Reduced Connexin26 in the Mature Cochlea Increases Susceptibility to Noise-Induced Hearing Loss in Mice
Abstract
:1. Introduction
2. Results
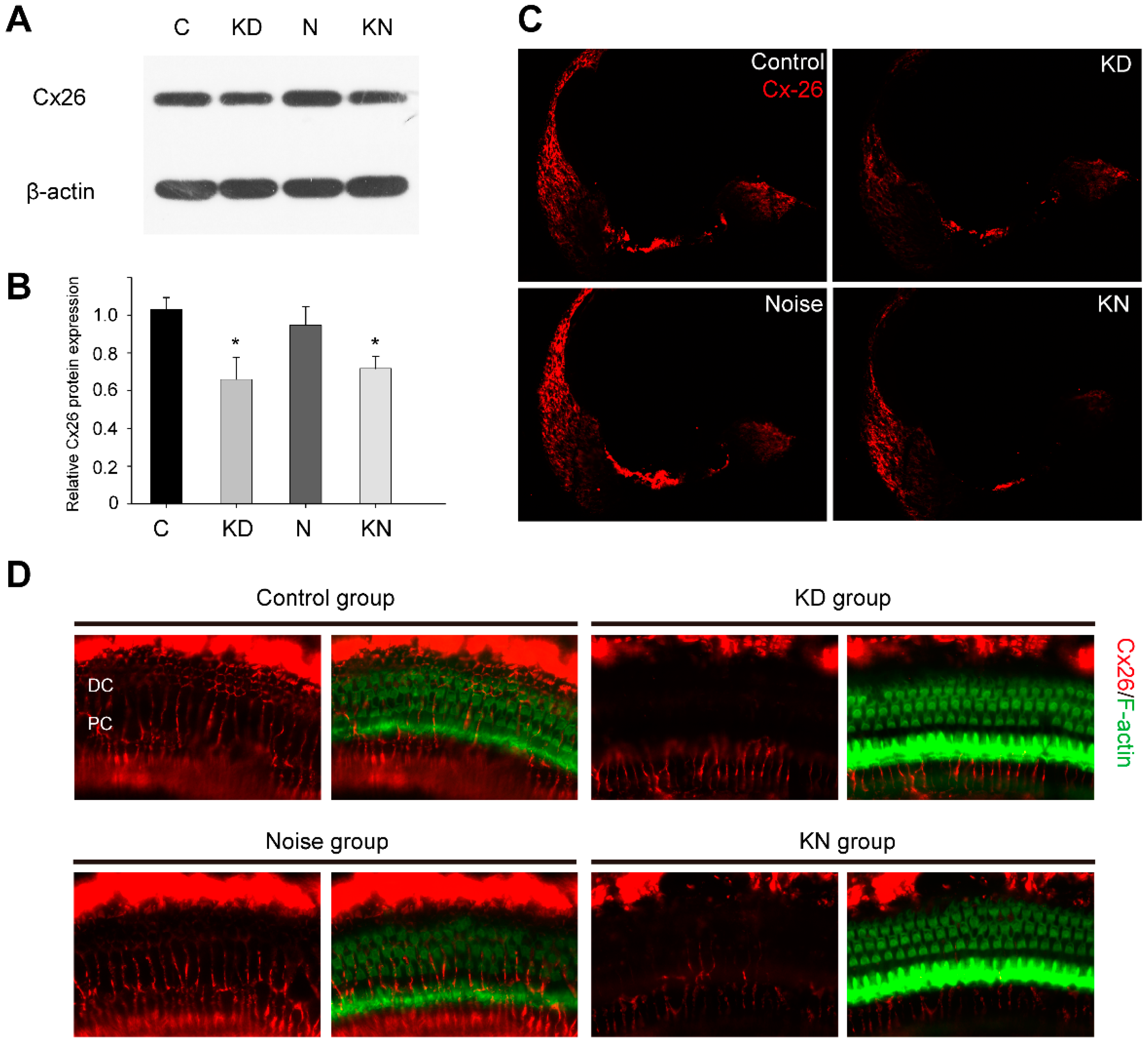
2.1. Connexin26 Deletion in Conditional Cx26 Knocked down Mice
2.2. Hearing Thresholds Shift after Cx26 Reduction and Noise Exposure
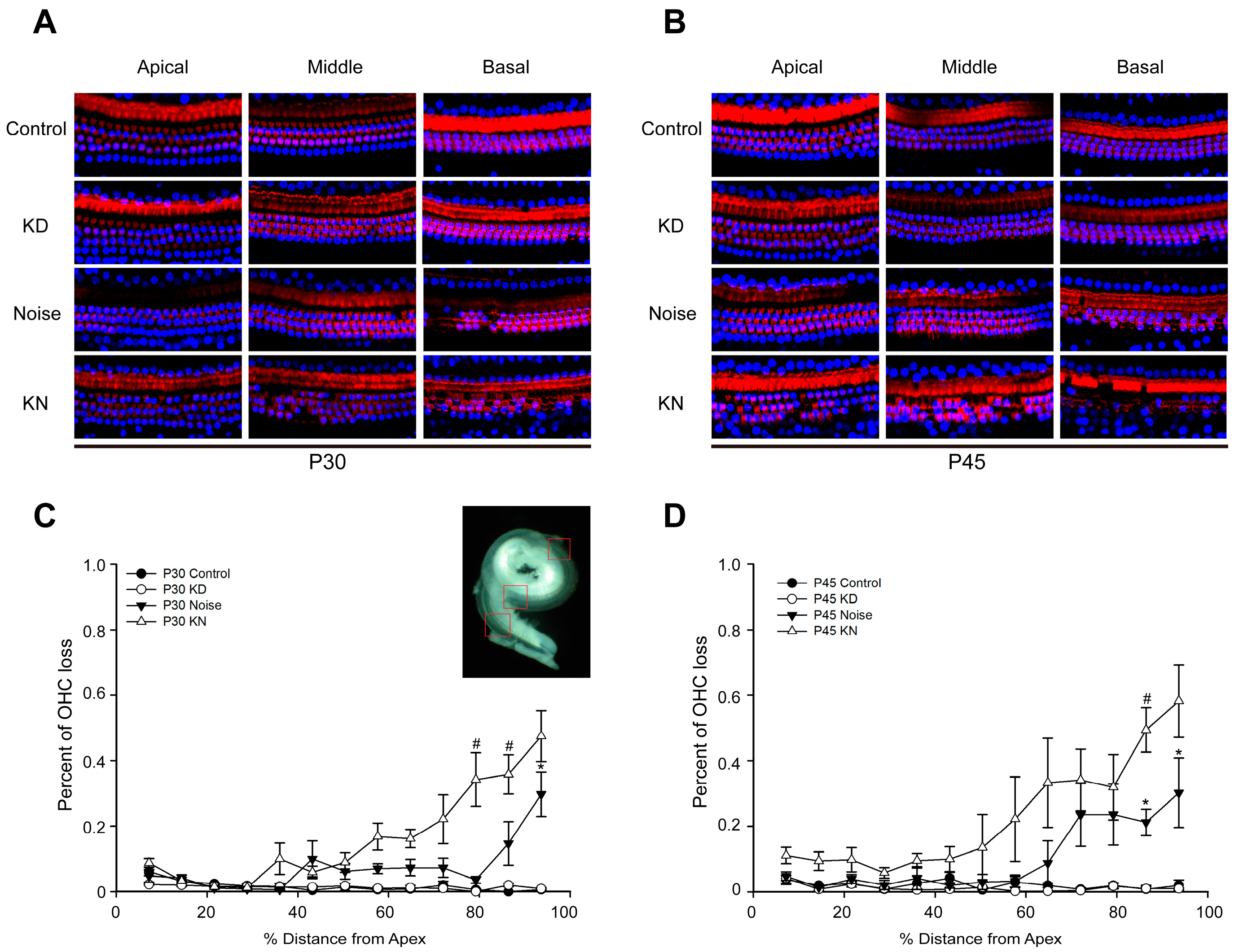
2.3. Cx26 Reduction Exacerbates Hair Cell Loss in the Knockdown + Noise Groups (KN Groups)
2.4. Cell Degeneration Pattern after Cx26 Reduction and Noise Exposure
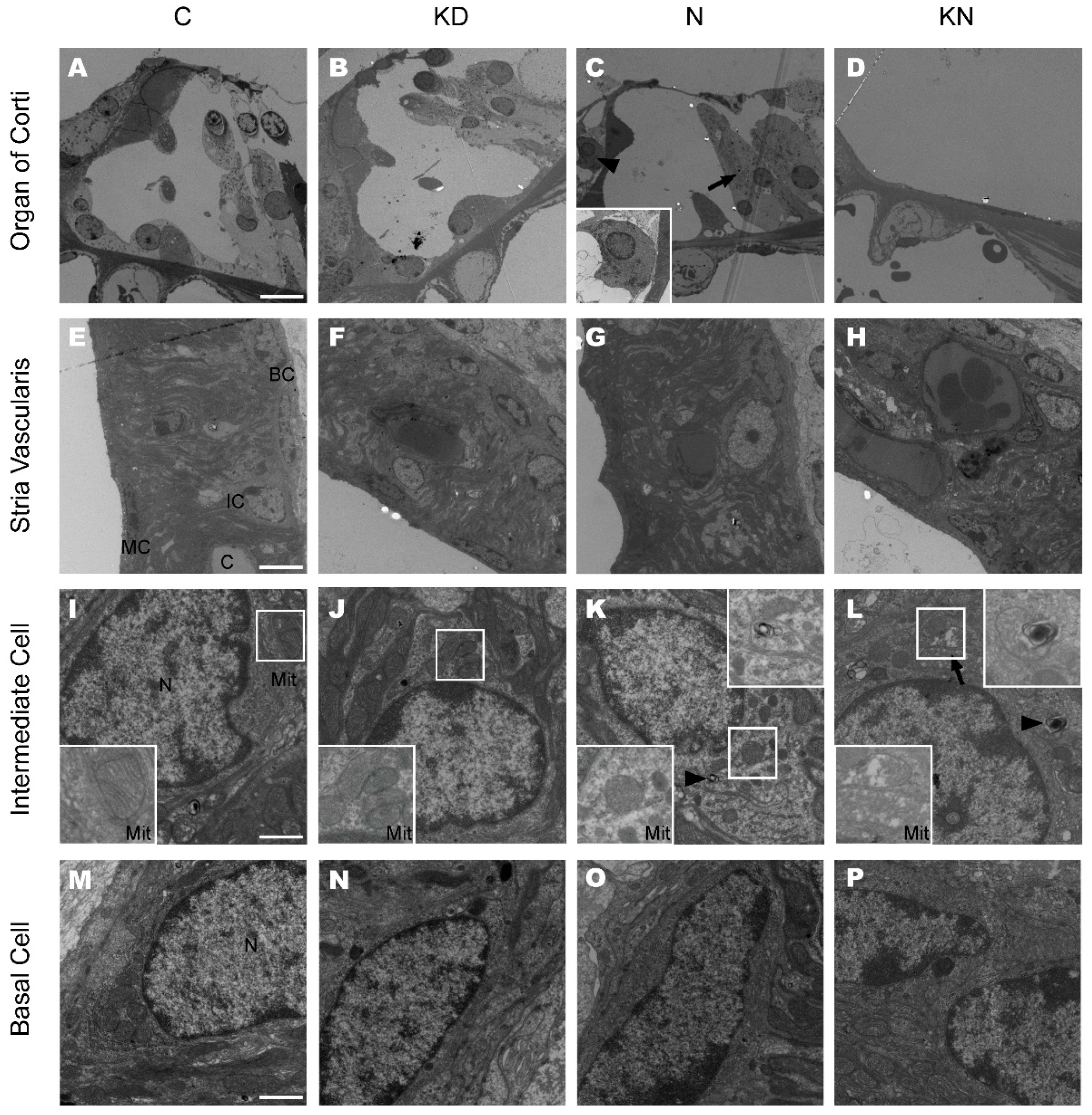
2.5. Ultrastructural Changes after Cx26 Reduction and Noise Exposure
3. Discussions
4. Materials and Methods
4.1. Generation of the Connexin26 (Cx26) Conditional Knockdown (KD) Mouse Model and Genotyping
4.2. Animal Treatment and Noise Exposure Procedure
4.3. Auditory Brainstem Recordings
4.4. Protein Extraction and Western Blot Analysis
4.5. Cochlear Tissue Preparation and Immunofluorescent Labeling
4.6. Quantification of Cochlear Hair Cells
4.7. Resin Sections and Transmission Electron Microscopy
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Abbreviations
| Cx26 | connexin26 |
| NIHL | noise-induced hearing loss |
| KD | knocked down |
| GJ | gap junction |
| SNPs | single nucleotide polymorphisms |
| SPF | specific-pathogen free |
| KN | knocked down + noise |
| HC | hair cell |
| OHC | outer hair cell |
| SV | stria vascularis |
| TC | tunnel of Corti |
| MC | marginal cells |
| OC | organ of Corti |
| BC | basal cell |
References
- Elfgang, C.; Eckert, R.; Lichtenberg-Frate, H.; Butterweck, A.; Traub, O.; Klein, R.A.; Hulser, D.F.; Willecke, K. Specific permeability and selective formation of gap junction channels in connexin-transfected hela cells. J. Cell Biol. 1995, 129, 805–817. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.B.; Yu, N.; Fleming, C.R. Gap junctional hemichannel-mediated atp release and hearing controls in the inner ear. Proc. Natl. Acad. Sci. USA 2005, 102, 18724–18729. [Google Scholar] [CrossRef] [PubMed]
