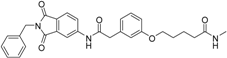
Abstract
Matrix metalloproteinases (MMPs) are a class of zinc dependent endopeptidases which play a crucial role in a multitude of severe diseases such as cancer and osteoarthritis. We employed MMP-13 as the target enzyme for the structure-based design and synthesis of inhibitors able to recognize the catalytic zinc ion in addition to an allosteric binding site in order to increase the affinity of the ligand. Guided by molecular modeling, we optimized an initial allosteric inhibitor by addition of linker fragments and weak zinc binders for recognition of the catalytic center. Furthermore we improved the lipophilic ligand efficiency (LLE) of the initial inhibitor by adding appropriate zinc binding fragments to lower the clogP values of the inhibitors, while maintaining their potency. All synthesized inhibitors showed elevated affinity compared to the initial hit, also most of the novel inhibitors displayed better LLE. Derivatives with carboxylic acids as the zinc binding fragments turned out to be the most potent inhibitors (compound 3 (ZHAWOC5077): IC50 = 134 nM) whereas acyl sulfonamides showed the best lipophilic ligand efficiencies (compound 18 (ZHAWOC5135): LLE = 2.91).
1. Introduction
As the most efficient contributor to degeneration of type II collagen within its superfamily of zinc-dependant endopeptidases, matrix metalloproteinase-13 (MMP-13) is a highly validated target for the treatment of diseases based on tissue degradation [1,2,3,4,5]. Unregulated MMP activity can lead to a variety of diseases such as tumor growth and metastasis, periodontal disease and osteoarthritis (OA) [6,7,8,9,10,11,12].
Initial success in developing inhibitors against MMP-13 was achieved over a decade ago by modifying the natural substrate close to the scissile amide bond. By implementing structural elements that cannot be hydrolyzed by the enzyme, peptidomimetics were obtained [13]. Potent inhibitors resulted by adding strong zinc binding groups such as hydroxamic acid to interact with the catalytic domain [14,15]. Those inhibitors lacked sufficient selectivity due to high structural similarity among the various MMPs. Because of toxicity and severe side effects arising from scarce selectivity no compound with strong zinc chelating groups such as hydroxamic acids has survived clinical trials so far [16,17].
Later generations of MMP-13 inhibitors did not bind to the catalytic zinc, but buried themselves deeper in the S1’ pocket, occupying the specificity loop leading to inhibitors that combine high potency with appealing selectivity profiles [18,19,20,21,22,23,24,25]. Adding weak zinc binding groups to those allosteric inhibitors can lead to selective inhibitors with higher potency due to a dual binding mode (allosteric pocket and catalytic center) [26].
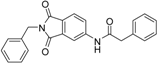
A previous study conducted in our laboratory led to the identification of compound 1 (Figure 1) with a lipophilic ligand efficiency (LLE = 1.07) which inhibits MMP-13 showing an IC50 value of 9.8 μM without chelating the catalytic zinc ion [27].
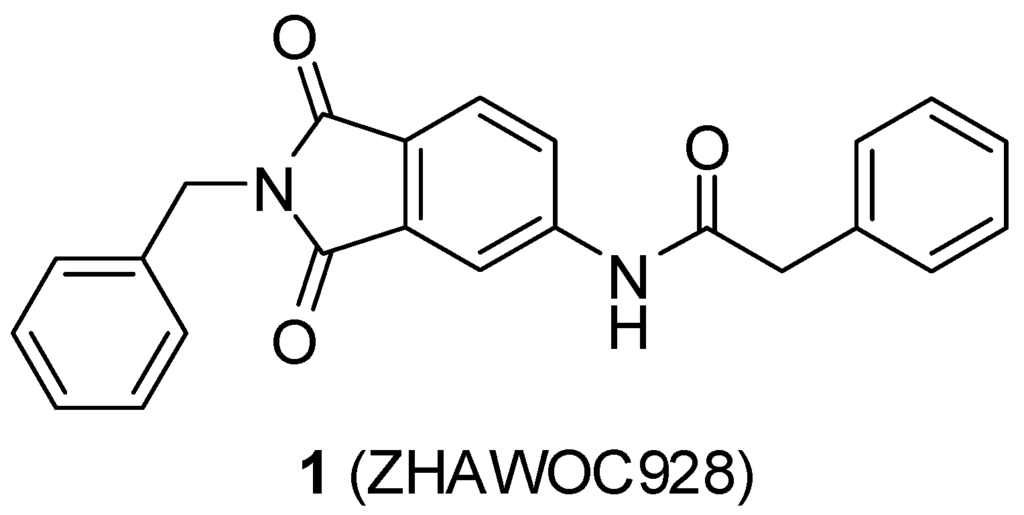
Figure 1.
Chemical structure of the previously identified inhibitor 1.
In this article we present a structure-based design approach to modify the allosteric MMP-13 inhibitor 1 to increase its potency by improving the molecular recognition between the enzyme and its ligand. This could be achieved by the introduction of a weak zinc binding fragment in order to interact with the catalytic center. Furthermore we enhanced the lipophilic ligand efficiency of the novel compounds compared to the allosteric inhibitor.
2. Results
2.1. Molecular Modeling
In the first step of the design process we had a close look on the binding mode of 1 in the allosteric binding pocket of MMP-13. We used the crystal structure PDB 2OW9 (Figure 2a) [24] to dock the molecule into the cavity defining a pharmacophore query to maintain the three hydrogen bonds to the backbone amino acids Thr224, Thr226 and Met232, formed by the oxygen atoms of the phthalimide scaffold (Figure 2b). It turned out that a zinc binding group could be attached to the molecule, favourably in meta position, to interact with the catalytic zinc ion (Figure 2c). As the distance that has to be overcome added up to more than 5 Å (Figure 2d) we decided to implement a linker consisting of four and more heavy atoms between the aromatic ring system and the chelating group.
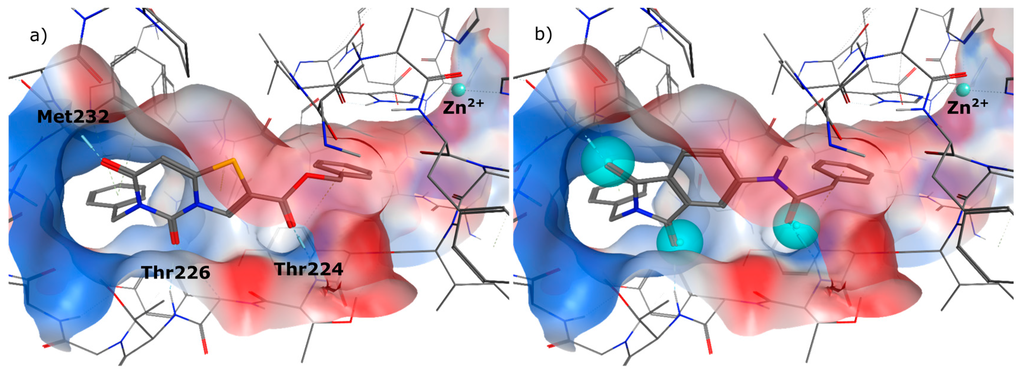
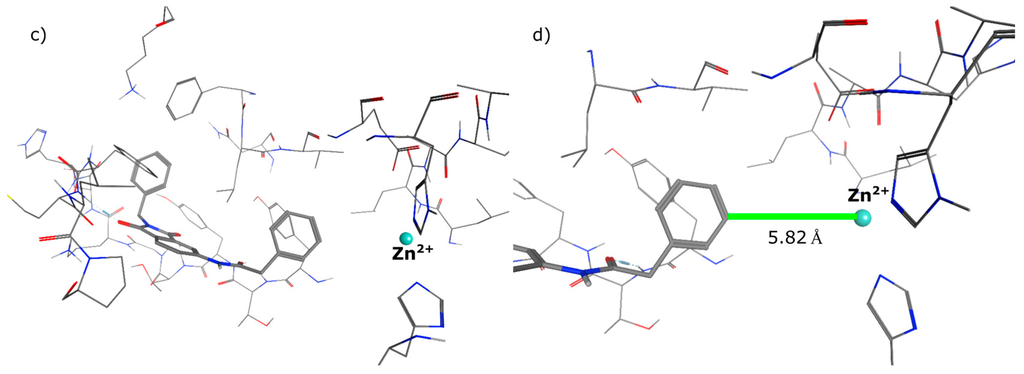
Figure 2.
(a) Co-crystal structure PDB 2OW9; (b) compound 1 docked to PDB 2OW9 with the pharmacophore being fulfilled; (c) possibility of attaching a weak zinc binder in meta position; (d) distance between ligand and zinc ion.
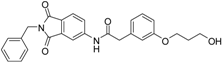
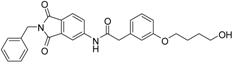
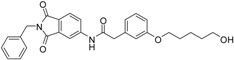
At the meta position of the aromatic ring an oxygen was added for synthetic reasons, followed by aliphatic linker chains between 3–6 carbon atoms in length as well as a terminal hydroxy group. The alcohol head group was implemented as a generic zinc chelator of small size to evaluate the optimal linker length (Figure 3).
