Abstract
Bifidobacterium longum is a very important gram-positive non-pathogenic bacterium in the human gastrointestinal tract for keeping the digestive and immune system healthy. Isocitrate dehydrogenase (IDH) from B. longum (BlIDH), a novel member in Type II subfamily, was overexpressed, purified and biochemically characterized in detail. The active form of BlIDH was an 83-kDa homodimer. Kinetic analysis showed BlIDH was a NADP+-dependent IDH (NADP-IDH), with a 567- and 193-fold preference for NADP+ over NAD+ in the presence of Mg2+ and Mn2+, respectively. The maximal activity for BlIDH occurred at 60 °C (with Mn2+) and 65 °C (with Mg2+), and pH 7.5 (with Mn2+) and pH 8.0 (with Mg2+). Heat-inactivation profiles revealed that BlIDH retained 50% of maximal activity after incubation at 45 °C for 20 min with either Mn2+ or Mg2+. Furthermore, the coenzyme specificity of BlIDH can be completely reversed from NADP+ to NAD+ by a factor of 2387 by replacing six residues. This current work, the first report on the coenzyme specificity conversion of Type II NADP-IDHs, would provide better insight into the evolution of NADP+ use by the IDH family.
1. Introduction
Isocitrate dehydrogenase (IDH) belongs to an ancient and ubiquitous metal-dependent β-decarboxylating dehydrogenase family that plays critical roles in amino acid biosynthesis, vitamin production and energy metabolism [1,2,3]. IDHs are key enzymes in the tricarboxylic acid cycle (TCA cycle) that catalyze the oxidative decarboxylation of isocitrate to α-ketoglutarate (α-KG) and CO2 with NAD+ or NADP+ as a coenzyme [4]. According to the different coenzyme dependencies, IDHs play a variety of roles in vivo. NAD+-dependent IDH (NAD-IDH, EC 1.1.1.41) provides the first connection between TCA cycle and electron-transport pathway by producing NADH, which participates in energy metabolism [5]. NADP+-dependent IDH (NADP-IDH, EC 1.1.1.42) generates NADPH, which provides the reducing power for biosynthesis, maintains the redox state of the cell, and takes part in CO2 assimilation. Therefore, NADP-IDH is essential in glutathione metabolism, fatty acids and steroids biosynthesis, and cellular antioxidation systems [6,7,8]. Recently, increasing attention has been paid to human NADP-IDHs. It has been reported that the mutations at some active sites can confer a new function on NADP-IDHs to reduce α-ketoglutarate to 2-hydroxyglutarate, which correlates closely with the incidence of tumors [9,10,11], such as gliomas, the most common type of human brain cancers.
Based on the phylogenetic analysis, IDHs can be divided into three subfamilies: Type I IDHs, Type II IDHs and monomeric IDHs [12]. Most bacterial homodimeric NAD(P)-IDHs, homotetrameric NAD-IDHs and mitochondrial heteroligomeric NAD-IDHs are clustered into the Type I subfamily. The homodimeric NADP-IDHs from eukaryotes (in cytoplasm and mitochondria) and some bacterial NADP-IDHs are categorized into the Type II subfamily. It was noted that, recently, several algae IDHs were found to be Type II NAD-IDHs, whose coenzyme specificity can be completely converted from NAD+ to NADP+ by rational mutagenesis [12,13]. All monomeric enzymes, either NAD+ or NADP+-dependent, fall into the third subfamily. Type II NADP-IDHs from eukaryotes, such as yeast, pig and human have been extensively studied, including biochemical properties, the crystal structures and catalytic mechanisms [14,15,16]. On the contrary, the information of Type II NADP-IDHs from bacteria is very limited and only a few of them have been preliminarily characterized.
Furthermore, it was proposed that NAD+ use is an ancestral trait and NADP+ use by bacterial IDHs arose on or about the time that eukaryotic mitochondria first appeared, some 3.5 billion years ago [17]. The switch in coenzyme dependency from NAD+ to NADP+ by IDHs was an ancient adaptation for bacterial survival on energy-poor compound (such as acetate). This hypothesis has been proved by the competition experiment in the laboratory for NADP+-dependent IDH from Escherichia coli (EcIDH), a typical member of Type I IDHs subfamily [17]. It is an interesting question that whether the selective mechanism of this ancient adaptation is also suitable to Type II IDHs subfamily , because the sequence similarity between two IDHs subfamilies is very low (<20%).
Bifidobacterium longum, a gram-positive and non-pathogenic bacterium, is one of the most popular probiotics in various dairy products to provide enormous health benefits for the healthy human gastrointestinal system, such as improving lactose tolerance, preventing diarrhea and inhibiting pathogen colonization [18,19,20,21]. Several studies have shown that B. longum plays a key role in modulating the immune system [22,23] and has certain guiding significance to cancer gene therapy [24,25].
In this work, the enzymology of a homodimeric IDH from B. longum (BlIDH) in Type II subfamily was investigated in detail. In addition, the coenzyme specificity of BlIDH was converted from NADP+ to NAD+ by site-directed mutagenesis, which may provide useful clues to explore the acquired cause of NADP+ dependency by Type II IDHs.
2. Results
2.1. Sequence Alignment
The coding sequence of BlIDH consisted of 1221 bp nucleotides with one open reading frame (ORF) encoding a protein of 406 amino acids. This enzyme showed a high level of sequence similarity to that of Type II NADP-IDHs, such as Mycobacterium tuberculosis IDH (73.5%), Clostridium thermocellum IDH (53.8%) and human cytosolic IDH (61.5%). Phylogenetic analysis was performed to further clarify the evolutionary relationship between BlIDH and other IDHs. The result revealed that BlIDH was clustered into the clade of Type II NADP-IDHs (Figure 1).