- Gossman, D.G.; Zhao, H.B. Hemichannel-mediated inositol 1,4,5-trisphosphate (IP3) release in the cochlea: A novel mechanism of IP3 intercellular signaling. Cell Commun. Adhes. 2008, 15, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Ahmad, S.; Chen, S.; Tang, W.; Zhang, Y.; Chen, P.; Lin, X. Cochlear gap junctions coassembled from Cx26 and 30 show faster intercellular Ca2+ signaling than homomeric counterparts. Am. J. Physiol. Cell Physiol. 2005, 288, C613–C623. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, T.; Kimura, R.S.; Paul, D.L.; Adams, J.C. Gap junctions in the rat cochlea: Immunohistochemical and ultrastructural analysis. Anat. Embryol. 1995, 191, 101–118. [Google Scholar] [CrossRef] [PubMed]
- Iliades, T.; Eleftheriades, N.; Iliadou, V.; Pampanos, A.; Voyiatzis, N.; Economides, J.; Leotsakos, P.; Neou, P.; Tsakanikos, M.; Antoniadi, T.; et al. Prelingual nonsyndromic hearing loss in greece. Molecular and clinical findings. ORL J. Oto-Rhino-Laryngol. Relat. Spec. 2002, 64, 321–323. [Google Scholar] [CrossRef]
- Zelante, L.; Gasparini, P.; Estivill, X.; Melchionda, S.; D′Agruma, L.; Govea, N.; Milá, M.; Monica, M.D.; Lutfi, J.; Shohat, M. Connexin 26 mutations associated with the most common form of non-syndromic neurosensory autosomal recessive deafness (DFNB1) in mediterraneans. Hum. Mol. Genet. 1997, 6, 1605–1609. [Google Scholar] [CrossRef] [PubMed]
- Murgia, A.; Orzan, E.; Polli, R.; Martella, M.; Vinanzi, C.; Leonardi, E.; Arslan, E.; Zacchello, F. Cx26 deafness: Mutation analysis and clinical variability. J. Med. Genet. 1999, 36, 829–832. [Google Scholar] [PubMed]
- Cohn, E.S.; Kelley, P.M.; Fowler, T.W.; Gorga, M.P.; Lefkowitz, D.M.; Kuehn, H.J.; Schaefer, G.B.; Gobar, L.S.; Hahn, F.J.; Harris, D.J.; et al. Clinical studies of families with hearing loss attributable to mutations in the connexin 26 gene (GJB2/DFNB1). Pediatrics 1999, 103, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, P.-I.; Borg, E.; Grip, L.; Dahl, N.; Bondeson, M.-L. Variability in noise susceptibility in a swedish population: The role of 35delg mutation in the connexin 26 (GJB2) gene. Audiol. Med. 2004, 2, 123–130. [Google Scholar] [CrossRef]