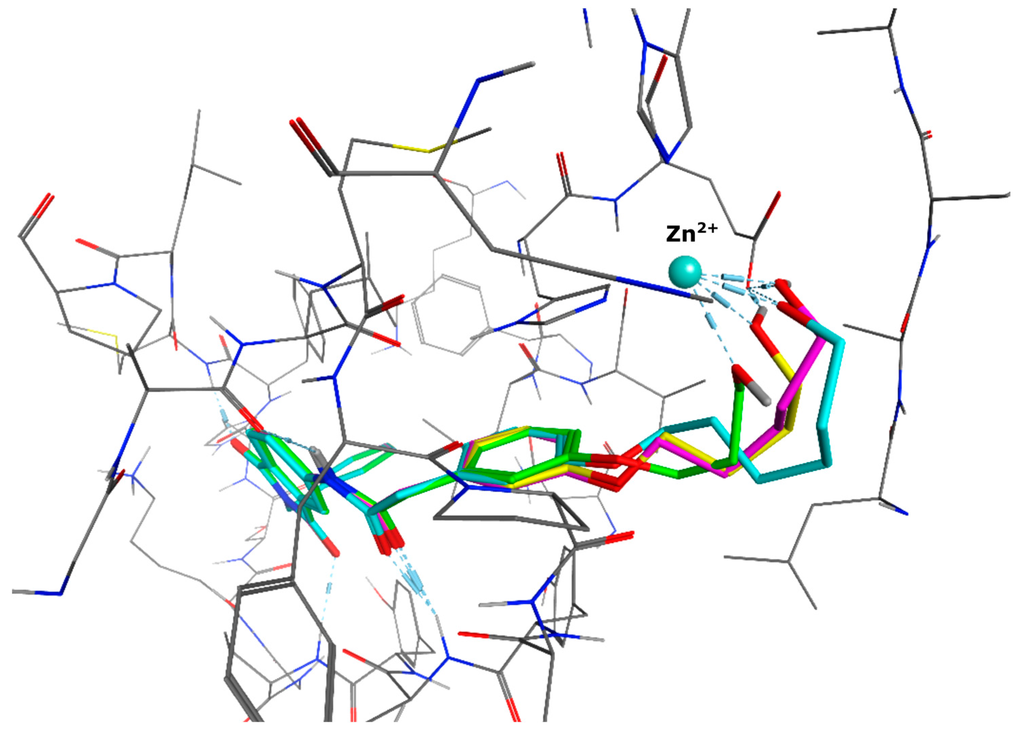
Figure 3.
Docking poses of four molecules with different linker lengths and the zinc-chelating hydroxy group (green: 3 CH2, yellow: 4 CH2, magenta: 5 CH2, cyan: 6 CH2).
We decided to focus on chain lengths of 4 and 5 carbon atoms (Figure 3, yellow and magenta) as they showed lower root mean square deviation (RMSD) values (1.16 yellow and 1.35 magenta) than the other two linkers (1.56 green and 2.57 cyan). This indicates better quality of the docking poses [28]. In addition, visual inspection of the docking poses indicated that chain lengths of 4 and 5 carbon atoms seemed to cover the distance more efficiently than the other two linkers. Subsequently we examined different head groups as potential zinc chelators. Therefore we modified structure 2 by implementing a carboxylic acid (3), an acyl sulfonamide (4) and an amide (5) to obtain a set of ligands for which the recognition of the catalytic zinc should increase their affinity to the protein and therefore enhance the inhibitors potency (Figure 4) [29,30].
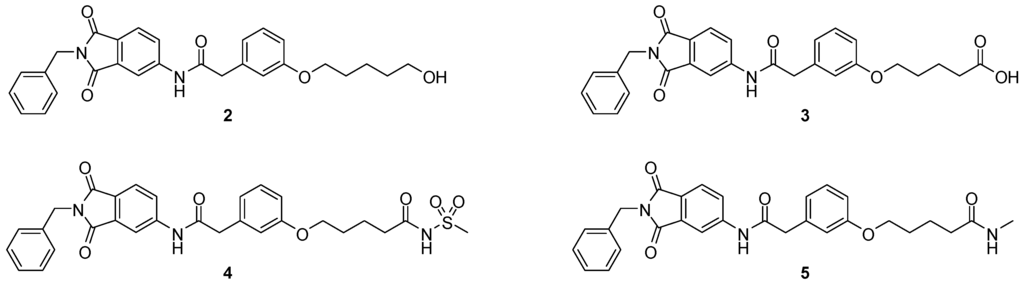
Figure 4.
Structural proposals for extended molecules to overcome the distance to the zinc ion, including different weak zinc binders.
For the purpose of this evaluation we executed docking experiments applying the identical pharmacophore as for the previously docked structure 1 (Figure 5a–d).

Figure 5.
Highest ranked docking pose of (a) alcohol 2; (b) carboxylic acid 3; (c) acyl sulfonamide 4; (d) amide 5, with the linker emphasized (green) and the head group showing the anticipated interaction with the zinc.
All of the four examined weak zinc binding head groups showed the postulated molecular recognition of the metal ion. The alcohol and the acid as well as the amide exhibit monodentate binding, whereas the acyl sulfonamide acts as a bidentate chelator. According to our modeling experiments, the carboxylic acid interacts as a monodentate ligand. This corresponds well with the reported binding mode of carboxylic acids in the active site of MMP-13 (Figure 6, crystal structure PDB 1ZTQ [31]).
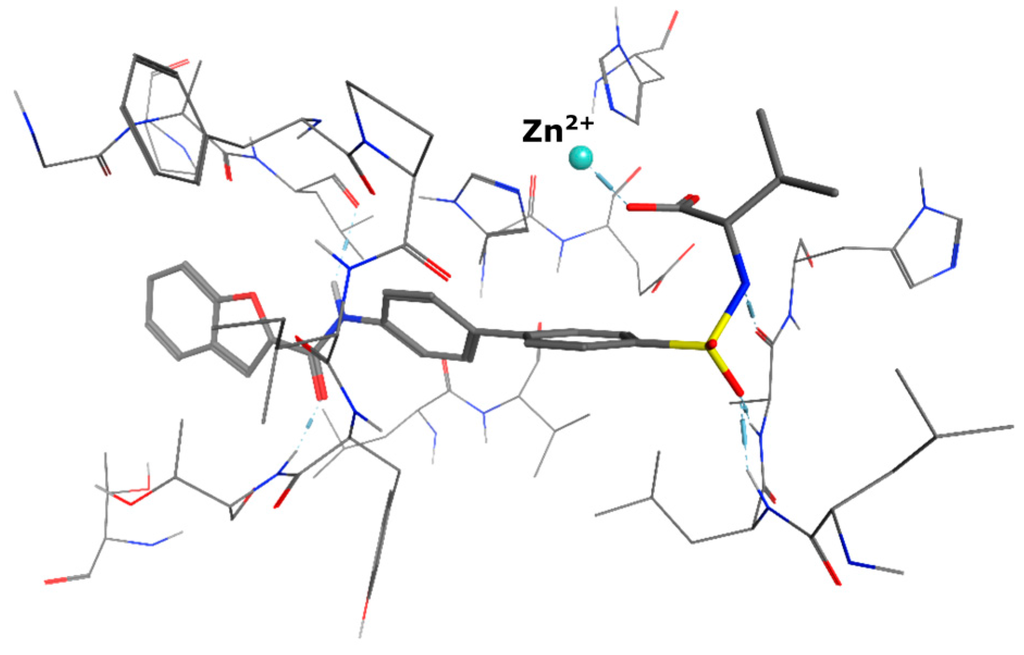
Figure 6.
Co-crystal structure of a carboxylic acid interacting with the zinc ion in the active site of MMP-13 involving only one of the two oxygen atoms.
The designed compounds 2–5 displayed better scoring values (in the range of −18) compared to 1 (−12.8). In consideration of the modeling results described above, we decided to synthesize a small library of this type of molecules.
2.2. Chemistry
Driven by the positive results of our computational design approach towards molecular recognition of the catalytic zinc ion, we synthesized compounds 2–5 according to the synthetic route summarized in Figure 7. The benzylated aminophthalimide derivative 6 was prepared according to a previously described procedure [27]. The acid 9 was obtained by saponification of 8 which was prepared by the nucleophilic substitution of 7 on benzyl-n-bromoalkylether. An amide coupling between the alkylated aminophthalimide 6 and the acid 9 led to compound 10 which could be hydrogenated to obtain the alcohol 2. By subsequent oxidation of the alcohol 2 the acid 3 was formed and finally converted to the compounds 4 and 5 by amide bond formation.
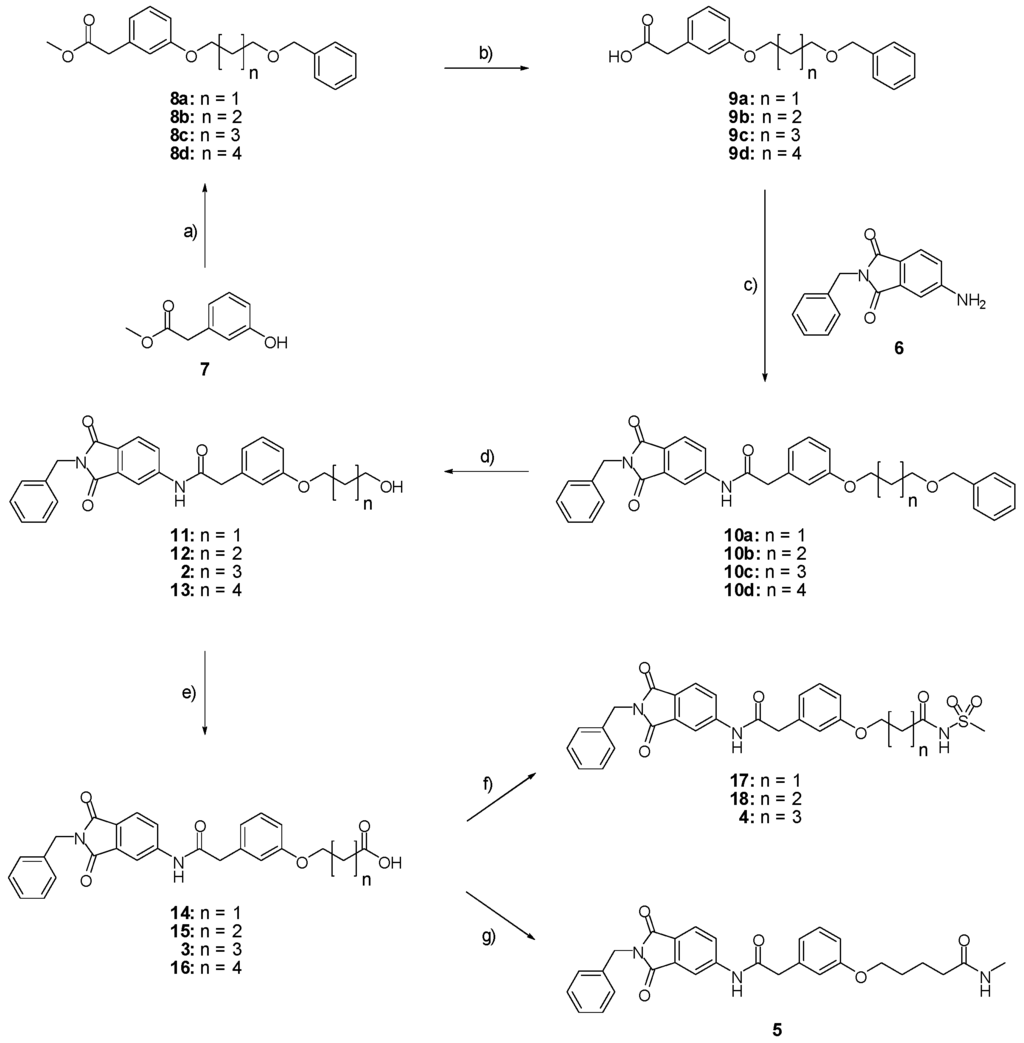
Figure 7.