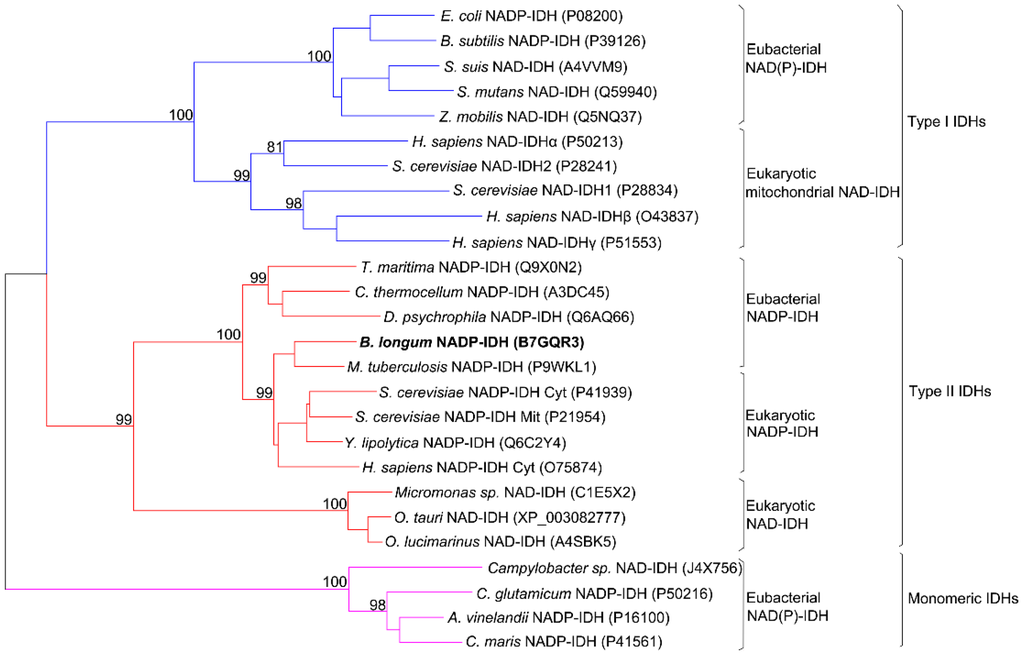
Figure 1.
Phylogenetic tree of 26 isocitrate dehydrogenases (IDHs). A neighbor-joining tree with 500 bootstrap was created using MEGA 6.06. The GenBank accession numbers were noted in the parentheses.
In order to evaluate potential substrate and coenzyme-binding sites, multiple sequence alignments were performed (Figure 2). All amino acids involved in substrate binding are highly conserved in both Type I and II subfamilies, but the residues responsible for coenzyme binding are significantly different in the two subfamilies. When compared with NADP-IDH in the Type II subfamily, Arg314 and His315 in the binary complex of human cytosolic NADP-IDH (HcIDH) are considered to be the major determinants of coenzyme specificity, which form salt bridges with the 2′-phosphate group of NADP+ [15].
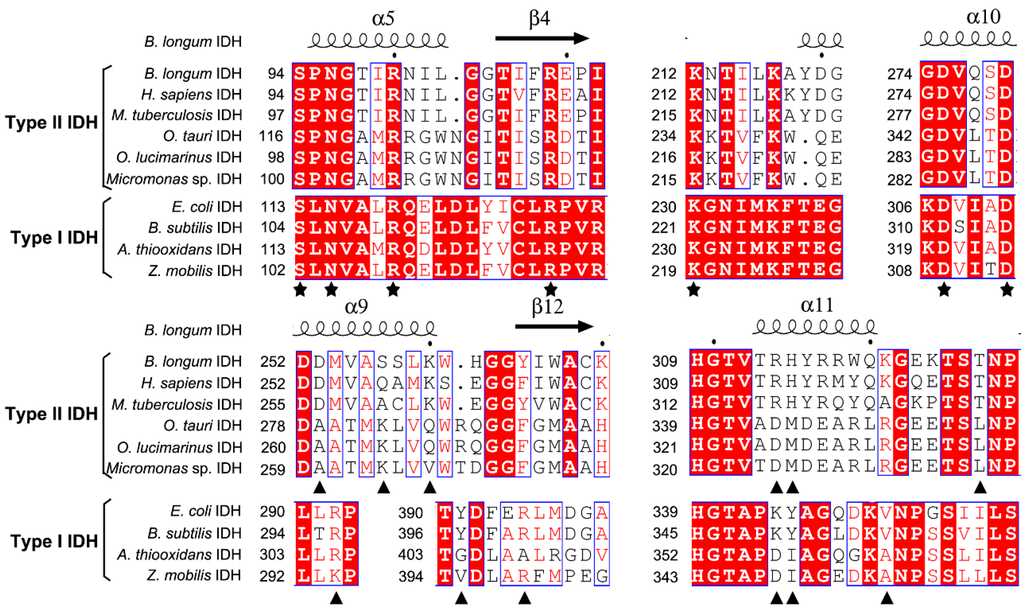
Figure 2.
Structure-based protein sequences alignment of isocitrate dehydrogenase (IDH) from B. longum (BlIDH) with other IDHs. The primary residues involved in substrate binding are indicated by pentagrams (★). The residues interact with the 2′-phosphate of NADP+ directly or indirectly are indicated by triangles (▲). The letters in blue boxes indicate conserved residues, and the white letters with red background in blue boxes indicate strictly conserved residues. The black letters in white boxes indicate similarity. The structure of BlIDH was generated by SWISS-MODEL server. The figure created by ESPript 3.0.
The corresponding residues are completely conserved in BlIDH (Arg314 and His315). In the quaternary complex of HcIDH (Figure 3), the side chains of Gln257′ and Lys260′ (the prime indicates the other subunit of the homodimer) form hydrogen bonds with the 2′-phosphate group of NADP+, which are homologous to Ser257′ and Lys260′ in BlIDH [15]. Furthermore, Arg314 in HcIDH forms a salt bridge with Asp253′ (equivalent to Asp253′ in BlIDH) and interacts with Arg249′ and Gln257′ [15]. As compared with NAD-IDH, Arg314 and His315 in BlIDH are replaced by Asp357 and Ile358 in Acidithiobacillus thiooxidans NAD-IDH (AtIDH) from Type I subfamily [26] and Asp344 and Met345 in Ostreococcus tauri NAD-IDH (OtIDH) from Type II subfamily [13]. Asp253′, Ser257′ and Lys260′ in BlIDH are substituted with Ala279′, Lys283′ and Gln286′ in OtIDH, as shown in Figure 3.
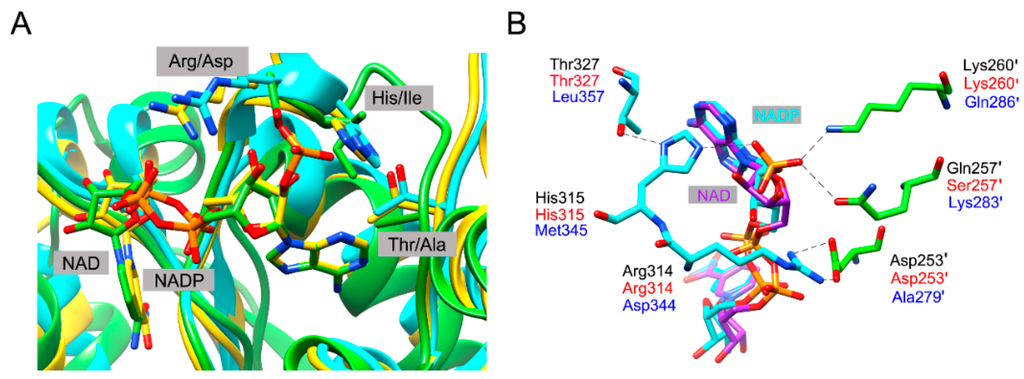
Figure 3.
