Addition of Adipose-Derived Stem Cells to Mesenchymal Stem Cell Sheets Improves Bone Formation at an Ectopic Site
Abstract
:1. Introduction
2. Results
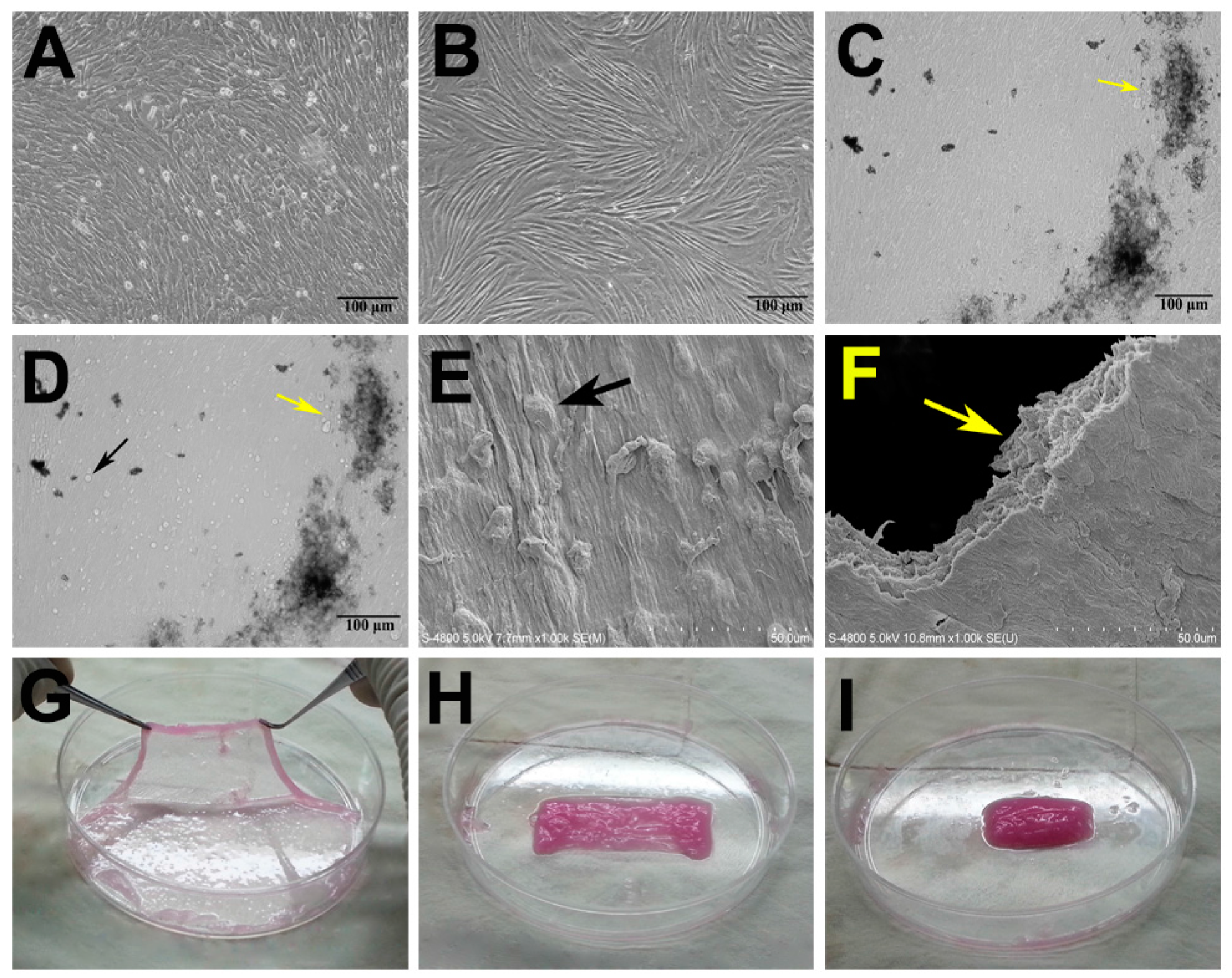
2.1. Characterization of Adipose-Derived Stem Cells (ADSCs) and Mesenchymal Stem Cell (MSC) Sheets
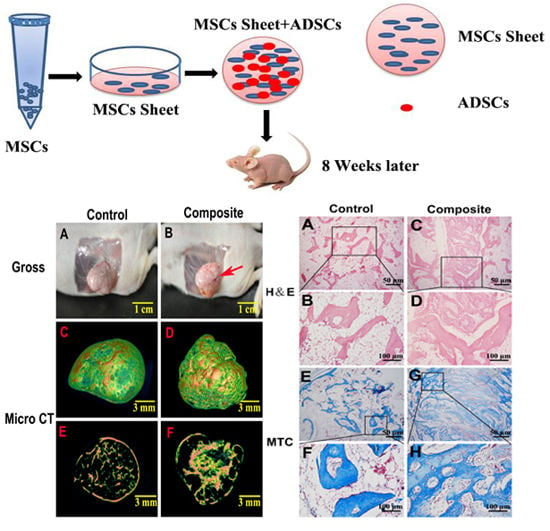
2.2. Gross Morphology and Micro Computed Tomography (CT) Analysis of Bone Formed in Vivo


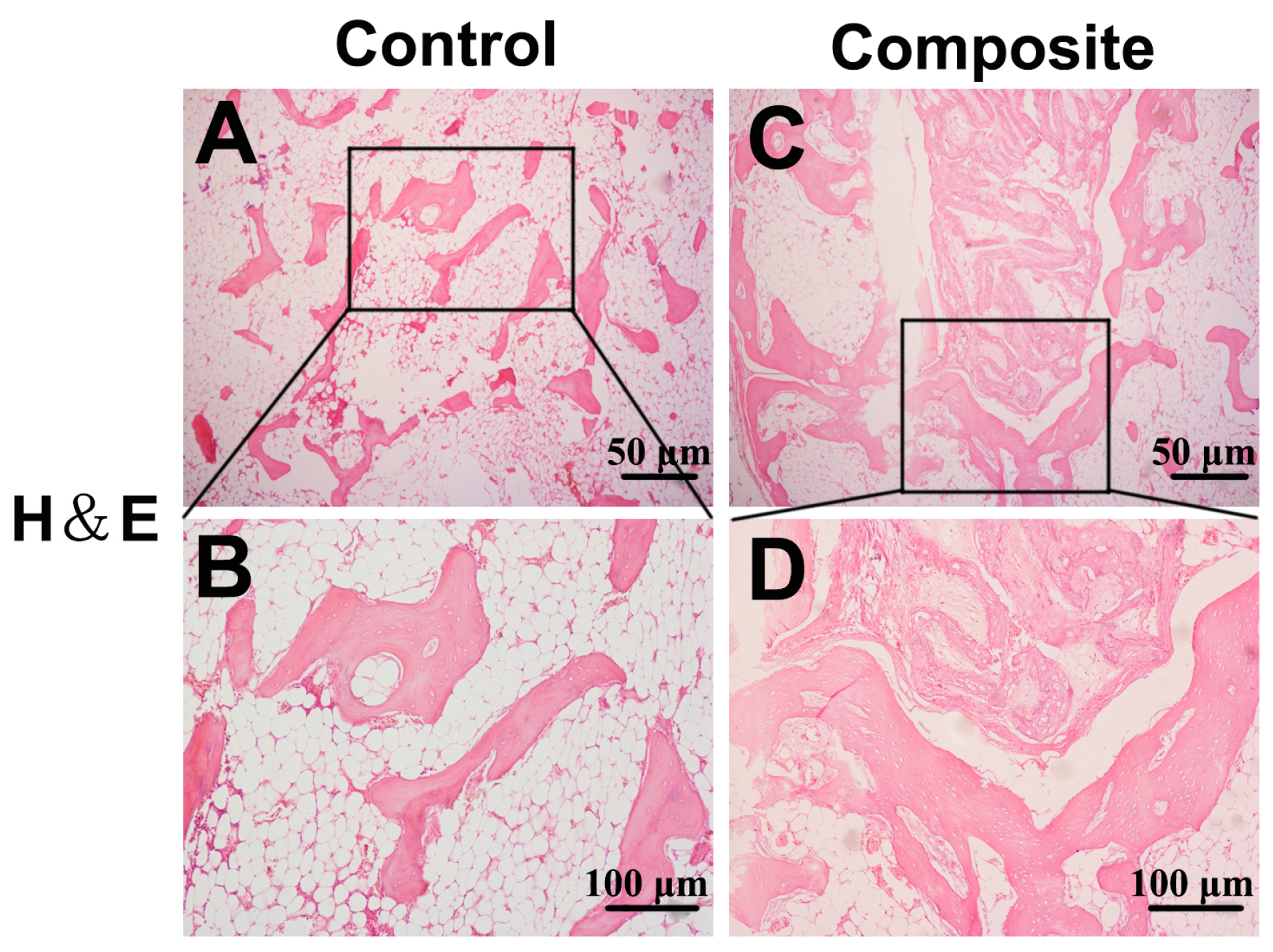
2.3. Histological Examination of Newly Formed Bone Tissue


3. Discussion
4. Experimental Section
4.1. Ethical Approval
4.2. MSC Isolation, Culture, and Sheet Preparation
4.3. ADSC Isolation and Culture
4.4. Preparation of MSC Sheet–ADSC Constructs
4.5. Surgical Protocol
4.6. Micro Computed Tomography (CT) Analysis
4.7. Histological Analysis of Implanted Constructs
4.8. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of interest
References
- O’Keefe, R.J.; Mao, J. Bone tissue engineering and regeneration: From discovery to the clinic—An overview. Tissue Eng. Part B Rev. 2011, 17, 389–392. [Google Scholar] [CrossRef] [PubMed]
- Saran, U.; Gemini Piperni, S.; Chatterjee, S. Role of angiogenesis in bone repair. Arch. Biochem. Biophys. 2014, 561, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Unger, R.E.; Dohle, E.; Kirkpatrick, C.J. Improving vascularization of engineered bone through the release of pro-angiogenic factors by co-culture systems. Adv. Drug Deliv. Rev. 2015, 94, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Gao, Z.; Geng, W.; Yan, X.; Chen, F.; Liu, Y. Engineering vascularized bone graft with osteogenic and angiogenic lineage differentiated bone marrow mesenchymal stem cells. Artif. Organs 2012, 36, 1036–1046. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wang, X.; Cao, J.; Ju, Z.; Ma, D.; Liu, Y.; Zhang, J. Coculture of peripheral blood CD34+ cell and mesenchymal stem cell sheets increase the formation of bone in calvarial critical-size defects in rabbits. Br. J. Oral Maxillofac. Surg. 2014, 52, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Liu, B.; Liu, G.; Zhang, W.; Cen, L.; Sun, J.; Yin, S.; Liu, W.; Cao, Y. Repair of cranial bone defects with adipose derived stem cells and coral scaffold in a canine model. Biomaterials 2007, 28, 5477–5486. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.N.; Li, L.; Leng, P.; Wang, Y.Z.; Lv, C.Y. Uninduced adipose-derived stem cells repair the defect of full-thickness hyaline cartilage. Chin. J. Traumatol. 2009, 12, 92–97. [Google Scholar] [PubMed]
- Kim, W.S.; Park, B.S.; Sung, J.H.; Yang, J.M.; Park, S.B.; Kwak, S.J.; Park, J.S. Wound healing effect of adipose-derived stem cells: A critical role of secretory factors on human dermal fibroblasts. J. Dermatol. Sci. 2007, 48, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Pieri, F.; Lucarelli, E.; Corinaldesi, G.; Aldini, N.N.; Fini, M.; Parrilli, A.; Dozza, B.; Donati, D.; Marchetti, C. Dose-dependent effect of adipose-derived adult stem cells on vertical bone regeneration in rabbit calvarium. Biomaterials 2010, 31, 3527–3535. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, S.T.; Asgari, A.; Lokmic, Z.; Sinclair, R.; Dusting, G.J.; Lim, S.Y.; Dilley, R.J. Comparative analysis of paracrine factor expression in human adult mesenchymal stem cells derived from bone marrow, adipose, and dermal tissue. Stem Cells Dev. 2012, 21, 2189–2203. [Google Scholar] [CrossRef] [PubMed]