Synthesis of compounds 2–18. (a) Benzyl-n-bromoalkylether, Cs2CO3, DMF, RT, 18 h, 8a: 80%, 8b: 85%, 8c: 83%, 8d: 84%; (b) KOH 10% in H2O, methanol, RT, 30 min., 9a: 89%, 9b: 92%, 9c: 100%, 9d: 94%; (c) SOCl2, DIPEA, THF, RT, 2 h, 10a: 74%, 10b: 64%, 10c: 66%, 10d: 55%; (d) Pd/C 10%, ethanol, RT, 2 h, 11: 57%, 12: 48%, 2: 83%, 13: 77%; (e) TEMPO, sodium phosphate 0.67 M in H2O pH 6.7, NaClO2, NaOCl, ACN, 40 °C, 4 h, 14: 51%, 15: 84%, 3: 53%, 16: 87%; (f) EDC·HCl, methanesulfonamide, DMAP, DCM, 0 °C, 20 min., RT, 12 h, 17: 19%, 18: 26%, 4: 41%; (g) DIPEA, HATU, methylamine 2 M in THF, THF, RT, 12 h, 5: 39%.
2.3. Biological Assays
Consecutively the synthesized compounds were tested for their inhibition activity against MMP-13 in in vitro assays. The values are averaged over triplicate determinations (Table 1).

Table 1.
MMP-13 inhibitory data for compounds 1–5 and 11–18.
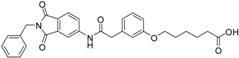
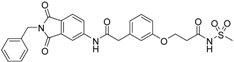
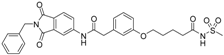
The carboxylic acids 3 and 16 turned out to be the most potent inhibitors within this series (IC50: 134 nM (3), 280 nM (16)). The acyl sulfonamides 4 and 18 show some of the best lipophilic ligand efficiencies (LLE: 2.53 (4), 2.91 (18)).
3. Discussion
We modified the allosteric inhibitor 1 of MMP-13 to increase its potency against the target enzyme and to elevate the LLE of the initial inhibitor. Increased potency could be achieved by the addition of a zinc binding fragment to the inhibitor. The carboxylic acid turned out to be the most potent option for the molecular recognition of the catalytic zinc(II) ion. The new inhibitors combine allosteric interaction with the recognition of the catalytic center to yield dual binding mode inhibitors. Compound 3 showed the highest affinity with an IC50 value of 134 nM. Increased LLE values were achieved by the implementation of acyl sulfonamide fragments to 1 which lowered the clogP value of the respective inhibitor. By comparing the acyl sulfonamide 18 with its carboxylic acid counterpart 15 one observes that the LLE improves from 2.16 to 2.91, which is in the range of lead compounds, while the IC50 values are comparable. Therefore acyl sulfonamides are good bioisosteres for substituting carboxylic acids as zinc binding functional groups if an increase of the LLE of MMP inhibitors is intended.
The results of the series with carboxylic acids as the zinc binding group clearly show that the selection of excessively short linkers leads to a loss in potency. Also linkers with six heavy atoms between the allosteric fragment and the zinc-recognizing acid cause diminished inhibition, leading to an optimal linker length of five heavy atoms. These findings harmonize with our modeling results as we observed better geometrical matches for linkers with medium lengths.
It should be mentioned that the critical observation of the generated docking poses with respect to binding angles and torsions was absolutely mandatory for the successful outcome of this study. The corresponding scoring values can only be compared for sets of molecules with comparable binding modes, but even then it is safer to examine the poses visually instead of using the scoring values for prioritizing compounds for synthesis.
4. Materials and Methods
4.1. General
All NMR spectra were recorded on a Bruker AVANCE III HD 500 One Bay spectrometer with a magnetic field of 11.75 T. For 1H NMR spectra a frequency of 500 MHz resulted. Chemical shifts are reported in ppm from tetramethylsilane as internal standard. Data are reported as follows: chemical shift, integration, multiplicity (s = singlet, d = doublet, t = triplet, q = quartet, quint. = quintet, br. = broad, m = multiplet), coupling constants (Hz). For 13C NMR spectra a frequency of 125 MHz resulted. Chemical shifts are reported in ppm from tetramethylsilane as internal standard. The multiplicities of the signals were determined by DEPT Measurements. High-resolution mass spectrometry was performed on an Agilent Technologies 6530 Q-TOF. NMR spectra, HRMS spectra and IC50 curves can be found in the supplementary materials.
4.2. Chemistry
All reagents and solvents used in the synthesis were purchased from Sigma Aldrich (Buchs, Switzerland) or TCI (Zwijndrecht, Belgium) and used as received. Solvents were stored over molecular sieves 4 Å.
Methyl 2-(3-{[5-(benzyloxy)pentyl]oxy}phenyl)acetate (8c; ZHAWOC4927): under an argon atmosphere, methyl 2-(3-hydroxyphenyl)acetate (7) (2.5 g, 15.05 mmol) and caesium carbonate (9.81 g, 30.11 mmol) were suspended in dimethylformamide (100 mL), the mixture was stirred at ambient temperature for 2 h. Benzyl-5-bromoamylether (4.26 g, 16.56 mmol) was added and it was stirred at ambient temperature for further 12 h. Water (250 mL) and ethyl acetate (250 mL) was added and the resulting phases separated. The organic phase was dried over sodium sulfate and concentrated in vacuum. Purification by chromatography on silica gel (Gradient: 0%–100% ethyl acetate in cyclohexane) afforded the title compound 8c as a white solid (4.30 g, 83% yield): 1H-NMR (500 MHz, [D6]DMSO, 25 °C, TMS): δ = 7.35–7.31 (4H; m; C-Haromatic), 7.29–7.24 (1H; m; C-Haromatic), 7.20 (1H; t; J = 7.9 Hz; C-Haromatic), 6.85–6.76 (3H; m; C-Haromatic), 4.50 (2H; s; CH2), 3.94 (2H; t; J = 6.5 Hz; CH2), 3.67 (3H; s; CH3), 3.57 (2H; s; CH2), 3.49 (2H; t; J = 6.3 Hz; CH2), 1.82–1.75 (2H; m; CH2), 1.71–1.65 (2H; m; CH2), 1.59–1.51 (2H; m; CH2) ppm. 13C-NMR (125 MHz, [D6]DMSO, 25 °C, TMS): δ = 171.96, 159.28, 138.66, 135.37, 129.56, 128.41, 127.67, 127.55, 121.46, 115.54, 113.19, 72.97, 70.27, 67.77, 52.08, 41.27, 29.57, 29.15, 22.86 ppm. HRMS-TOF: m/z [M + H]+ calculated for C21H26O4: 343.1909, found: 343.1898.
In analogy to 8c the following derivatives were synthesized:
Methyl 2-(3-{[3-(benzyloxy)propyl]oxy}phenyl)acetate (8a; ZHAWOC4557): The title compound 8a was obtained as a white solid in 80% yield: 1H-NMR (500 MHz, [D6]DMSO, 25 °C, TMS): δ = 7.35–7.29 (4H; m; C-Haromatic), 7.29–7.24 (1H; m; C-Haromatic), 7.24–7.18 (1H; t; m; C-Haromatic), 6.86–6.76 (3H; m; C-Haromatic), 4.51 (2H; s; CH2), 4.06 (2H; t; J = 6.3 Hz; CH2), 3.67 (3H; s; CH3), 3.65 (2H; t; J = 6.3 Hz; CH2), 3.57 (2H; s; CH2), 2.07 (2H; quint; J = 6.19 Hz; CH2) ppm. 13C-NMR (125 MHz, [D6]DMSO, 25 °C, TMS): δ = 171.93, 159.20, 138.47, 135.39, 129.56, 128.41, 127.65, 127.59, 121.56, 115.63, 113.21, 73.07, 66.87, 64.82, 52.06, 41.25, 29.80 ppm. MS: m/z [M + Na]+ 337.