- Van Eyken, E.; van Laer, L.; Fransen, E.; Topsakal, V.; Hendrickx, J.-J.; Demeester, K.; van de Heyning, P.; Mäki-Torkko, E.; Hannula, S.; Sorri, M. The contribution of GJB2 (connexin 26) 35delg to age-related hearing impairment and noise-induced hearing loss. Otol. Neurotol. 2007, 28, 970–975. [Google Scholar] [PubMed]
- Abreu-Silva, R.S.; Rincon, D.; Horimoto, A.R.; Sguillar, A.P.; Ricardo, L.A.; Kimura, L.; Batissoco, A.C.; Auricchio, M.T.; Otto, P.A.; Mingroni-Netto, R.C. The search of a genetic basis for noise-induced hearing loss (NIHL). Ann. Hum. Biol. 2011, 38, 210–218. [Google Scholar] [PubMed]
- Pawelczyk, M.; van Laer, L.; Fransen, E.; Rajkowska, E.; Konings, A.; Carlsson, P.I.; Borg, E.; van Camp, G.; Sliwinska-Kowalska, M. Analysis of gene polymorphisms associated with k ion circulation in the inner ear of patients susceptible and resistant to noise-induced hearing loss. Ann. Hum. Genet. 2009, 73, 411–421. [Google Scholar] [PubMed]
- Wang, S.L.; Yu, L.G.; Liu, R.P.; Zhu, W.Z.; Gao, W.M.; Xue, L.P.; Jiang, X.; Zhang, Y.H.; Yi, D.; Chen, D. Gene-gene interaction of GJB2, SOD2, and cat on occupational noise-induced hearing loss in chinese han population. Biomed. Environ. Sci.: BES 2014, 27, 965–968. [Google Scholar] [PubMed]
- Inoshita, A.; Iizuka, T.; Okamura, H.O.; Minekawa, A.; Kojima, K.; Furukawa, M.; Kusunoki, T.; Ikeda, K. Postnatal development of the organ of corti in dominant-negative GJB2 transgenic mice. Neuroscience 2008, 156, 1039–1047. [Google Scholar] [PubMed]
- Cohen-Salmon, M.; Ott, T.; Michel, V.; Hardelin, J.P.; Perfettini, I.; Eybalin, M.; Wu, T.; Marcus, D.C.; Wangemann, P.; Willecke, K.; et al. Targeted ablation of connexin26 in the inner ear epithelial gap junction network causes hearing impairment and cell death. Curr. Biol.: CB 2002, 12, 1106–1111. [Google Scholar] [PubMed]