Comparison of the NADP+-binding sites among the human cytosolic NADP-IDH (HcIDH), BlIDH, Acidithiobacillus thiooxidans NAD-IDH (AtIDH) and Ostreococcus tauri NAD-IDH (OtIDH). (A) Overlay of the subunits of HcIDH (yellow, PDB code: 1T0L), modelled BlIDH (cyan) and AtIDH (green, PDB code: 2D4V) highlighting the selected coenzyme binding sites in these three IDHs. The NADP+ molecule (with yellow C atoms) and NAD+ molecule (with green C atoms) were represented by the stick. The model of BlIDH was generated by SWISS-MODEL server; (B) A close-up view showing the selected residues involving in NADP+ binding in HcIDH (labelled by black). The equivalent residues in BlIDH, targeted by site-directed mutagenesis, and in OtIDH were labelled by red and blue, respectively.
2.2. Expression and Purification
Recombinant BlIDH with 6×His tag was successfully heterologously expressed in E. coli Rosetta (DE3) and purified to homogeneity by Co2+ affinity chromatography. Molecular mass of the recombinant protein was determined to be approximately 45 kDa by SDS-PAGE, which compared well with the predicted value (45 kDa) (Figure 4A) and was confirmed by Western blotting by probing with anti-6×His antibody (Figure 4B). The oligomeric status of BlIDH was determined by size exclusion chromatography (SEC), and a single symmetrical peak was observed (Figure 4C) while the native molecular mass of BlIDH was calculated to be 83 kDa, suggesting that the native enzyme forms a homodimer in solution.
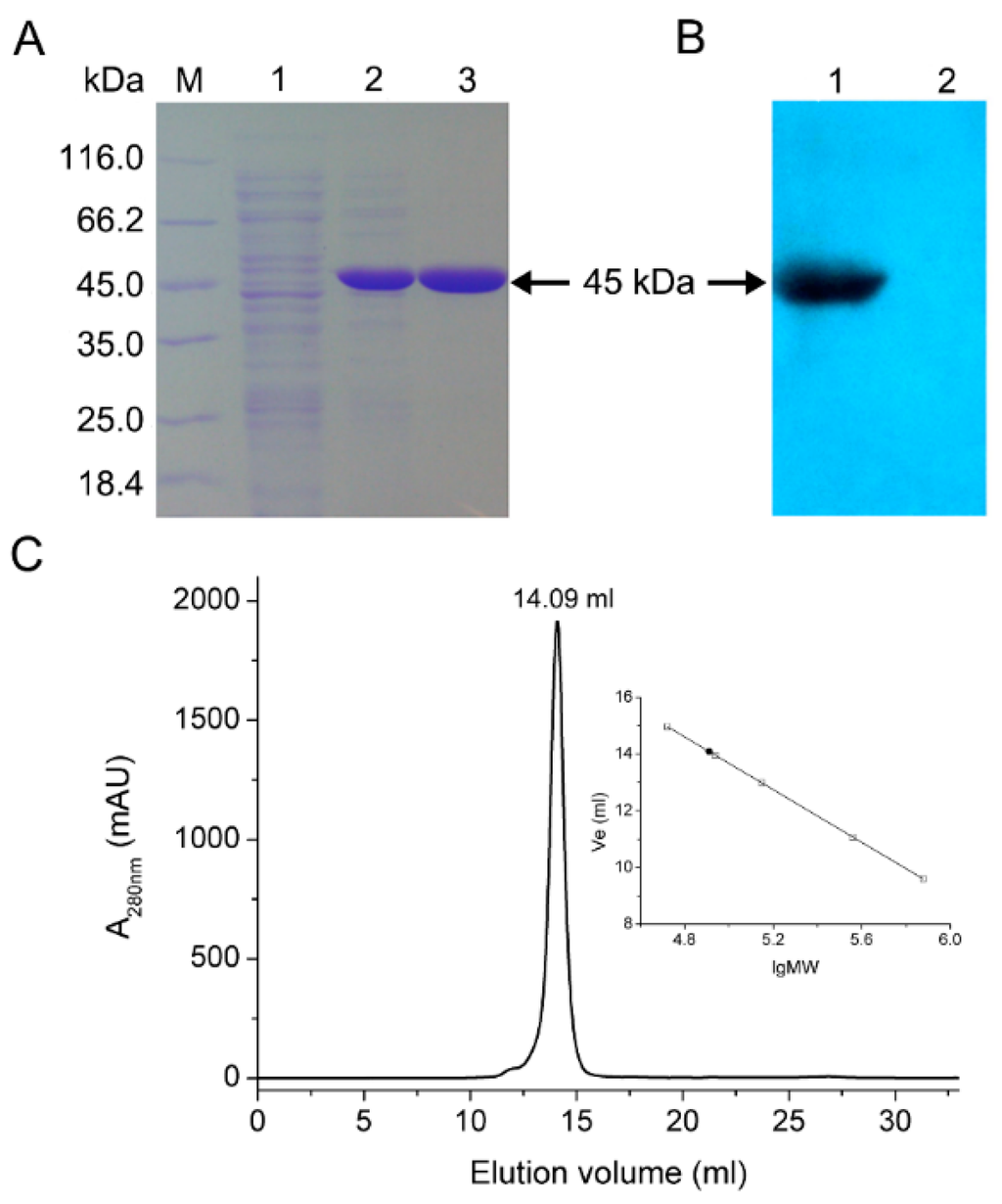
Figure 4.
Overexpression and molecular mass determination of BlIDH. (A) SDS-PAGE analysis. M, molecular mass marker; lane 1, crude extract from cells transformed by pET-28b(+) with IPTG treatment; lane 2, crude extract from cells transformed by recombinant plasmid pET-BlIDH with IPTG treatment; lane 3, purified BlIDH; (B) Western blot analysis. Lane 1, purified BlIDH; lane2, negative control, crude extract from cells transformed by pET-28b(+) with IPTG treatment; (C) Size exclusion chromatography (SEC) analysis of BlIDH.
2.3. Kinetic Characterization
The optimal pH values of the purified recombinant BlIDH were 7.5 with Mn2+ and 8.0 with Mg2+ (Figure 5A), similar to the homodimeric NADP-IDH from Leptospira interrogans (pH 7.0 with Mn2+ and pH 8.0 with Mg2+) [27], but apparently lower than the monomeric IDHs from Corynebacterium glutamicum (pH 9.0 with Mg2+) [28] and Chlorobium limicola (pH 9.0 with Mg2+) [29].