Methyl 2-(3-{[4-(benzyloxy)butyl]oxy}phenyl)acetate (8b; ZHAWOC4558): The title compound 8b was obtained as a white solid in 85% yield: 1H-NMR (500 MHz, [D6]DMSO, 25 °C, TMS): δ = 7.35–7.30 (4H; m; C-Haromatic), 7.30–7.23 (1H; m; C-Haromatic), 7.22–7.16 (1H; t; m; C-Haromatic), 6.85–6.74 (3H; m; C-Haromatic), 4.50 (2H; s; CH2), 3.95 (2H; t; J = 6.14 Hz; CH2), 3.66 (3H; s; CH3), 3.56 (2H; s; CH2), 3.52 (2H; t; J = 6.14 Hz; CH2), 1.93–1.72 (4H; m; CH2) ppm. 13C-NMR (125 MHz, [D6]DMSO, 25 °C, TMS): δ = 171.93, 159.26, 138.63, 135.39, 129.56, 128.41, 127.67, 127.57, 121.49, 115.58, 113.20, 72.94, 69.98, 67.60, 52.04, 41.26, 26.42, 26.22 ppm. MS: m/z [M + Na]+ 351.
Methyl 2-(3-{[6-(benzyloxy)hexyl]oxy}phenyl)acetate (8d; ZHAWOC4928): The title compound 8d was obtained as a white solid in 84% yield: 1H-NMR (500 MHz, [D6]DMSO, 25 °C, TMS): δ = 7.35–7.31 (4H; m; C-Haromatic), 7.29–7.25 (1H; m; C-Haromatic), 7.21 (1H; t; J = 7.88 Hz; C-Haromatic), 6.85–6.77 (3H; m; C-Haromatic), 4.50 (2H; s; CH2), 3.93 (2H; t; J = 6.5 Hz; CH2), 3.68 (3H; s; CH3), 3.58 (2H; s; CH2), 3.48 (2H; t; J = 6.5 Hz; CH2), 1.81–1.74 (2H; m; CH2), 1.68–1.62 (2H; m; CH2), 1.51–1.42 (4H; m; CH2) ppm. 13C-NMR (125 MHz, [D6]DMSO, 25 °C, TMS): δ = 171.97, 159.29, 138.69, 135.34, 129.54, 128.38, 127.66, 127.52, 121.43, 115.53, 113.19, 72.92, 70.35, 67.81, 52.07, 41.27, 29.74, 29.27, 26.03, 25.96 ppm. MS: m/z [M + Na]+ 379.
2-(3-{[5-(benzyloxy)pentyl]oxy}phenyl)acetic acid (9c; ZHAWOC4929): The ester (8c) (4.30 g, 12.57 mmol) was dissolved in methanol (220 mL) and stirred at ambient temperature. Potassium hydroxide 10% in water (220 mL) was added over 10 min. and the mixture was stirred for another 20 min. Methanol was removed in vacuum and the aqueous phase extracted with diethyl ether (200 mL). The aqueous phase was acidified with concentrated hydrochloric acid and extracted with diethyl ether (200 mL). The second organic phase was dried over sodium sulfate and concentrated in vacuum to obtain the title compound 9c as a white solid (4.13 g, 100% yield) : 1H-NMR (500 MHz, [D6]DMSO, 25 °C, TMS): δ = 7.35–7.31 (4H; m; C-Haromatic), 7.30–7.25 (1H; m; C-Haromatic), 7.21 (1H; t; J = 7.9 Hz; C-Haromatic), 6.86–6.77 (3H; m; C-Haromatic), 4.51 (2H; s; CH2), 3.94 (2H; t; J = 6.6 Hz; CH2), 3.59 (2H; s; CH2), 3.50 (2H; t; J = 6.6 Hz; CH2), 1.82–1.75 (2H; m; CH2), 1.72–1.65 (2H; m; CH2), 1.58–1.50 (2H; m; CH2) ppm. 13C-NMR (125 MHz, [D6]DMSO, 25 °C, TMS): δ = 177.07, 159.27, 138.54, 134.73, 129.61, 128.40, 127.70, 127.57, 121.56, 115.66, 113.37, 72.94, 70.24, 67.77, 41.09, 29.50, 29.10, 22.80 ppm. HRMS-TOF: m/z [M + H]+ calculated for C20H24O4: 329.1753, found: 329.1748.
In analogy to 9c the following derivatives were synthesized:
2-(3-{[3-(benzyloxy)propyl]oxy}phenyl)acetic acid (9a; ZHAWOC4559): The title compound 9a was obtained as a white solid in 89% yield: 1H-NMR (500 MHz, [D6]DMSO, 25 °C, TMS): δ = 8.46 (1H; br. s.; COOH), 7.33–7.17 (6H; m; C-Haromatic), 6.87–6.77 (3H; m; C-Haromatic), 4.51 (2H; s; CH2), 4.05 (2H; t; J = 6.2 Hz; CH2), 3.64 (2H; t; J = 6.2 Hz; CH2), 3.57 (2H; s; CH2), 2.06 (2H; quint; J = 6.2 Hz ; CH2) ppm. 13C-NMR (125 MHz, [D6]DMSO, 25 °C, TMS): δ = 177.35, 159.20, 138.33, 134.79, 129.63, 128.43, 127.72, 127.65, 121.70, 115.82, 113.40, 73.06, 66.87, 64.84, 41.13, 29.73 ppm. MS: m/z [M − H]− 299.
2-(3-{[4-(benzyloxy)butyl]oxy}phenyl)acetic acid (9b; ZHAWOC4560): The title compound 9b was obtained as a white solid in quantitative yield: 1H-NMR (500 MHz, [D6]DMSO, 25 °C, TMS): δ = 7.35–7.31 (4H; m; C-Haromatic), 7.30–7.25 (1H; m; C-Haromatic), 7.21 (1H; t; J = 7.8 Hz; C-Haromatic), 6.86–6.77 (3H; m; C-Haromatic), 4.51 (2H; s; CH2), 3.96 (2H; t; J = 6.4 Hz; CH2), 3.59 (2H; s; CH2), 3.54 (2H; t; J = 6.4 Hz; CH2), 1.90–1.83 (2H; m; CH2), 1.82–1.75 (2H; m; CH2) ppm. 13C-NMR (125 MHz, [D6]DMSO, 25 °C, TMS): δ = 177.27, 159.24, 138.50, 134.69, 129.62, 128.41, 127.71, 127.59, 121.59, 115.68, 113.39, 72.92, 69.93, 67.59, 41.08, 26.35, 26.15 ppm. MS: m/z [M − H]− 313.
2-(3-{[6-(benzyloxy)hexyl]oxy}phenyl)acetic acid (9d; ZHAWOC4930): The title compound 9d was obtained as a white solid in 94% yield: 1H-NMR (500 MHz, [D6]DMSO, 25 °C, TMS): δ = 7.35–7.31 (4H; m; C-Haromatic), 7.29–7.24 (1H; m; C-Haromatic), 7.21 (1H; t; J = 7.8 Hz; C-Haromatic), 6.85–6.78 (3H; m; C-Haromatic), 4.50 (2H; s; CH2), 3.92 (2H; t; J = 6.6 Hz; CH2), 3.58 (2H; s; CH2), 3.47 (2H; t; J = 6.6 Hz; CH2), 1.80-1.73 (2H; m; CH2), 1.67–1.61 (2H; m; CH2), 1.50–1.38 (4H; m; CH2) ppm. 13C-NMR (125 MHz, [D6]DMSO, 25 °C, TMS): δ = 177.17, 159.30, 138.55, 134.80, 129.61, 128.41, 127.73, 127.59, 121.57, 115.68, 113.40, 72.89, 70.34, 67.84, 41.15, 29.67, 29.24, 26.00, 25.94 ppm. MS: m/z [M − H]− 341.
N-(2-benzyl-1,3-dioxo-2,3-dihydro-1H-isoindol-5-yl)-2-(3-{[5-(benzyloxy)pentyl]oxy}phenyl)acetamide (10c; ZHAWOC5606): The acid (9c) (4.10 g, 12.49 mmol) was stirred in an excess of thionylchloride at 55 °C for 1 h. After removal of excess thionylchloride in vacuum the acid chloride was dissolved in tetrahydrofuran (10 mL) and added to a solution of 5-amino-2-benzylisoindoline-1,3-dione (6) (2.42 g, 9.60 mmol) in tetrahydrofuran (200 mL) under argon at ambient temperature. Diisopropylethylamine (1.90 g, 14.70 mmol) was added and the mixture was stirred at ambient temperature for 2 h. After removal of the tetrahydrofuran in vacuum, ethyl acetate (200 mL) and 10% citric acid (200 mL) were added and the resulting phases were separated. The organic phase was washed with 10% sodium bicarbonate (200 mL) and brine (200 mL), dried over sodium sulfate and concentrated in vacuum. Purification by chromatography on silica gel (Gradient: 0%–100% ethyl acetate in cyclohexane) afforded the title compound 10c as a yellow solid (3.56 g, 66% yield): 1H-NMR (500 MHz, [D6]DMSO, 25 °C, TMS): δ = 7.89 (1H; d; J = 2.1 Hz; C-Haromatic), 7.84 (1H; dd; J = 8.2 Hz, 2.1 Hz; C-Haromatic), 7.74 (1H; d; J = 8.2 Hz; C-Haromatic), 7.71 (1H; br. s; N-H), 7.43–7.39 (2H; m; C-Haromatic), 7.37–7.24 (9H; m; C-Haromatic), 6.93–6.81 (3H; m; C-Haromatic), 4.82 (2H; s; CH2), 4.53 (2H; m; CH2), 3.98 (2H; t; J = 6.5 Hz; CH2), 3.74 (2H; s; CH2), 3.53 (2H; t; J = 6.5 Hz; CH2), 1.87–1.77 (2H; m; CH2), 1.76–1.67 (2H; m; CH2), 1.63––.53 (2H; m; CH2) ppm. 13C-NMR (125 MHz, [D6]DMSO, 25 °C, TMS): δ = 171.22, 169.33, 167.53, 159.83, 143.21, 136.35, 130.47, 128.66, 128.49, 128.38, 127.79, 127.65, 127.56, 124.45, 121.41, 115.83, 114.08, 113.76, 72.97, 70.23, 67.88, 44.88, 41.64, 29.52, 29.06, 22.84 ppm. HRMS-TOF: m/z [M + H]+ calculated for C35H34N2O5: 563.2546, found: 563.2542.