- Wang, Y.; Chang, Q.; Tang, W.; Sun, Y.; Zhou, B.; Li, H.; Lin, X. Targeted connexin26 ablation arrests postnatal development of the organ of corti. Biochem. Biophys. Res. Commun. 2009, 385, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Sun, Y.; Lin, X.; Kong, W. Down regulated connexin26 at different postnatal stage displayed different types of cellular degeneration and formation of organ of corti. Biochem. Biophys. Res. Commun. 2014, 445, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, J.; Zhu, Y.; Liang, C.; Zhao, H.-B. Deafness induced by connexin 26 (GJB2) deficiency is not determined by endocochlear potential (EP) reduction but is associated with cochlear developmental disorders. Biochem. Biophys. Res. Commun. 2014, 448, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Chen, J.; Liang, C.; Zong, L.; Jones, R.O.; Zhao, H.B. Connexin26 (GJB2) deficiency reduces active cochlear amplification leading to late-onset hearing loss. Neuroscience 2015, 284, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Chang, Q.; Tang, W.; Kim, Y.; Lin, X. Timed conditional null of connexin26 in mice reveals temporary requirements of connexin26 in key cochlear developmental events before the onset of hearing. Neurobiol. Dis. 2015, 73, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Tang, W.; Ahmad, S.; Lu, J.; Colby, C.C.; Zhu, J.; Yu, Q. Applications of targeted gene capture and next-generation sequencing technologies in studies of human deafness and other genetic disabilities. Hear. Res. 2012, 288, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Tang, W.; Chang, Q.; Wang, Y.; Kong, W.; Lin, X. Connexin30 null and conditional connexin26 null mice display distinct pattern and time course of cellular degeneration in the cochlea. J. Comp. Neurol. 2009, 516, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, T.; Kamiya, K.; Gotoh, S.; Sugitani, Y.; Suzuki, M.; Noda, T.; Minowa, O.; Ikeda, K. Perinatal GJB2 gene transfer rescues hearing in a mouse model of hereditary deafness. Hum. Mol. Genet. 2015, 24, 3651–3661. [Google Scholar] [CrossRef] [PubMed]