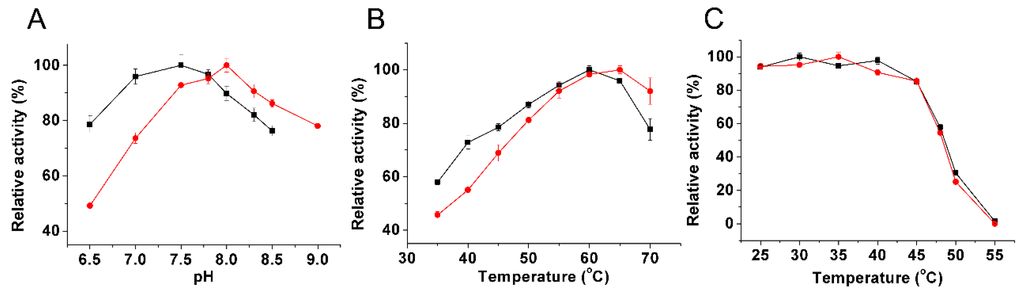
Figure 5.
Effect of pH and temperature on the activity of BlIDH in the presence of Mg2+ (●) and Mn2+ (■), respectively. (A) Effect of pH on the activity of BlIDH; (B) Effect of temperature on the activity of BlIDH; (C) Heat-inactivation profiles of the BlIDH. The values indicate the means of at least three independent measurements.
The recombinant BlIDH exhibited the maximal activity around 60 and 65 °C in the presence of Mn2+ and Mg2+, respectively (Figure 5B), similar to that of L. interrogan IDH (60 °C with Mn2+ and Mg2+) [27], but higher than those of NAD-IDHs from Congregibacter litoralis (35 °C with Mn2+ and Mg2+) [30] and Zymomonas mobilis IDH (55 °C with Mn2+ and Mg2+) [31]. Heat-inactivation studies revealed that the recombinant BlIDH was stable under 45 °C, but rapidly lost activity above this temperature, and only 50% activity remained after a 20 min incubation at 48 °C (Figure 5C).
The effects of 11 metal ions on BlIDH activity were also examined. The activity of recombinant BlIDH was entirely dependent on the presence of a divalent cation, such as Mn2+, the most effective activator for BlIDH catalysis (Table 1). However, several divalent metal ions, including Ni2+, Co2+, Zn2+, Cu2+ and Ca2+, inhibited the activity in the presence of Mn2+ or Mg2+, where Zn2+ showed the most inhibitory effects on BlIDH activity.

Table 1.
Effects of metal ions on the activity of recombinant BlIDH.
The kinetic parameters for recombinant BlIDH were determined in both NAD+- and NADP+-dependent forms (Table S1). The Km values of BlIDH for NADP+ were 19.45 μM with Mg2+ and 58.29 μM with Mn2+ (Figure S1A,B). The apparent Km values of BlIDH for NAD+ were over 184-fold and nine-fold higher than those for NADP+ in the presence of Mg2+ and Mn2+, respectively. As a result, the catalytic efficiency (kcat/Km) of BlIDH showed 567-fold (Mg2+) and 193-fold (Mn2+) preference for NADP+ over NAD+, respectively. Evidently, BlIDH has remarkably high coenzyme specificity toward NADP+ although it is a dual-specificity enzyme.
When compared with other homodimeric NADP-IDHs, the Km values for NADP+ of BlIDH (19.45 μM with Mg2+) was similar to those of L. interrogan IDH (21.1 μM) [27] and E. coli IDH (17 μM) [32], but higher than that of Rattus norvegicus cytosolic IDH (11.5 μM) [33] and much lower than those of Yarrowia lipolytica IDH (59 μM) [34] and Helicobacter pylori IDH (176 μM) [35]. The kcat/Km values for NADP+ of BlIDH (1.87 s−1·μM−1 with Mg2+) was higher than that of H. pylori IDH (0.704 s−1·μM−1) [35], a member of Type I IDHs subfamily. When compared with other monomeric NADP-IDHs, in the presence of NADP+, the coenzyme affinity (1/Km) and the catalytic efficiency (1.87 s−1·μM−1 with Mg2+ and 0.83 s−1·μM−1 with Mn2+) of BlIDH were quite a bit lower than those of monomeric IDHs, such as C. glutamicum IDH (21.75 s−1·μM−1) [28] and Colwellia maris IDH (8.9 s−1·μM−1) [36].
2.4. Switch of Coenzyme Specificity
Amino acid sequence alignment, homology modeling and structural analysis of Type II NADP-IDHs revealed that several amino acid residues in BlIDH may interact with 2′-phosphate moiety of NADP+ directly or indirectly, including Arg314, His315, Thr327, Asp253, Ser257 and Lys260 (Figure 3). To switch coenzyme specificity of BlIDH, these residues were selected as targets for site-directed mutagenesis. Here, only three mutants, single mutant R314D, triple mutant R314D/H315I/T327A and sextuple mutant D253A/S257K/K260Q/R314D/H315I/T327A, displayed detectable activity. Furthermore, circular dichroism (CD) spectra of mutants were very similar to that of wild type (Figure S2), indicating that these six mutations did not cause the conformational alterations of BlIDH.
Kinetic analysis demonstrated that single mutant R314D resulted in an approximate 57-fold increase, from 19.45 to 1102 μM, in Km for NADP+, with no significant change in Km for NAD+ (Table 2). Furthermore, R314D showed a dramatic decrease (984-fold) in kcat/Km toward NADP+ coupled with a 16.5-fold decrease in kcat/Km toward NAD+. Thus, the single mutant R314D is still a dual-specificity enzyme with an approximate 9.5-fold preference for NADP+ over NAD+, although the wild-type BlIDH exhibited 567-fold preference for NADP+. When another two amino acids (His315 and Thr327) were substituted with Ile315 and Ala327, the triple mutant showed no detectable activity for NADP+ but only for NAD+-linked reaction. However, the catalytic efficiency of R314D/H315I/T327A was very poor as compared to wild type (Table 2).

Table 2.
The kinetic parameters of wild-type BlIDH and mutants.
Mutations at six sites caused a 17-fold increase in Km for NADP+ and a 28-fold decrease in Km for NAD+ (Figure S1C,D). Furthermore, the catalytic efficiency of sextuple mutant was increased 1.2-fold for NAD+ while it retained only about 0.05% catalytic efficiency for NADP+. As a result, the sextuple mutant showed an approximately four-fold preference for NAD+ over NADP+ [(kcat/Km) NAD/(kcat/Km) NADP], which clearly indicated that the coenzyme specificity of BlIDH was completely converted from NADP+ to NAD+ by a factor of 2387 via six-residue replacement.
