Nitta, H.; Imai, K.; Hayashi, H.; Chikamoto, A.; Yamamoto, K.; Ikeshima, S.; Kuramoto, M.; et al. Predictive factors of pathological vascular invasion in hepatocellular carcinoma within 3 cm and 3 nodules without radiological vascular invasion. Hepatol. Res. 2015. [Google Scholar] [CrossRef] [PubMed]
- Alvaro, T.; Lejeune, M.; Salvado, M.T.; Bosch, R.; Garcia, J.F.; Jaen, J.; Banham, A.H.; Roncador, G.; Montalban, C.; Piris, M.A. Outcome in Hodgkin’s lymphoma can be predicted from the presence of accompanying cytotoxic and regulatory T cells. Clin. Cancer Res. 2005, 11, 1467–1473. [Google Scholar] [CrossRef] [PubMed]
- Kuang, D.M.; Zhao, Q.; Wu, Y.; Peng, C.; Wang, J.; Xu, Z.; Yin, X.Y.; Zheng, L. Peritumoral neutrophils link inflammatory response to disease progression by fostering angiogenesis in hepatocellular carcinoma. J. Hepatol. 2011, 54, 948–955. [Google Scholar] [CrossRef] [PubMed]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [PubMed]




| Characteristics | CD68+ TAMs | CD86+ TAMs | CD206+ TAMs | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Low | High | p # | Low | High | p # | Low | High | p # | |
| Age (years) | |||||||||
| ≤51 | 50 | 81 | 0.522 | 86 | 45 | 0.602 | 80 | 51 | 0.796 |
| >51 | 52 | 70 | - | 76 | 46 | - | 77 | 45 | - |
| Gender | |||||||||
| Female | 17 | 16 | 0.184 | 142 | 78 | 0.699 | 21 | 12 | 1.000 |
| Male | 85 | 135 | - | 20 | 13 | - | 136 | 84 | - |
| HBsAg | |||||||||
| Negative | 4 | 8 | 0.767 | 8 | 4 | 1.000 | 8 | 4 | 1.000 |
| Positive | 98 | 143 | - | 154 | 87 | - | 149 | 92 | - |
| HCVAb | |||||||||
| Negative | 101 | 146 | 0.406 | 158 | 89 | 1.000 | 153 | 94 | 1.000 |
| Positive | 1 | 5 | - | 4 | 2 | - | 4 | 2 | - |
| AFP (ng/mL) | |||||||||
| ≤20 | 44 | 55 | 0.296 | 61 | 38 | 0.592 | 58 | 41 | 0.426 |
| >20 | 58 | 96 | - | 101 | 53 | - | 99 | 55 | - |
| ALT (U/L) | |||||||||
| ≤75 | 88 | 137 | 0.309 | 150 | 75 | 0.020 | 141 | 84 | 0.680 |
| >75 | 14 | 14 | - | 12 | 16 | - | 16 | 12 | - |
| γ-GT (U/L) | |||||||||
| ≤54 | 47 | 64 | 0.606 | 67 | 44 | 0.294 | 72 | 39 | 0.436 |
| >54 | 55 | 87 | - | 95 | 47 | - | 85 | 57 | - |
| Liver cirrhosis | |||||||||
| NO | 15 | 18 | 0.570 | 23 | 10 | 0.561 | 22 | 11 | 0.701 |
| YES | 87 | 133 | - | 139 | 81 | - | 135 | 85 | - |
| Tumor size (cm) | |||||||||
| ≤5 | 59 | 81 | 0.522 | 86 | 54 | 0.359 | 90 | 50 | 0.436 |
| >5 | 43 | 70 | - | 76 | 37 | - | 67 | 46 | - |
| Tumor number | |||||||||
| Single | 82 | 118 | 0.753 | 137 | 63 | 0.006 | 131 | 69 | 0.038 |
| Multiple | 20 | 33 | - | 25 | 28 | - | 26 | 27 | - |
| Vascular invasion | |||||||||
| No | 65 | 95 | 1.000 | 106 | 54 | 0.345 | 109 | 51 | 0.011 |
| Yes | 37 | 56 | - | 56 | 37 | - | 48 | 45 | - |
| Tumor encapsulation | |||||||||
| None | 45 | 66 | 1.000 | 73 | 38 | 0.692 | 80 | 31 | 0.004 |
| Complete | 57 | 85 | - | 89 | 53 | - | 77 | 65 | - |
| Tumor differentiation | |||||||||
| I + II | 71 | 105 | 1.000 | 113 | 63 | 1.000 | 106 | 70 | 0.400 |
| III + IV | 81 | 46 | - | 49 | 28 | - | 51 | 26 | - |
| TNM stage | |||||||||