- Beltramello, M.; Piazza, V.; Bukauskas, F.F.; Pozzan, T.; Mammano, F. Impaired permeability to ins (1,4,5) P3 in a mutant connexin underlies recessive hereditary deafness. Nat. Cell Biol. 2005, 7, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Kelsell, D.P.; Dunlop, J.; Stevens, H.P.; Lench, N.J.; Liang, J.N.; Parry, G.; Mueller, R.F.; Leigh, I.M. Connexin 26 mutations in hereditary non-syndromic sensorineural deafness. Nature 1997, 387, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Pujol, R.; Lavigne-Rebillard, M.; Lenoir, M. Development of sensory and neural structures in the mammalian cochlea. In Development of the Auditory System; Springer: New York, NY, USA, 1998; pp. 146–192. [Google Scholar]
- Liang, C.; Zhu, Y.; Zong, L.; Lu, G.-J.; Zhao, H.-B. Cell degeneration is not a primary causer for connexin26 (GJB2) deficiency associated hearing loss. Neurosci. Lett. 2012, 528, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liang, C.; Chen, J.; Zong, L.; Chen, G.D.; Zhao, H.B. Active cochlear amplification is dependent on supporting cell gap junctions. Nat. Commun. 2013, 4, 1786. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hirose, K.; Liberman, M.C. Dynamics of noise-induced cellular injury and repair in the mouse cochlea. J. Assoc. Res. Otolaryngol. 2002, 3, 248–268. [Google Scholar] [CrossRef] [PubMed]
- Konings, A.; van Laer, L.; van Camp, G. Genetic studies on noise-induced hearing loss: A review. Ear Hear. 2009, 30, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Vicente-Torres, M.A.; Schacht, J. A bad link to mitochondrial cell death in the cochlea of mice with noise-induced hearing loss. J. Neurosci. Res. 2006, 83, 1564–1572. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-D.; Fechter, L.D. The relationship between noise-induced hearing loss and hair cell loss in rats. Hear. Res. 2003, 177, 81–90. [Google Scholar] [CrossRef]