3. Discussion
3.1. Coenzyme Specificity Determinants of BlIDH
IDH specificity is governed by residue interactions at three layers [37]. The “First Layer” residues directly contact with the unique 2′-hydroxyl and 2′-phosphate groups of NAD(P)+; The “Second Layer” residues are more distant amino acids, which can modulate the effects of the first group but not in contact with the unique cofactor moieties; The “Third Layer” residues are far from the cofactor binding site and form long-range interactions, which might promote the formation of a low-energy conformation.
Based on sequence alignment and available structural information, Arg314 and His315 in BlIDH are considered to be the “First Layer” residues, two putative coenzyme specificity determinants, by directly interacting with 2′-phosphate group of NADP+. As expected, the single mutant R314D decreased the affinity for NADP+ by approximately 57-fold due to the removal of a salt bridge between Arg314 and 2′-phosphate group of NADP+ together with the removal of the electrostatic repulsion between negatively charged Asp and 2′-phosphate group of NADP+. When adjacent His315 was simultaneously mutated to Met or Ile, the resulting double mutant R314D/H315I and R314D/H315M lost activity completely. The loss in NADP+-dependent activity might result from the removal of favorable interactions between Arg314 or His315 and the 2′-phosphate group.
To achieve the NAD+-dependent activity, a third mutation (Thr327Ala) was introduced into the double mutant, creating R314D/H315I/T327A. Here, the residue Thr327 in BlIDH is equivalent to Val351 in EcIDH and therefore considered to be the “Second Layer” residue. The crystal structure of an EcIDH mutant has revealed several essential indirect effects of Val351Ala on specificity alteration, such as to avoid obstructing adenine ring shift and to accommodate the favorable repacking in the adenosine-binding pocket [37]. The similar result has also been reported for Haloferax volcanii IDH that the replacement of Val by Ala was crucial for its engineered enzyme to obtain the activity with NAD+ as a coenzyme [38]. As a result, the triple mutant R314D/H315I/T327A restored partial activity for NAD+ (Table 3).

Table 3.
Comparison of kinetic parameters between sextuple mutant and other IDHs.
In order to improve the affinity and catalytic activity of R314D/H315I/T327A, a putative residue Asp253 forming hydrogen bond with Arg314 and two putative residues (Ser257 and Lys260) forming extra hydrogen bonds with 2′-phosphate group of NADP+ were engineered to the corresponding residues in NAD+-dependent OtIDH (Ala279, Lys283 and Gln286), generating D253A/S257K/K260Q/R314D/H315I/T327A. Compared with the triple mutant (R314D/H315I/T327A), this sextuple mutant showed approximately a 34-fold increase in affinity and a 1.3-fold increase in catalytic activity, resulting in a 40-fold increase in catalytic efficiency for NAD+. With respect to the wild-type enzyme, the sextuple mutant displayed a 27.6-fold increase in affinity and a 1.2-fold increase in catalytic efficiency for NAD+. As a consequence, the sextuple mutant showed an approximately four-fold preference for NAD+ over NADP+ [(kcat/Km) NAD/(kcat/Km) NADP]. Therefore, the coenzyme specificity of BlIDH was converted from a 567-fold preference for NADP+ to a four-fold preference for NAD+ through rational engineering of the major determinants for coenzyme specificity.
3.2. Evolutionary Implications for NADP+ Use by Prokaryotic Type II IDHs
Phylogenetic analysis and competition experiments have revealed that NADP+ use by bacterial IDHs of Type I subfamily evolved from NAD+ use about 3.5 billion years ago, and the cause of selection is the NADPH demand for bacterial adaptation to anabolic niches such as acetate [17]. For IDHs of Type I subfamily, their coenzyme specificity can be completely converted from NADP+ to NAD+ such as NADP-IDHs from E. coli and H. volcanii [32,38], or from NAD+ to NADP+ such as NAD-IDH from Pyrococcus furiosus [40].
The evolutionary origin of Type II subfamily is still obscure because all its members are NADP-dependent IDHs. As more and more genomic sequences have become available, some novel NAD-dependent IDHs are clustered into Type II subfamily after reconstructing the phylogenetic tree of IDHs. We deduce that the ancestor of Type II subfamily is also a NAD-dependent enzyme, the same as that of Type I subfamily. Several NAD-dependent IDHs have converted their coenzyme specificities from NAD+ to NADP+ by strategic amino acid replacements, such as NAD-IDHs from Micromonas sp. [12], O. tauri [13] and C. litoralis [30].
The information about successful inversion of coenzyme specificity in NADP-IDHs of Type II subfamily is very limited. In a previous report, only a single mutant of Yarrowia lipolytica NADP-IDH (YlIDH, a Type II member), R322D, displayed poor activity for NAD+ [34]. In this work, the coenzyme specificity of BlIDH mutant was converted from NADP+ to NAD+ by a factor of 2387, which is the first case for complete alteration of the coenzyme specificity of Type II NADP-IDHs. However, due to the absence of a putative gene encoding isocitrate lyase in B. longum genome, it is unclear whether the coenzyme specificity change of BlIDH was also caused by the increased demand of NADPH under carbon starvation.
4. Experimental Section
4.1. Sequence Analysis
The X-ray structure of human cytosolic IDH (HcIDH) was downloaded from the Protein Data Bank database (available online: http://www.rcsb.org/pdb/). SWISS-MODEL server (avaible online: http://swissmodel.expasy.org/) was employed to create the homology model of BlIDH using HcIDH structure (PDB code: 1T0L) as a template. Multiple protein sequence alignment was performed using ClustalW program and ESPript 3.0 web tool (available online: http://espript.ibcp.fr/ESPript/cgi-bin/ESPript.cgi). For phylogenetic analysis, 26 protein sequences (including BlIDH) were aligned with ClustalW program. A Neighbor-Joining tree with 500 bootstrap was created via MEGA 6.06 program [41]. Protein structural figures were conducted using PyMOL [42] and UCSF Chimera [43].
4.2. Bacterial Srains and Reagents
The E. coli strains, DH5α and Rosetta (DE3), and plasmid pET-28b(+) were stored at low temperature in our laboratory. PrimeSTARTM HS DNA polymerase was purchased from TaKaRa Biotechnology Co., Ltd. (Dalian, China). Restriction endonucleases, T4 DNA ligase and protein molecular weight standards were obtained from Thermo Scientific (Shanghai, China).
4.3. Recombinant Plasmid Construction
Genomic DNA of B. longum subsp. infantis ATCC 15697 was bought from Leibniz Institute DSMZ-German Collection of Microorganisms and Cell Cultures (Braunschweig, Germany). According to the genomic sequence of B. longum (NCBI Reference Sequence: NC_011593.1), one pair of primers (Table S2) were designed to amplify the complete icd gene. Initial denaturing step of polymerase chain reaction (PCR) was 3 min at 95 °C, followed by 35 cycles of 95 °C for 30 s, 65 °C for 30 s, and 72 °C for 1 min and 30 s. After Nde I and Not I digestion, the PCR product was cloned into the expression vector pET-28b(+) which has a 6×His tag coding sequence upstream of the multiple cloning site. The icd gene in the recombinant plasmid pET-BlIDH was sequenced (GenScript, Nanjing, China).