In analogy to 10c the following derivatives were synthesized:
N-(2-benzyl-1,3-dioxo-2,3-dihydro-1H-isoindol-5-yl)-2-(3-{[3-(benzyloxy)propyl]oxy}phenyl)acetamide (10a; ZHAWOC4754): The title compound 10a was obtained as a yellow solid in 74% yield: 1H-NMR (500 MHz, [D6]DMSO, 25 °C, TMS): δ = 8.08 (1H; s; NH), 7.90 (1H; d; J = 1.8 Hz; C-Haromatic), 7.82 (1H; dd; J = 8.2 Hz, 1.8 Hz; C-Haromatic), 7.68 (1H; d; J = 8.2 Hz; C-Haromatic), 7.39–7.19 (11H; m; C-Haromatic), 6.89–6.76 (3H; m; C-Haromatic), 4.78 (2H; s; CH2), 4.50 (2H; m; CH2), 4.05 (2H; t; J = 6.2 Hz; CH2), 3.67 (2H; s; CH2), 3.64 (2H; t; J = 6.2 Hz; CH2), 2.11–2.02 (2H; m; CH2) ppm. 13C-NMR (125 MHz, [D6]DMSO, 25 °C, TMS): δ = 171.27, 169.55, 167.62, 167.57, 159.62, 143.46, 138.33, 136.36, 135.22, 133.51, 130.29, 128.66, 128.43, 128.39, 127.79, 127.61, 126.78, 124.42, 123.85, 121.47, 115.82, 114.14, 113.64, 73.04, 66.78, 64.92, 44.72, 41.62, 29.68 ppm. MS: m/z [M − H]− 533; [M + Na]+ 557.
N-(2-benzyl-1,3-dioxo-2,3-dihydro-1H-isoindol-5-yl)-2-(3-{[4-(benzyloxy)butyl]oxy}phenyl)acetamide (10b; ZHAWOC4755): The title compound 10b was obtained as a yellow solid in 64% yield: 1H-NMR (500 MHz, [D6]DMSO, 25 °C, TMS): δ = 8.21 (1H; s; NH), 7.91 (1H; d; J = 1.9 Hz; C-Haromatic), 7.83 (1H; dd; J = 8.2 Hz, 1.9 Hz; C-Haromatic), 7.68 (1H; d; J = 8.2 Hz; C-Haromatic), 7.40–7.19 (11H; m; C-Haromatic), 6.87–6.77 (3H; m; C-Haromatic), 4.78 (2H; s; CH2), 4.50 (2H; m; CH2), 3.94 (2H; t; J = 6.0 Hz; CH2), 3.66 (2H; s; CH2), 3.53 (2H; t; J = 6.0 Hz; CH2), 1.92–1.71 (4H; m; CH2) ppm. 13C-NMR (125 MHz, [D6]DMSO, 25 °C, TMS): δ = 169.63, 167.63, 167.58, 159.64, 143.53, 138.48, 136.37, 135.26, 133.49, 130.22, 128.66, 128.42, 128.38, 127.78, 127.64, 127.57, 126.74, 124.40, 123.86, 121.38, 115.79, 114.15, 113.51, 72.92, 69.93, 47.66, 44.68, 41.61, 26.34, 26.15 ppm. MS: m/z [M − H]− 547; [M + Na]+ 571.
N-(2-benzyl-1,3-dioxo-2,3-dihydro-1H-isoindol-5-yl)-2-(3-{[4-(benzyloxy)butyl]oxy}phenyl)acetamide (10d): The title compound 10d was prepared from the acid 9d and used directly for further synthesis without purification.
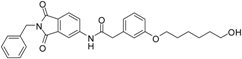
N-(2-benzyl-1,3-dioxo-2,3-dihydro-1H-isoindol-5-yl)-2-{3-[(5-hydroxypentyl)oxy]phenyl}acetamide (2; ZHAWOC5041): The benzyl ether (10c) (3.30 g, 5.87 mmol) was dissolved in ethanol (300 mL). Palladium 10% on activated charcoal (0.70 g, 0.59 mmol) was added and a hydrogen atmosphere was applied at 1 bar. After stirring at ambient temperature for 2 h the mixture was filtered over celite and concentrated in vacuum. Purification by chromatography on silica gel (Gradient: 0%–100% ethyl acetate in cyclohexane) afforded the title compound 2 as a white solid (2.30 g, 83% yield): 1H-NMR (500 MHz, [D6]DMSO, 25 °C, TMS): δ = 8.99 (1H; s; N-H), 7.98 (1H; d; J = 2 Hz; C-Haromatic), 7.83 (1H; dd; J = 8.2 Hz, 2 Hz; C-Haromatic), 7.64 (1H; d; J = 8.2 Hz; C-Haromatic), 7.37–7.32 (2H; m; C-Haromatic), 7.28–7.12 (4H; m; C-Haromatic), 6.84–6.69 (3H; m; C-Haromatic), 4.76 (2H; s; CH2), 3.85 (2H; t; J = 6.3 Hz; CH2), 3.64 (4H; br. s; 2 x CH2), 2.21 (1H; br. s; O-H), 1.77–1.69 (2H, m; CH2), 1.63–1.55 (2H; m; CH2), 1.53–1.44 (2H; m; CH2) ppm. 13C-NMR (125 MHz, [D6]DMSO, 25 °C, TMS): δ = 170.14, 167.68, 167.65, 159.38, 143.87, 136.33, 135.48, 133.34, 129.90, 128.61, 128.33, 127.74, 124.28, 123.93, 121.28, 112.72, 114.22, 113.08, 67.69, 62.48, 44.41, 41.54, 32.31, 28.94, 22.40 ppm. HRMS-TOF: m/z [M + H]+ calculated for C28H28N2O5: 473.2076, found: 473.2076.
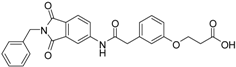
5-(3-{[(2-benzyl-1,3-dioxo-2,3-dihydro-1H-isoindol-5-yl)carbamoyl]methyl}phenoxy)pentanoic acid (3; ZHAWOC5077): The alcohol (2) (2.20 g, 4.66 mmol), TEMPO (0.07 g, 0.352 mmol) and 0.67 M aqueous sodium phosphate (22 mL) in acetonitrile (26.4 mL) were stirred and heated to 40 °C. NaClO2 (1.12 g, 9.90 mmol in 5 mL water) and 10% NaOCl (2.2 mL) were added in parallel dropwise and it was stirred for 4 h. After cooling to ambient temperature water (20 mL) was added and the mixture was poured in an ice could solution of Na2SO3 (1.8 g in 30 mL water). The aqueous phase was extracted with diethylether (80 mL), acidified with 10% citric acid and again extracted with diethylether (2 × 100 mL). The organic phases were dried over sodium sulfate and concentrated in vacuum to obtain the title compound 3 as a white solid (1.20 g, 53% yield): 1H-NMR (500 MHz, [D6]DMSO, 25 °C, TMS): δ = 12.05 (1H; br. s; COOH), 10.77 (1H; s; N-H), 8.22 (1H; d; J = 1.6 Hz; C-Haromatic), 7.90 (1H; dd; J = 8.2 Hz, 1.6 Hz; C-Haromatic), 7.84 (1H; d; J = 8.2 Hz; C-Haromatic), 7.35–7.21 (6H; m; C-Haromatic), 6.93–6.80 (3H; m; C-Haromatic), 4.74 (2H; s; CH2), 3.96 (2H; t; J = 6.2 Hz; CH2), 3.69 (2H; s; CH2), 2.28 (2H; t; J = 7.5 Hz; CH2), 1.76–1.69 (2H; m; CH2), 1.68–1.60 (2H; m; CH2) ppm. 13C-NMR (125 MHz, [D6]DMSO, 25 °C, TMS): δ = 174.82, 170.44, 167.93, 167.76, 159.08, 145.23, 137.21, 137.14, 129.85, 129.04, 127.85, 127.82, 125.78, 124.93, 123.86, 121.75, 116.00, 113.36, 113.04, 67.48, 43.86, 41.31, 33.79, 28.61, 21.70 ppm. HRMS-TOF: m/z [M + H]+ calculated for C28H26N2O6: 487.1869, found: 487.1850.