- Yu, J.; Wang, Y.; Liu, P.; Li, Q.; Sun, Y.; Kong, W. Mitochondrial DNA common deletion increases susceptibility to noise-induced hearing loss in a mimetic aging rat model. Biochem. Biophys. Res. Commun. 2014, 453, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Zilberstein, Y.; Liberman, M.C.; Corfas, G. Inner hair cells are not required for survival of spiral ganglion neurons in the adult cochlea. J. Neurosci. 2012, 32, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Wang, Y.F.; Wang, S.Y.; Liu, S.F.; Yu, Z.; Xi, L.; Li, H.W. Ultrastructural pathological changes in the cochlear cells of connexin 26 conditional knockout mice. Mol. Med. Rep. 2013, 8, 1029–1036. [Google Scholar] [PubMed]
- Suzuki, T.; Matsunami, T.; Hisa, Y.; Takata, K.; Takamatsu, T.; Oyamada, M. Roles of gap junctions in glucose transport from glucose transporter 1-positive to -negative cells in the lateral wall of the rat cochlea. Histochem. Cell Biol. 2009, 131, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Matsunami, T.; Suzuki, T.; Hisa, Y.; Takata, K.; Takamatsu, T.; Oyamada, M. Gap junctions mediate glucose transport between GLUT1-positive and-negative cells in the spiral limbus of the rat cochlea. Cell. Commun. Adhes. 2006, 13, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Chang, Q.; Tang, W.; Ahmad, S.; Zhou, B.; Lin, X. Gap junction mediated intercellular metabolite transfer in the cochlea is compromised in connexin30 null mice. PLoS ONE 2008, 3, e4088. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.-Q.; Zheng, H.-W.; Hill, K.; Sha, S.-H. Traumatic noise activates Rho-family Gtpases through transient cellular energy depletion. J. Neurosci. 2012, 32, 12421–12430. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, R.; Yamaguchi, T.; Kuramoto, N.; Ogita, K. Acoustic overstimulation activates 5′-AMP-activated protein kinase through a temporary decrease in ATP level in the cochlear spiral ligament prior to permanent hearing loss in mice. Neurochem. Int. 2011, 59, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Yamane, H.; Nakai, Y.; Takayama, M.; Konishi, K.; Iguchi, H.; Nakagawa, T.; Shibata, S.; Kato, A.; Sunami, K.; Kawakatsu, C. The emergence of free radicals after acoustic trauma and strial blood flow. Acta Oto-Laryngol. Suppl. 1995, 519, 87–92. [Google Scholar] [CrossRef]