4.4. Site-Directed Mutagenesis
In order to identify the determinants and convert the coenzyme specificity of BlIDH, six mutants were created by site-directed mutagenesis and overlap extension PCR technique [44]. The oligonucleotides for constructing the mutants were reported in Table S2. The mutated genes were constructed by sequential PCR steps. In the first step, two fragments containing the desired mutation were amplified with the following primers: BlIDH-S and one of the antisense primers including the point mutation; one of the sense primers including the point mutation and BlIDH-As. Then, the two overlapping fragments were purified and used as templates to amplify the full-length fragment using BlIDH-S and BlIDH-As. The final PCR products were digested by Nde I and Not I and ligated into the expression vector pET-28b(+). DNA sequencing was performed in both directions to verify all sequences of the mutated genes (GenScript, Nanjing, China).
4.5. Overexpression and Purification
The recombinant expression plasmids were transformed into E. coli Rosetta (DE3) strains, and grown overnight at 37 °C in Luria-Bertani (LB) medium supplemented with kanamycin and chloramphenicol to a final concentration of 30 μg/mL. The cultures were inoculated into 100 mL fresh LB media with the same antibiotics. When the optical density at 600 nm (OD600) of culture reached 0.4, the isopropyl-1-thio-β-d-galactopyranoside (IPTG) was added to a final concentration of 0.5 mM, followed by continuous growth at 20 °C for 20 h. The cells were collected by centrifugation at 5000× g for 5 min at 4 °C, and sonicated in lysis buffer (50 mM NaH2PO4, 300 mM NaCl, pH 7.8). The debris was removed by centrifugation at 12,000× g for 20 min at 4 °C. Finally, the recombinant proteins fused with 6×His-tag were purified with BD TALON metal affinity resin according to the manufacturer’s instructions (Clontech, Palo Alto, CA, USA).
4.6. SDS-PAGE and Western Blotting
The purity and molecular weight of BlIDH were determined by 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). For the Western blotting analysis, the recombinant enzymes were separated and transferred electrophoretically onto a nitrocellulose membrane. Then, the membrane was blocked for 1 h at room temperature in two solutions, TBS-T buffer (0.2% Tween-20, 150 mM NaCl and 50 mM Tris–HCl at pH 7.5) and 5% skim milk. Next, the membrane was immunoblotted with each antibody for 1 h, anti-6×His-tagpolyclonal antibody (cat#2365, Cell Signaling Technology, Danvers, MA, USA, 1:1000) followed by anti-rabbit IgG secondary antibody (cat#S3731, Promega, Madison, WI, USA, 1:2500). After that, the membrane was washed with TBS-T buffer for 10 min for three times. Finally, the chemiluminescence signal was observed by exposing the blot to X-ray film for an appropriate time period in a dark room.
4.7. Gel Filtration Chromatography
Molecular mass and oligomeric states of the recombinant BlIDH were measured by gel filtration chromatography on a 10/300 Superdex 200 column (Amersham Biosciences, Freiburg, Germany) with equilibration buffer (50 mM NaH2PO4 and 150 mM NaCl at pH 7.2). The following standards were employed: Ovalbumin (44,000 Da), Conalbumin (75,000 Da), Aldolase (158,000 Da), Ferritin (440,000 Da) and Thyroglobulin (669,000 Da).
4.8. Circular Dichroism Spectroscopy
Circular dichroism (CD) spectra of the recombinant enzymes were measured with a Jasco model J-810 spectropolarimeter. Each sample were prepared in 75 mM Na2SO4 and 20 mM NaH2PO4 at pH 7.5 and then diluted to a final concentration of 0.2 mg/mL. The ellipticity (θ) was generated by averaging 3 scans of the protein solution between 195 and 260 nm at 0.5-nm increments. The mean molar ellipticity, [θ] (deg cm2 dmole−1), was calculated from [θ] = θ/10nCl, where the relationship has been described previously [45]. The θ is the measurement, n is the number of residues per subunit of protein (411 amino acids for BlIDH), C is the molar concentration of the samples, and l is the cell path length (0.1 cm).
4.9. Enzyme Assays and Kinetic Characterization
The enzyme assay was described by Cvitkovitch et al. [46]. The activity assays were carried out at 25 °C in 1 mL reaction mixture containing 35 mM Tris-HCl at pH 8.0, 2 mM MgCl2 or MnCl2, 1 mM d,l-isocitric acid, and 0.5 mM NADP+ or 5 mM NAD+. The NAD(P)H production was monitored at 340 nm (ε340 = 6.22 mM−1·cm−1) using Cary300 UV-Vis spectrophotometer (Varian, Palo Alto, CA, USA). One unit of enzyme activity refers to 1 μM of NAD(P)H formed per minute. The enzyme concentration was detected by using Quick Start Bradford Protein Assay kit (Bio-Rad, Hercules, CA, USA).
To measure the Km values of the recombinant enzymes for NAD(P)+, the concentration of isocitrate was fixed at 1.0 mM with varying coenzyme concentrations. Apparent Km values for NAD(P)+ were calculated by nonlinear regression with GraphPad Prism 5.0 software (Prism, San Diego, CA, USA) and Origin 8.0 (OriginLab, Northampton, MA, USA). All kinetic parameters were measured in at least three independent experiments.
The effects of temperature, pH and metal ions on BlIDH activity were measured using the standard assay method. The optimum pH was determined in the range of 6.0–9.0, and the optimal temperature was measured in the range of 35–60 °C. The half-life of BlIDH was tested after incubation of enzyme aliquots at 25–55 °C for 20 min. The effects of metal ions on BlIDH activity were also detected, including 2 mM monovalent metal cations (K+, Li+, Na+, and Rb+) and divalent metal cations (Mg2+, Mn2+, Co2+, Ca2+, Cu2+, Zn2+, and Ni2+).
Supplementary Materials
Supplementary materials can be found at http://www.mdpi.com/1422-0067/17/3/296/s1.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (31570010, 31400003), the Natural Science Foundation of Anhui Province of China (1308085QC67), and the Provincial Project of Natural Science Research for Colleges and Universities of Anhui Province of China (KJ2013A128), Innovation Team of Scientific Research Platform in Anhui Universities and Key Laboratory of the Biotic Environment and Ecological Safety in Anhui Province.