5-(3-{[(2-benzyl-1,3-dioxo-2,3-dihydro-1H-isoindol-5-yl)carbamoyl]methyl}phenoxy)-N-methanesulfonylpentanamide (4; ZHAWOC5079): The acid (3) (106 mg, 0.218 mmol) was dissolved in dichloromethane (5 mL) and cooled to 0 °C. EDC·HCl (63 mg, 0.327 mmol), methansulfonamide (23 mg, 0.240 mmol) and DMAP (40 mg, 0.327 mmol) were added and stirred at 0 °C for further 20 min. After warming up to ambient temperature it was stirred for another 12 h. The reaction was quenched by adding 10% citric acid (5 mL). The precipitate was filtered off and dissolved in DMSO (1 mL). Purification by reversed phase chromatography on silica gel C18 (Gradient: 0%–100% methanol in water) afforded the title compound 4 as a white solid (50 mg, 41% yield): 1H-NMR (500 MHz, [D6]DMSO, 25 °C, TMS): δ = 11.68 (1H; s; N-H), 10.76 (1H; s; N-H), 8.22 (1H; d: J = 1.9 Hz; C-Haromatic), 7.90 (1H; dd; J = 8.5 Hz, 1.9 Hz; C-Haromatic), 7.85 (1H; d; J = 8.5 Hz; C-Haromatic), 7.35–7.21 (6H; m; C-Haromatic), 6.94–6.81 (3H; m; C-Haromatic), 4.74 (2H; s; CH2), 3.96 (2H; t; J = 5.7 Hz; CH2), 3.69 (2H; s; CH2), 3.22 (3H; s ; CH3), 2.35 (2H; t; J = 7.2 Hz; CH2), 1.76–1.60 (4H; m; 2 × CH2) ppm. 13C-NMR (125 MHz, [D6]DMSO, 25 °C, TMS): δ = 172.94, 170.43, 167.93, 167.76, 159.05, 145.22, 137.21, 137.15, 133.55, 129.85, 129.04, 127.86, 127.83, 125.79, 124.94, 123.85, 121.80, 115.99, 113.35, 113.06, 67.37, 43.84, 41.45, 41.32, 35.47, 28.42, 21.29 ppm. HRMS-TOF: m/z [M + H]+ calculated for C29H29N3O7S: 564.1804, found: 564.1808.
5-(3-{[(2-benzyl-1,3-dioxo-2,3-dihydro-1H-isoindol-5-yl)carbamoyl]methyl}phenoxy)-N-methylpentanamide (5; ZHAWOC5080): The acid (3) (100 mg, 0.206 mmol) was dissolved in tetrahydrofuran (3 mL) and diisopropylethylamine (138 mg, 1.070 mmol), HATU (215 mg, 0.565 mmol) such as methylamine 2 M in THF (0.206 mL, 0.412 mmol) were added. The mixture was stirred at ambient temperature for 18 h before it was concentrated in vacuum and purified by chromatography on silica gel (Gradient: 0%–100% ethyl acetate in Cyclohexane) to obtain the title compound 5 as a white solid (40 mg, 39% yield): 1H-NMR (500 MHz, [D6]DMSO, 25 °C, TMS): δ = 10.76 (1H; s; N-H), 8.21 (1H; d; J = 1.9 Hz; C-Haromatic), 7.90 (1H; dd; J = 8.2 Hz, 1.9 Hz; C-Haromatic), 7.84 (1H; d; J = 8.2 Hz; C-Haromatic), 7.71 (1H; br. q; J = 4.5 Hz; N-H), 7.35–7.20 (6H; m; C-Haromatic), 6.92–6.79 (3H; m; C-Haromatic), 4.74 (2H; s; CH2), 3.94 (2H; t; J = 6 Hz; CH2), 3.68 (2H; s; CH2), 2.55 (3H; d; J = 4.5 Hz; CH3), 2.11 (2H; t; J = 7.3 Hz; CH2), 1.72–1.58 (4H; m; 2 × CH2) ppm. 13C-NMR (125 MHz, [D6]DMSO, 25 °C, TMS): δ = 172.71, 170.44, 167.93 167.76, 159.09, 145.23, 137.21, 137.14, 129.84, 129.04, 127.86, 127.83, 125.79, 124.93, 123.85, 121.74, 115.98, 113.36, 113.05, 67.46, 43.85, 41.32, 35.38, 28.78, 25.87, 22.36 ppm. HRMS-TOF: m/z [M + H]+ calculated for C29H29N3O5: 500.2185, found: 500.2181.
The following alcohols were synthesized according to compound (2):
N-(2-benzyl-1,3-dioxo-2,3-dihydro-1H-isoindol-5-yl)-2-[3-(3-hydroxypropoxy)phenyl]acetamide (11; ZHAWOC4756): The title compound was isolated as a white solid in 57% yield: 1H-NMR (500 MHz, [D6]DMSO, 25 °C, TMS): δ = 8.95 (1H; s; N-H), 7.95 (1H; d; J = 1.7 Hz; C-Haromatic), 7.77 (1H; dd; J = 8.2 Hz, 1.7 Hz; C-Haromatic), 7.60 (1H; d; J = 8.2 Hz; C-Haromatic), 7.38–7.05 (6H; m; C-Haromatic), 6.82–6.64 (3H; m; C-Haromatic), 4.74 (2H; s; CH2), 3.97 (2H; t; J = 6 Hz; CH2), 3.77 (2H; t; J = 5.9 Hz; CH2), 3.58 (2H; s; CH2), 2.95 (1H; br. s; O-H), 1.94 (2H; quint.; J = 5.9 Hz; CH2) ppm. 13C-NMR (125 MHz, [D6]DMSO, 25 °C, TMS): δ = 170.31, 167.82, 167.73, 159.19, 143.74, 136.27, 135.46, 133.32, 130.02, 128.69, 128.34, 127.84, 126.55, 124.36, 124.08, 121.57, 115.70, 114.32, 113.20, 65.09, 59.68, 44.37, 41.62, 31.97 ppm. HRMS-TOF: m/z [M + H]+ calculated for C26H24N2O5: 445.1763, found: 445.1776.
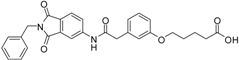
N-(2-benzyl-1,3-dioxo-2,3-dihydro-1H-isoindol-5-yl)-2-[3-(4-hydroxybutoxy)phenyl]acetamide (12; ZHAWOC4757): The title compound was isolated as a white solid in 48% yield: 1H-NMR (500 MHz, [D6]DMSO, 25 °C, TMS): δ = 8.93 (1H; s; N-H), 7.96 (1H; d; J = 1.75 Hz; C-Haromatic), 7.80 (1H; dd; J = 8.2 Hz, 1.75 Hz; C-Haromatic), 7.62 (1H; d; J = 8.2 Hz; C-Haromatic), 7.38–7.10 (6H; m; C-Haromatic), 6.85–6.66 (3H; m; C-Haromatic), 4.74 (2H; s; CH2), 3.87 (2H; t; J = 6 Hz; CH2), 3.66 (2H; t; J = 6.3 Hz; CH2), 3.61 (2H; s; CH2), 2.84 (1H; br. s; O-H), 1.85–1.73 (2H; m; CH2), 1.73–1.60 (2H; m; CH2) ppm. 13C-NMR (125 MHz, [D6]DMSO, 25 °C, TMS): δ = 170.26, 167.82, 167.72, 159.27, 143.78, 136.28, 135.47, 133.35, 130.04, 128.68, 128.34, 127.83, 126.55, 124.38, 124.06, 121.50, 115.83, 114.32, 113.18, 67.70, 62.35, 44.42, 41.61, 29.30, 25.76 ppm. HRMS-TOF: m/z [M + H]+ calculated for C27H26N2O5: 459.1920, found: 459.1922.
N-(2-benzyl-1,3-dioxo-2,3-dihydro-1H-isoindol-5-yl)-2-{3-[(6-hydroxyhexyl)oxy]phenyl}acetamide (13; ZHAWOC5042): The title compound was isolated as a white solid in 77% yield: 1H-NMR (500 MHz, [D6]DMSO, 25 °C, TMS): δ = 8.74 (1H; s; N-H), 7.94 (1H; d; J = 1.8 Hz; C-Haromatic), 7.82 (1H; dd; J = 8.2 Hz, 1.8 Hz; C-Haromatic), 7.65 (1H; d; J = 8.2 Hz; C-Haromatic), 7.38–7.32 (2H; m; C-Haromatic), 7.29–7.15 (4H; m; C-Haromatic), 6.85–6.72 (3H; m; C-Haromatic), 4.76 (2H; s; CH2), 3.86 (2H; t; J = 6.4 Hz; CH2), 3.64 (2H; s; CH2), 3.63 (2H; t; J = 6.5 Hz; CH2), 2.41 (1H; br. s; O-H), 1.76–1.67 (2H; m; ; CH2), 1.60–1.53 (2H; m; ; CH2), 1.47–1.33 (4H; m; ; CH2) ppm. 13C-NMR (125 MHz, [D6]DMSO, 25 °C, TMS): δ = 170.08, 167.74, 167.67, 159.51, 143.74, 136.30, 135.38, 133.38, 130.06, 128.67, 128.36, 127.80, 126.58, 124.38, 121.33, 115.76, 114.25, 113.30, 67.78, 62.71, 44.53, 41.61, 32.58, 29.13, 25.82, 25.53 ppm. HRMS-TOF: m/z [M + H]+ calculated for C29H30N2O5: 487.2233, found: 487.2211.
The following acids were synthesized according to compound (3):
3-(3-{[(2-benzyl-1,3-dioxo-2,3-dihydro-1H-isoindol-5-yl)carbamoyl]methyl}phenoxy)propanoic acid (14; ZHAWOC4767): The title compound was isolated as a white solid in 51% yield: 1H-NMR (500 MHz, [D6]DMSO, 25 °C, TMS): δ = 12.35 (1H; br. s; COOH), 10.76 (1H; s; N-H), 8.21 (1H; d; J = 1.7 Hz; C-Haromatic), 7.90 (1H; dd; J = 8.2 Hz, 1.7 Hz; C-Haromatic), 7.84 (1H; d; J = 8.2 Hz; C-Haromatic), 7.36–7.19 (6H; m; C-Haromatic), 6.96–6.78 (3H; m; C-Haromatic), 4.74 (2H; s; CH2), 4.15 (2H; t; J = 6 Hz; CH2), 3.69 (2H; s; CH2), 2.69 (2H; t; J = 6 Hz; CH2) ppm. 13C-NMR (125 MHz, [D6]DMSO, 25 °C, TMS): δ = 172.71, 170.42, 167.93, 167.76, 158.80, 145.23, 137.21, 133.55, 129.89, 129.04, 127.83, 125.79, 124.93, 123.86, 122.00, 115.91, 113.37, 113.10, 63.95, 43.83, 41.31, 34.56 ppm. HRMS-TOF: m/z [M + H]+ calculated for C26H22N2O6: 459.1556, found: 459.1557.