| I | 65 | 92 | 0.693 | 113 | 49 | 0.001 | 108 | 49 | 0.005 |
| III + IV | 37 | 59 | - | 44 | 47 | - | 49 | 47 | - |
| Variables | OS | TTR | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||
| p | HR | 95% CI | p | p | HR | 95% CI | p | |
| Age, years (>51 vs. ≤51) | 0.624 | - | - | NA | 0.437 | - | - | NA |
| Gender (male vs. female) | 0.273 | - | - | NA | 0.990 | - | - | NA |
| HBsAg (positive vs. negative) | 0.594 | - | - | NA | 0.389 | - | - | NA |
| HCVAb (positive vs. negative) | 0.180 | - | - | NA | 0.307 | - | - | NA |
| Serum AFP, ng/mL (>20 vs. ≤20) | <0.001 | 2.095 | 1.323–3.318 | 0.002 | 0.027 | - | - | NS |
| Serum ALT, U/L (>75 vs. ≤75) | 0.945 | - | - | NA | 0.725 | - | - | NA |
| Serum γ-GT, U/L (>54 vs. ≤54) | <0.001 | 1.799 | 1.124–2.881 | 0.014 | 0.001 | 1.537 | 1.028–2.298 | 0.036 |
| Liver cirrhosis (yes vs. no) | 0.320 | - | - | NA | 0.751 | - | - | NA |
| Tumor size (cm) (>5 vs. ≤5) | <0.001 | 2.609 | 1.635–4.162 | <0.001 | <0.001 | 1.735 | 1.151–2.616 | 0.008 |
| Tumor multiplicity (multiple vs. single) | 0.362 | - | - | NA | 0.566 | - | - | NA |
| Tumor encapsulation (none vs. complete) | 0.493 | - | - | NA | 0.153 | - | - | NA |
| Tumor differentiation (poor vs. well) | <0.001 | 2.166 | 1.436–3.267 | <0.001 | 0.008 | - | - | NS |
| Vascular invasion (yes vs. no) | <0.001 | 1.756 | 1.139–2.709 | 0.011 | <0.001 | 1.696 | 1.136–2.531 | 0.016 |
| TNM stage (III-II vs. I b) | 0.028 | - | - | NS | 0.103 | - | - | NA |
| CD68+ TAMs (High vs. Low b) | 0.095 | - | - | NA | 0.169 | - | - | NA |
| CD86+ TAMs (Low vs. High b) | 0.029 | 2.178 | 1.333–3.558 | 0.002 | 0.040 | 1.810 | 1.181–2.776 | 0.006 |
| CD206+ TAMs (High vs. Low b) | 0.026 | 1.584 | 1.053–2.385 | 0.027 | 0.033 | 1.872 | 1.065–3.290 | 0.030 |
| CD86/CD206 signature a | 0.002 | - | - | 0.002 | 0.004 | - | - | 0.007 |
| II vs. I b | 0.020 | 2.343 | 1.173–4.681 | 0.016 | 0.033 | 1.872 | 1.065–3.290 | 0.029 |
| III vs. I b | 0.020 | 2.685 | 1.218–5.920 | 0.014 | 0.047 | 1.703 | 0.833–3.483 | 0.144 |
| IV vs. I b | 0.002 | 3.358 | 1.622–6.952 | 0.001 | 0.004 | 2.377 | 1.305–4.330 | 0.005 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, P.; Ma, L.; Liu, L.; Zhao, G.; Zhang, S.; Dong, L.; Xue, R.; Chen, S. CD86+/CD206+, Diametrically Polarized Tumor-Associated Macrophages, Predict Hepatocellular Carcinoma Patient Prognosis. Int. J. Mol. Sci. 2016, 17, 320. https://doi.org/10.3390/ijms17030320
Dong P, Ma L, Liu L, Zhao G, Zhang S, Dong L, Xue R, Chen S. CD86+/CD206+, Diametrically Polarized Tumor-Associated Macrophages, Predict Hepatocellular Carcinoma Patient Prognosis. International Journal of Molecular Sciences. 2016; 17(3):320. https://doi.org/10.3390/ijms17030320
Chicago/Turabian StyleDong, Pingping, Lijie Ma, Longzi Liu, Guangxi Zhao, Si Zhang, Ling Dong, Ruyi Xue, and She Chen. 2016. "CD86+/CD206+, Diametrically Polarized Tumor-Associated Macrophages, Predict Hepatocellular Carcinoma Patient Prognosis" International Journal of Molecular Sciences 17, no. 3: 320. https://doi.org/10.3390/ijms17030320