Author Contributions
Peng Wang and Guo-Ping Zhu conceived and designed the experiments; Shi-Ping Huang and Hong-Mei Cheng performed the experiments; Shi-Ping Huang, Hong-Mei Cheng and Guo-Ping Zhu analyzed the data; Shi-Ping Huang and Guo-Ping Zhu wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| PCR | polymerase chain reaction |
| LB | Luria-Bertani |
| IPTG | isopropyl-1-thio-β-d-galactopyranoside |
| SDS-PAGE | sodium dodecyl sulfate polyacrylamide gel electrophoresis |
| CD | circular dichroism |
| SEC | size exclusion chromatography |
| IDH | isocitrate dehydrogenase |
| NADP+ | nicotinamide adenine dinucleotide phosphate |
| NAD+ | nicotinamide adenine dinucleotide |
| NADP-IDH | NADP+-dependent isocitrate dehydrogenase |
| NAD-IDH | NAD+-dependent isocitrate dehydrogenase |
| BlIDH | isocitrate dehydrogenase from Bifidobacterium longum |
| EcIDH | NADP-IDH from Escherichia coli |
| HcIDH | human cytosolic NADP-IDH |
| AtIDH | NAD-IDH from Acidithiobacillus thiooxidans |
| OtIDH | NAD-IDH from Ostreococcus tauri |
| Km | Michaelis constant |
| kcat | catalytic rate constant |
References
- Sivaraman, J.; Li, Y.; Banks, J.; Cane, D.E.; Matte, A.; Cygler, M. Crystal structure of Escherichia coli PdxA, an enzyme involved in the pyridoxal phosphate biosynthesis pathway. J. Biol. Chem. 2003, 278, 43682–43690. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, J.; Kobashi, N.; Nishiyama, M.; Yamane, H. Characterization of homoisocitrate dehydrogenase involved in lysine biosynthesis of an extremely thermophilic bacterium, Thermus thermophilus HB27, and evolutionary implication of β-decarboxylating dehydrogenase. J. Biol. Chem. 2003, 278, 1864–1871. [Google Scholar] [CrossRef] [PubMed]
- Tipton, P.A.; Beecher, B.S. Tartrate dehydrogenase, a new member of the family of metal-dependent decarboxylating R-hydroxyacid dehydrogenases. Arch. Biochem. Biophys. 1994, 313, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Dean, A.M.; Koshland, D.E., Jr. Kinetic mechanism of Escherichia coli isocitrate dehydrogenase. Biochemistry 1993, 32, 9302–9309. [Google Scholar] [CrossRef] [PubMed]
- Fernie, A.R.; Carrari, F.; Sweetlove, L.J. Respiratory metabolism: Glycolysis, the TCA cycle and mitochondrial electron transport. Curr. Opin. Plant Biol. 2004, 7, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Spaans, S.K.; Weusthuis, R.A.; van der Oost, J.; Kengen, S.W. NADPH-generating systems in bacteria and archaea. Front. Microbiol. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Koh, H.J.; Park, D.C.; Song, B.J.; Huh, T.L.; Park, J.W. Cytosolic NADP+-dependent isocitrate dehydrogenase status modulates oxidative damage to cells. Free Radic. Biol. Med. 2002, 32, 1185–1196. [Google Scholar] [CrossRef]
- Jo, S.H.; Son, M.K.; Koh, H.J.; Lee, S.M.; Song, I.H.; Kim, Y.O.; Lee, Y.S.; Jeong, K.S.; Kim, W.B.; Park, J.W.; et al. Control of mitochondrial redox balance and cellular defense against oxidative damage by mitochondrial NADP+-dependent isocitrate dehydrogenase. J. Biol. Chem. 2001, 276, 16168–16176. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.L.; Holmen, S.L.; Colman, H. IDH1 and IDH2 mutations in gliomas. Curr. Neurol. Neurosci. Rep. 2013, 13, 345. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Liu, Y.; Lu, C.; Cross, J.R.; Morris, J.P.; Shroff, A.S.; Ward, P.S.; Bradner, J.E.; Thompson, C.; Lowe, S.W. Cancer-associated IDH2 mutants drive an acute myeloid leukemia that is susceptible to Brd4 inhibition. Genes Dev. 2013, 27, 1974–1985. [Google Scholar] [CrossRef] [PubMed]
- Dang, L.; White, D.W.; Gross, S.; Bennett, B.D.; Bittinger, M.A.; Driggers, E.M.; Fantin, V.R.; Jang, H.G.; Jin, S.; Keenan, M.C.; et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature 2009, 462, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Lv, C.; Zhu, G. Novel type II and monomeric NAD+ specific isocitrate dehydrogenases: Phylogenetic affinity, enzymatic characterization, and evolutionary implication. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.G.; Song, P.; Cao, Z.Y.; Wang, P.; Zhu, G.P. A unique homodimeric NAD+-linked isocitrate dehydrogenase from the smallest autotrophic eukaryote Ostreococcus tauri. FASEB J. 2015, 29, 2462–2472. [Google Scholar] [CrossRef]
- Peng, Y.; Zhong, C.; Huang, W.; Ding, J. Structural studies of Saccharomyces cerevesiae mitochondrial NADP-dependent isocitrate dehydrogenase in different enzymatic states reveal substantial conformational changes during the catalytic reaction. Protein Sci. 2008, 17, 1542–1554. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhao, J.; Xu, Z.; Peng, B.; Huang, Q.; Arnold, E.; Ding, J. Structures of human cytosolic NADP-dependent isocitrate dehydrogenase reveal a novel self-regulatory mechanism of activity. J. Biol. Chem. 2004, 279, 33946–33957. [Google Scholar] [CrossRef] [PubMed]
- Ceccarelli, C.; Grodsky, N.B.; Ariyaratne, N.; Colman, R.F.; Bahnson, B.J. Crystal structure of porcine mitochondrial NADP+-dependent isocitrate dehydrogenase complexed with Mn2+ and isocitrate. Insights into the enzyme mechanism. J. Biol. Chem. 2002, 277, 43454–43462. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Golding, G.B.; Dean, A.M. The selective cause of an ancient adaptation. Science 2005, 307, 1279–1282. [Google Scholar] [CrossRef] [PubMed]
- Srutkova, D.; Spanova, A.; Spano, M.; Drab, V.; Schwarzer, M.; Kozakova, H.; Rittich, B. Efficiency of PCR-based methods in discriminating Bifidobacterium longum ssp. longum and Bifidobacterium longum ssp. infantis strains of human origin. J. Microbiol. Methods 2011, 87, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Zhu, L.; Liu, X.; Li, T.; Zhang, Y.; Ying, T.; Wang, B.; Wang, J.; Dong, H.; Feng, E.; et al. A proteome reference map and proteomic analysis of Bifidobacterium longum NCC2705. Mol. Cell. Proteom. 2006, 5, 1105–1118. [Google Scholar] [CrossRef] [PubMed]