- Ohlemiller, K.K.; Wright, J.S.; Dugan, L.L. Early elevation of cochlear reactive oxygen species following noise exposure. Audiol. Neuro-Otol. 1999, 4, 229–236. [Google Scholar] [CrossRef]
- Feine, I.; Pinkas, I.; Salomon, Y.; Scherz, A. Local oxidative stress expansion through endothelial cells—A key role for gap junction intercellular communication. PLoS ONE 2012, 7, e41633. [Google Scholar] [CrossRef] [PubMed]
- Le, H.T.; Sin, W.C.; Lozinsky, S.; Bechberger, J.; Vega, J.L.; Guo, X.Q.; Saez, J.C.; Naus, C.C. Gap junction intercellular communication mediated by connexin43 in astrocytes is essential for their resistance to oxidative stress. J. Biol. Chem. 2014, 289, 1345–1354. [Google Scholar] [CrossRef] [PubMed]


© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.-X.; Chen, S.; Xie, L.; Ji, Y.-Z.; Wu, X.; Wang, W.-W.; Yang, Q.; Yu, J.-T.; Sun, Y.; Lin, X.; et al. Reduced Connexin26 in the Mature Cochlea Increases Susceptibility to Noise-Induced Hearing Loss in Mice. Int. J. Mol. Sci. 2016, 17, 301. https://doi.org/10.3390/ijms17030301
Zhou X-X, Chen S, Xie L, Ji Y-Z, Wu X, Wang W-W, Yang Q, Yu J-T, Sun Y, Lin X, et al. Reduced Connexin26 in the Mature Cochlea Increases Susceptibility to Noise-Induced Hearing Loss in Mice. International Journal of Molecular Sciences. 2016; 17(3):301. https://doi.org/10.3390/ijms17030301
Chicago/Turabian StyleZhou, Xing-Xing, Sen Chen, Le Xie, Yu-Zi Ji, Xia Wu, Wen-Wen Wang, Qi Yang, Jin-Tao Yu, Yu Sun, Xi Lin, and et al. 2016. "Reduced Connexin26 in the Mature Cochlea Increases Susceptibility to Noise-Induced Hearing Loss in Mice" International Journal of Molecular Sciences 17, no. 3: 301. https://doi.org/10.3390/ijms17030301
APA StyleZhou, X.-X., Chen, S., Xie, L., Ji, Y.-Z., Wu, X., Wang, W.-W., Yang, Q., Yu, J.-T., Sun, Y., Lin, X., & Kong, W.-J. (2016). Reduced Connexin26 in the Mature Cochlea Increases Susceptibility to Noise-Induced Hearing Loss in Mice. International Journal of Molecular Sciences, 17(3), 301. https://doi.org/10.3390/ijms17030301