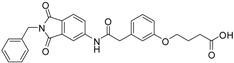
4-(3-{[(2-benzyl-1,3-dioxo-2,3-dihydro-1H-isoindol-5-yl)carbamoyl]methyl}phenoxy)butanoic acid (15; ZHAWOC4768): The title compound was isolated as a white solid in 84% yield: 1H-NMR (500 MHz, [D6]DMSO, 25 °C, TMS): δ = 12.12 (1H; br. s; COOH), 10.76 (1H; s; N-H), 8.21 (1H; d; J = 1.7 Hz; C-Haromatic), 7.90 (1H; dd; J = 8.2 Hz, 1.7 Hz; C-Haromatic), 7.84 (1H; d; J = 8.2 Hz; C-Haromatic), 7.37–7.18 (6H; m; C-Haromatic), 6.95–6.78 (3H; m; C-Haromatic), 4.74 (2H; s; CH2), 3.97 (2H; t; J = 6.4 Hz; CH2), 3.68 (2H; s; CH2), 2.38 (2H; t; J = 7.4 Hz; CH2), 1.93 (2H; quint.; J = 6.7 Hz; CH2) ppm. 13C-NMR (125 MHz, [D6]DMSO, 25 °C, TMS): δ = 174.62, 170.44, 167.93, 167.76, 158.97, 145.24, 137.21, 137.17, 129.86, 129.04, 127.85, 127.82, 124.92, 123.86, 121.83, 115.95, 113.37, 113.11, 66.97, 43.84, 41.31, 30.72, 24.80 ppm. HRMS-TOF: m/z [M + H]+ calculated for C27H24N2O6: 473.1712, found: 473.1709.
6-(3-{[(2-benzyl-1,3-dioxo-2,3-dihydro-1H-isoindol-5-yl)carbamoyl]methyl}phenoxy)hexanoic acid (16; ZHAWOC5078): The title compound was isolated as a white solid in 87% yield: 1H-NMR (500 MHz, [D6]DMSO, 25 °C, TMS): δ = 11.82 (1H; br. s; COOH), 10.78 (1H; s; N-H), 8.21 (1H; d; J = 1.8 Hz; C-Haromatic), 7.90 (1H; dd; J = 8.2 Hz, 1.8 Hz; C-Haromatic), 7.83 (1H; d; J = 8.2 Hz; C-Haromatic), 7.35–7.19 (6H; m; C-Haromatic), 6.93–6.78 (3H; m; C-Haromatic), 4.73 (2H; s; CH2), 3.93 (2H; t; J = 6.3 Hz; CH2), 3.68 (2H; s; CH2), 2.22 (2H; t; J = 7.3 Hz; CH2), 1.73–1.66 (2H; m; CH2), 1.59–1.51 (2H; m; CH2), 1.45–1.37 (2H; m; CH2) ppm. 13C-NMR (125 MHz, [D6]DMSO, 25 °C, TMS): δ = 174.94, 170.45, 167.92, 167.75, 159.11, 145.24, 137.20, 137.14, 129.84, 129.04, 127.85, 127.82, 125.77, 124.91, 123.85, 121.72, 115.96, 113.36, 113.05, 67.67, 43.86, 41.31, 34.19, 28.93, 25.65, 24.79 ppm. HRMS-TOF: m/z [M + H]+ calculated for C29H28N2O6: 501.2025, found: 501.2022.
The following acyl sulfonamides were synthesized according to compound (4):
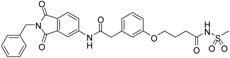
3-(3-{[(2-benzyl-1,3-dioxo-2,3-dihydro-1H-isoindol-5-yl)carbamoyl]methyl}phenoxy)-N-methanesulfonylpropanamide (17; ZHAWOC5136): The title compound was isolated as a white solid in 19% yield: 1H-NMR (500 MHz, [D6]DMSO, 25 °C, TMS): δ = 11.86 (1H; s; N-H), 10.77 (1H; s; N-H), 8.21 (1H; d: J = 1.9 Hz; C-Haromatic), 7.89 (1H; dd; J = 8.2 Hz, 1.9 Hz; C-Haromatic), 7.84 (1H; d; J = 8.2 Hz; C-Haromatic), 7.35–7.21 (6H; m; C-Haromatic), 6.94–6.81 (3H; m; C-Haromatic), 4.74 (2H; s; CH2), 4.18 (2H; t; J = 6 Hz; CH2), 3.69 (2H; s; CH2), 3.22 (3H; s ; CH3), 2.75 (2H; t; J = 6 Hz; CH2) ppm. 13C-NMR (125 MHz, [D6]DMSO, 25 °C, TMS): δ = 170.90, 170.41, 167.93, 167.76, 158.72, 145.22, 137.21, 133.55, 129.90, 129.04, 127.86, 127.82, 125.80, 124.94, 123.86, 122.10, 115.99, 113.16, 63.33, 43.82, 41.46, 41.32, 36.15 ppm. HRMS-TOF: m/z [M + H]+ calculated for C27H25N3O7S: 536.1491, found: 536.1483.
4-(3-{[(2-benzyl-1,3-dioxo-2,3-dihydro-1H-isoindol-5-yl)carbamoyl]methyl}phenoxy)-N-methanesulfonylbutanamide (18; ZHAWOC5135): The title compound was isolated as a white solid in 26% yield: 1H-NMR (500 MHz, [D6]DMSO, 25 °C, TMS): δ = 11.71 (1H; s; N-H), 10.77 (1H; s; N-H), 8.21 (1H; d: J = 1.8 Hz; C-Haromatic), 7.89 (1H; dd; J = 8.2 Hz, 1.8 Hz; C-Haromatic), 7.84 (1H; d; J = 8.2 Hz; C-Haromatic), 7.35–7.21 (6H; m; C-Haromatic), 6.93–6.80 (3H; m; C-Haromatic), 4.74 (2H; s; CH2), 3.96 (2H; t; J = 6.2 Hz; CH2), 3.68 (2H; s; CH2), 3.21 (3H; s ; CH3), 2.44 (2H; t; J = 7.4 Hz; CH2), 1.95 (2H; quint.; J = 6.7 Hz; CH2) ppm. 13C-NMR (125 MHz, [D6]DMSO, 25 °C, TMS): δ = 172.77, 170.42, 167.93, 167.76, 158.92, 145.22, 137.21, 137.18, 133.55, 129.87, 129.04, 127.86, 127.83, 125.80, 124.94, 123.86, 121.90, 115.93, 113.36, 113.13, 66.89, 43.83, 41.46, 41.32, 32.55, 24.15 ppm. HRMS-TOF: m/z [M + H]+ calculated for C28H27N3O7S: 550.1648, found: 550.1640.
4.3. In Silico Studies
Molecular modeling experiments were performed using the Molecular Operating Environment MOE 2013.08 from Chemical Computing Group (www.chemcomp.com). Co-crystal structures of MMP-13 are available from the Protein Data Bank (www.rcsb.org). For the actual work PDB code 2OW9 was selected for the computational studies. In MOE the duplicates were eliminated using the sequence editor and the pocket was prepared for the docking studies via the Protonate 3D method applying the default values for temperature 300 K, pH 7 and salt 0.1. The ligands to be docked to the protein were imported from structural data files (SD files) to receive a MOE compatible molecular database. As the SD files did not contain 3D coordinates, they were generated directly using MOE rebuild3D with a root mean square deviation (RMSD) gradient of 0.1. For docking experiments the Amber10:EHT force field [32,33] was used. The pharmacophore placement was applied with the pharmacophore set of three hydrogen bond acceptors (Acc1) used in the query. The docked poses were subsequently analyzed with respect to their scores and interactions with the catalytic zinc ion.
4.4. In Vitro Assays
IC50 values were determined at Reaction Biology Corporation, Malvern, PA, USA in triplicates using 10 concentrations starting at 10 μM with 3 fold dilution. The substrate used for the determinations was the (5-FAM/QXLTM) FRET peptide. The buffer consisted of 50 mM HEPES at pH 7.5 with 10 mM CaCl2 and 0.01% Brij-35. 0.1 mg/mL BSA was added before use. As a control inhibitor GM6001 was used.
5. Conclusions
In conclusion, we could employ molecular recognition for evolving an allosteric inhibitor through the addition of properly arranged weak zinc binding fragments to obtain an inhibitor with dual binding mode. In addition, the lipophilic ligand efficiency could be improved to lead-like level.
For the definite confirmation of the binding mode, a co-crystal structure of the complex is required. This work is currently ongoing in our lab.
Supplementary Materials
Supplementary materials can be found at http://www.mdpi.com/1422-0067/17/3/314/s1.
Acknowledgments
The authors are grateful to the Zurich University of Applied Sciences (ZHAW) for financial support. We thank Roland Josuran for HRMS measurements.