- Schell, M.A.; Karmirantzou, M.; Snel, B.; Vilanova, D.; Berger, B.; Pessi, G.; Zwahlen, M.C.; Desiere, F.; Bork, P.; Delley, M.; et al. The genome sequence of Bifidobacterium longum reflects its adaptation to the human gastrointestinal tract. Proc. Natl. Acad. Sci. USA 2002, 99, 14422–14427. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.Y.; Chang, F.J. Antioxidative effect of intestinal bacteria Bifidobacterium longum ATCC 15708 and Lactobacillus acidophilus ATCC 4356. Dig. Dis. Sci. 2000, 45, 1617–1622. [Google Scholar] [CrossRef] [PubMed]
- Iwabuchi, N.; Xiao, J.Z.; Yaeshima, T.; Iwatsuki, K. Oral administration of Bifidobacterium longum ameliorates influenza virus infection in mice. Biol. Pharm. Bull. 2011, 34, 1352–1355. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.Z.; Kondo, S.; Yanagisawa, N.; Miyaji, K.; Enomoto, K.; Sakoda, T.; Iwatsuki, K.; Enomoto, T. Clinical efficacy of probiotic Bifidobacterium longum for the treatment of symptoms of Japanese cedar pollen allergy in subjects evaluated in an environmental exposure unit. Allergol. Int. 2007, 56, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Sivan, A.; Corrales, L.; Hubert, N.; Williams, J.B.; Aquino-Michaels, K.; Earley, Z.M.; Benyamin, F.W.; Lei, Y.M.; Jabri, B.; Alegre, M.L.; et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 2015, 350, 1084–1089. [Google Scholar] [CrossRef] [PubMed]
- Yazawa, K.; Fujimori, M.; Amano, J.; Kano, Y.; Taniguchi, S. Bifidobacterium longum as a delivery system for cancer gene therapy: Selective localization and growth in hypoxic tumors. Cancer Gene Ther. 2000, 7, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Imada, K.; Tamura, T.; Takenaka, R.; Kobayashi, I.; Namba, K.; Inagaki, K. Structure and quantum chemical analysis of NAD+-dependent isocitrate dehydrogenase: Hydride transfer and co-factor specificity. Proteins 2008, 70, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, P.; Zhu, G.; Wang, B.; Zhu, G. Enzymatic characterization of a type II isocitrate dehydrogenase from pathogenic Leptospira interrogans serovar Lai strain 56601. Appl. Biochem. Biotechnol. 2014, 172, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Yang, H. A highly specific monomeric isocitrate dehydrogenase from Corynebacterium glutamicum. Arch. Biochem. Biophys. 2000, 383, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Kanao, T.; Kawamura, M.; Fukui, T.; Atomi, H.; Imanaka, T. Characterization of isocitrate dehydrogenase from the green sulfur bacterium Chlorobium limicola. Eur. J. Biochem. 2002, 269, 1926–1931. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.C.; Tian, C.Q.; Cheng, H.M.; Xu, L.; Wang, P.; Zhu, G.P. A novel type II NAD+-specific isocitrate dehydrogenase from the marine bacterium Congregibacter litoralis KT71. PLoS ONE 2015, 10, e0125229. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Jin, M.; Zhu, G. Biochemical and molecular characterization of NAD+-dependent isocitrate dehydrogenase from the ethanologenic bacterium Zymomonas mobilis. FEMS Microbiol. Lett. 2012, 327, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Dean, A.M.; Golding, G.B. Protein engineering reveals ancient adaptive replacements in isocitrate dehydrogenase. Proc. Natl. Acad. Sci. USA 1997, 94, 3104–3109. [Google Scholar] [CrossRef] [PubMed]
- Jennings, G.T.; Minard, K.I.; McAlister-Henn, L. Expression and mutagenesis of mammalian cytosolic NADP+-specific isocitrate dehydrogenase. Biochemistry 1997, 36, 13743–13747. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, P.; Ge, Y.; Wang, W.; Abbas, A.; Zhu, G. NADP+-specific isocitrate dehydrogenase from oleaginous yeast Yarrowia lipolytica CLIB122: Biochemical characterization and coenzyme sites evaluation. Appl. Biochem. Biotechnol. 2013, 171, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Liu, J.; Shen, G. Cloning, expression, and enzymatic characterization of isocitrate dehydrogenase from Helicobacter pylori. Protein J. 2009, 28, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Yasutake, Y.; Tanaka, I.; Takada, Y. Elucidation of stability determinants of cold-adapted monomeric isocitrate dehydrogenase from a psychrophilic bacterium, Colwellia maris, by construction of chimeric enzymes. Microbiology 2005, 151, 1083–1094. [Google Scholar] [CrossRef] [PubMed]
- Hurley, J.H.; Chen, R.; Dean, A.M. Determinants of cofactor specificity in isocitrate dehydrogenase: Structure of an engineered NADP+→NAD+ specificity-reversal mutant. Biochemistry 1996, 35, 5670–5678. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Arnedo, A.; Camacho, M.; Llorca, F.; Bonete, M.J. Complete reversal of coenzyme specificity of isocitrate dehydrogenase from Haloferax volcanii. Protein J. 2005, 24, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Jin, M.; Su, R.; Song, P.; Wang, M.; Zhu, G. Enzymatic characterization of isocitrate dehydrogenase from an emerging zoonotic pathogen Streptococcus suis. Biochimie 2011, 93, 1470–1475. [Google Scholar] [CrossRef] [PubMed]
- Steen, I.H.; Lien, T.; Madsen, M.S.; Birkeland, N.K. Identification of cofactor discrimination sites in NAD-isocitrate dehydrogenase from Pyrococcus furiosus. Arch. Microbiol. 2002, 178, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Bramucci, E.; Paiardini, A.; Bossa, F.; Pascarella, S. PyMod: Sequence similarity searches, multiple sequence-structure alignments, and homology modeling within PyMOL. BMC Bioinform. 2012, 13. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.N.; Hunt, H.D.; Horton, R.M.; Pullen, J.K.; Pease, L.R. Site-directed mutagenesis by overlap extension using the polymerase chain-reaction. Gene 1989, 77, 51–59. [Google Scholar] [CrossRef]
- Pace, C.N.; Vajdos, F.; Fee, L.; Grimsley, G.; Gray, T. How to measure and predict the molar absorption coefficient of a protein. Protein Sci. 1995, 4, 2411–2423. [Google Scholar] [CrossRef] [PubMed]
- Cvitkovitch, D.G.; Gutierrez, J.A.; Bleiweis, A.S. Role of the citrate pathway in glutamate biosynthesis by Streptococcus mutans. J. Bacteriol. 1997, 179, 650–655. [Google Scholar] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).