Author Contributions
Thomas Fischer was involved in the structure-based design and synthesis of the inhibitors and in writing this article. Rainer Riedl was involved in the conception and the supervision of the research and in writing this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Attur, M.; Yang, Q.; Shimada, K.; Tachida, Y.; Nagase, H.; Mignatti, P.; Statman, L.; Palmer, G.; Kirsch, T.; Beier, F.; et al. Elevated expression of periostin in human osteoarthritis cartilage and its potential role in matrix degradation via MMP-13. Osteoarthr. Cartil. 2015, 23, A135–A136. [Google Scholar] [CrossRef]
- Supuran, C.; Winum, J.Y.; Wang, B. Drug Design of Zinc-Enzyme Inhibitors: Functional, Structural, and Disease Applications; Wiley: New York, NY, USA, 2009. [Google Scholar]
- Nagase, H.; Woessner, J.F. Matrix Metalloproteinases. J. Biol. Chem. 1999, 274, 21491–21494. [Google Scholar] [CrossRef] [PubMed]
- Bertini, I.; Calderone, V.; Fragai, M.; Luchinat, C.; Maletta, M.; Yeo, K.J. Snapshots of the Reaction Mechanism of Matrix Metalloproteinases. Angew. Chem. Int. Ed. 2006, 45, 7952–7955. [Google Scholar] [CrossRef] [PubMed]
- Cawston, T.E.; Wilson, A.J. Understanding the role of tissue degrading enzymes and their inhibitors in development and disease. Best Pract. Res. Clin. Rheumatol. 2006, 20, 983–1002. [Google Scholar] [CrossRef] [PubMed]
- Rowan, A.D.; Litherland, G.J.; Hui, W.; Milner, J.M. Metalloproteases as potential therapeutic targets in arthritis treatment. Expert Opin. Ther. Targets 2008, 12, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Carl, R.F. Novel Therapies in OA. Curr. Drug Targets 2010, 11, 614–619. [Google Scholar]
- Li, N.G.; Shi, Z.H.; Tang, Y.P.; Wang, Z.J.; Song, S.L.; Qian, L.H.; Qian, D.W.; Duan, J.A. New Hope for the Treatment of Osteoarthritis Through Selective Inhibition of MMP-13. Curr. Med. Chem. 2011, 18, 977–1001. [Google Scholar] [CrossRef] [PubMed]
- Stamenkovic, I. Matrix metalloproteinases in tumor invasion and metastasis. Semin. Cancer Biol. 2000, 10, 415–433. [Google Scholar] [CrossRef] [PubMed]
- Zigrino, P.; Kuhn, I.; Bäuerle, T.; Zamek, J.; Fox, J.W.; Neumann, S.; Licht, A.; Schorpp-Kistner, M.; Angel, P.; Mauch, C. Stromal Expression of MMP-13 Is Required for Melanoma Invasion and Metastasis. J. Investig. Dermatol. 2009, 129, 2686–2693. [Google Scholar] [CrossRef] [PubMed]
- Kiili, M.; Cox, S.W.; Chen, H.W.; Wahlgren, J.; Maisi, P.; Eley, B.M.; Salo, T.; Sorsa, T. Collagenase-2 (MMP-8) and collagenase-3 (MMP-13) in adult periodontitis: molecular forms and levels in gingival crevicular fluid and immunolocalisation in gingival tissue. J. Clin. Periodontol. 2002, 29, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Sorsa, T.; Tjäderhane, L.; Salo, T. Matrix metalloproteinases (MMPs) in oral diseases. Oral Dis. 2004, 10, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.F.; Mobashery, S. Recent advances in MMP inhibitor design. Cancer Metastasis Rev. 2006, 25, 115–136. [Google Scholar] [CrossRef] [PubMed]
- Babine, R.E.; Bender, S.L. Molecular Recognition of Protein−Ligand Complexes:Applications to Drug Design. Chem. Rev. 1997, 97, 1359–1472. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, M.; Floyd, C.D.; Brown, P.; Gearing, A.J.H. Design and Therapeutic Application of Matrix Metalloproteinase Inhibitors. Chem. Rev. 1999, 99, 2735–2776. [Google Scholar] [CrossRef] [PubMed]
- Tommasi, R.A.; Weiler, S.; McQuire, L.W.; Rogel, O.; Chambers, M.; Clark, K.; Doughty, J.; Fang, J.; Ganu, V.; Grob, J.; et al. Potent and selective 2-naphthylsulfonamide substituted hydroxamic acid inhibitors of matrix metalloproteinase-13. Bioorg. Med. Chem. Lett. 2011, 21, 6440–6445. [Google Scholar] [CrossRef] [PubMed]
- Sparano, J.A.; Bernardo, P.; Stephenson, P.; Gradishar, W.J.; Ingle, J.N.; Zucker, S.; Davidson, N.E. Randomized Phase III Trial of Marimastat Versus Placebo in Patients With Metastatic Breast Cancer Who Have Responding or Stable Disease After First-Line Chemotherapy: Eastern Cooperative Oncology Group Trial E2196. J. Clin. Oncol. 2004, 22, 4683–4690. [Google Scholar] [CrossRef] [PubMed]
- Engel, C.K.; Pirard, B.; Schimanski, S.; Kirsch, R.; Habermann, J.; Klingler, O.; Schlotte, V.; Weithmann, K.U.; Wendt, K.U. Structural Basis for the Highly Selective Inhibition of MMP-13. Chem. Biol. 2005, 12, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Gege, C.; Bao, B.; Bluhm, H.; Boer, J.; Gallagher, B.M.; Korniski, B.; Powers, T.S.; Steeneck, C.; Taveras, A.G.; Baragi, V.M. Discovery and Evaluation of a Non-Zn Chelating, Selective Matrix Metalloproteinase 13 (MMP-13) Inhibitor for Potential Intra-articular Treatment of Osteoarthritis. J. Med. Chem. 2012, 55, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Roth, J.; Minond, D.; Darout, E.; Liu, Q.; Lauer, J.; Hodder, P.; Fields, G.B.; Roush, W.R. Identification of novel, exosite-binding matrix metalloproteinase-13 inhibitor scaffolds. Bioorg. Med. Chem. Lett. 2011, 21, 7180–7184. [Google Scholar] [CrossRef] [PubMed]
- Savi, C.D.; Morley, A.D.; Ting, A.; Nash, I.; Karabelas, K.; Wood, C.M.; James, M.; Norris, S.J.; Karoutchi, G.; Rankine, N.; et al. Selective non zinc binding inhibitors of MMP13. Bioorg. Med. Chem. Lett. 2011, 21, 4215–4219. [Google Scholar] [CrossRef] [PubMed]
- Schnute, M.E.; O’Brien, P.M.; Nahra, J.; Morris, M.; Howard Roark, W.; Hanau, C.E.; Ruminski, P.G.; Scholten, J.A.; Fletcher, T.R.; Hamper, B.C.; et al. Discovery of (pyridin-4-yl)-2H-tetrazole as a novel scaffold to identify highly selective matrix metalloproteinase-13 inhibitors for the treatment of osteoarthritis. Bioorg. Med. Chem. Lett. 2010, 20, 576–580. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.J.; Abeywardane, A.; Liang, S.; Muegge, I.; Padyana, A.K.; Xiong, Z.; Hill-Drzewi, M.; Farmer, B.; Li, X.; Collins, B.; et al. Fragment-Based Discovery of Indole Inhibitors of Matrix Metalloproteinase-13. J. Med. Chem. 2011, 54, 8174–8187. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.R.; Pavlovsky, A.G.; Ortwine, D.F.; Prior, F.; Man, C.F.; Bornemeier, D.A.; Banotai, C.A.; Mueller, W.T.; McConnell, P.; Yan, C.; et al. Discovery and Characterization of a Novel Inhibitor of Matrix Metalloprotease-13 That Reduces Cartilage Damage in Vivo without Joint Fibroplasia Side Effects. J. Biol. Chem. 2007, 282, 27781–27791. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, S.; Nuti, E.; Cercignani, G.; Regina, G.L.; Silvestri, R.; Supuran, C.T.; Rossello, A. Kinetic characterization of 4,4′-biphenylsulfonamides as selective non-zinc binding MMP inhibitors. J. Enzym. Inhib. Med. Chem. 2015, 30, 947–954. [Google Scholar] [CrossRef] [PubMed]
- Lanz, J.; Riedl, R. Merging Allosteric and Active Site Binding Motifs: De novo Generation of Target Selectivity and Potency via Natural-Product-Derived Fragments. Chemmedchem 2015, 10, 451–454. [Google Scholar] [CrossRef] [PubMed]
- Fischer, T.; Riedl, R. Strategic Targeting of Multiple Water-Mediated Interactions: A Concise and Rational Structure-Based Design Approach to Potent and Selective MMP-13 Inhibitors. Chemmedchem 2013, 8, 1457–1461. [Google Scholar] [CrossRef] [PubMed]
- Liebeschuetz, J.W.; Cole, J.C.; Korb, O. Pose prediction and virtual screening performance of GOLD scoring functions in a standardized test. J. Comput. Aided Mol. Des. 2012, 26, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, J.A.; Major Jourden, J.L.; Miller, M.T.; Cohen, S.M. To bind zinc or not to bind zinc: An examination of innovative approaches to improved metalloproteinase inhibition. Biochim. Biophys. Acta BBA Mol. Cell. Res. 2010, 1803, 72–94. [Google Scholar] [CrossRef] [PubMed]
- Rossello, A.; Nuti, E.; Orlandini, E.; Balsamo, A.; Panelli, L. Inhibitors of Zinc Proteases Thioaryl Substituted and Their Use. WO2008015139 (A2), 7 February 2008. [Google Scholar]
- Wu, J.; Rush, T.S., III; Hotchandani, R.; Du, X.; Geck, M.; Collins, E.; Xu, Z.B.; Skotnicki, J.; Levin, J.I.; Lovering, F.E. Identification of potent and selective MMP-13 inhibitors. Bioorg. Med. Chem. Lett. 2005, 15, 4105–4109. [Google Scholar] [CrossRef] [PubMed]
- Case, D.A.; Cheatham, T.E.; Darden, T.; Gohlke, H.; Luo, R.; Merz, K.M.; Onufriev, A.; Simmerling, C.; Wang, B.; Woods, R.J. The Amber biomolecular simulation programs. J. Comput. Chem. 2005, 26, 1668–1688. [Google Scholar] [CrossRef] [PubMed]
- Gerber, P.R.; Müller, K. MAB, a generally applicable molecular force field for structure modelling in medicinal chemistry. J. Comput. Aided Mol. Des. 1995, 9, 251–268. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).