- Nakao, N.; Nakayama, T.; Yahata, T.; Muguruma, Y.; Saito, S.; Miyata, Y.; Yamamoto, K.; Naoe, T. Adipose tissue-derived mesenchymal stem cells facilitate hematopoiesis in vitro and in vivo: Advantages over bone marrow-derived mesenchymal stem cells. Am. J. Pathol. 2010, 177, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Ertas, G.; Ural, E.; Ural, D.; Aksoy, A.; Kozdag, G.; Gacar, G.; Karaoz, E. Comparative analysis of apoptotic resistance of mesenchymal stem cells isolated from human bone marrow and adipose tissue. Sci. World J. 2012, 2012, 105698. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, H.; Cho, H.; Bae, Y.; Suh, K.; Jung, J. Direct comparison of human mesenchymal stem cells derived from adipose tissues and bone marrow in mediating neovascularization in response to vascular ischemia. Cell. Physiol. Biochem. 2007, 20, 867–876. [Google Scholar] [CrossRef] [PubMed]
- Demirbag, B.; Huri, P.Y.; Kose, G.T.; Buyuksungur, A.; Hasirci, V. Advanced cell therapies with and without scaffolds. Biotechnol. J. 2011, 6, 1437–1453. [Google Scholar] [CrossRef] [PubMed]
- Tsumanuma, Y.; Iwata, T.; Washio, K.; Yoshida, T.; Yamada, A.; Takagi, R.; Ohno, T.; Lin, K.; Yamato, M.; Ishikawa, I.; et al. Comparison of different tissue-derived stem cell sheets for periodontal regeneration in a canine 1-wall defect model. Biomaterials 2011, 32, 5819–5825. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Zhong, C.; Yao, H.; Liu, Y.; Chen, F.; Li, J.; Zhao, J.; Mao, T.; Ren, L. Engineering injectable bone using bone marrow stromal cell aggregates. Stem Cells Dev. 2011, 20, 989–999. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.; Akahane, M.; Shigematsu, H.; Tadokoro, M.; Morita, Y.; Ohgushi, H.; Dohi, Y.; Imamura, T.; Tanaka, Y. Cell sheet transplantation of cultured mesenchymal stem cells enhances bone formation in a rat nonunion model. Bone 2010, 46, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yamato, M.; Nishida, K.; Ohki, T.; Kanzaki, M.; Sekine, H.; Shimizu, T.; Okano, T. Cell delivery in regenerative medicine: The cell sheet engineering approach. J. Control. Release 2006, 116, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Y.C.; Lee, W.Y.; Yu, C.L.; Hwang, S.M.; Chung, M.F.; Hsu, L.W.; Chang, Y.; Lin, W.W.; Tsai, M.S.; Wei, H.J.; et al. Cardiac repair with injectable cell sheet fragments of human amniotic fluid stem cells in an immune-suppressed rat model. Biomaterials 2010, 31, 6444–6453. [Google Scholar] [CrossRef] [PubMed]
- Labbe, B.; Marceau-Fortier, G.; Fradette, J. Cell sheet technology for tissue engineering: The self-assembly approach using adipose-derived stromal cells. Methods Mol. Biol. 2011, 702, 429–441. [Google Scholar] [PubMed]
- Akahane, M.; Nakamura, A.; Ohgushi, H.; Shigematsu, H.; Dohi, Y.; Takakura, Y. Osteogenic matrix sheet-cell transplantation using osteoblastic cell sheet resulted in bone formation without scaffold at an ectopic site. J. Tissue Eng. Regen. Med. 2008, 2, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Weng, Y.; Lu, S.; Zong, C.; Qiu, J.; Liu, Y.; Liu, B. Osteoblastic mesenchymal stem cell sheet combined with choukroun platelet-rich fibrin induces bone formation at an ectopic site. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 103, 1204–1216. [Google Scholar] [CrossRef] [PubMed]
- Sterodimas, A.; de Faria, J.; Nicaretta, B.; Pitanguy, I. Tissue engineering with adipose-derived stem cells (ADSCs): Current and future applications. J. Plast. Reconstr. Aesthet. Surg. 2010, 63, 1886–1892. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Shim, M.S.; Shim, S.H.; Yang, H.N.; Jeon, S.Y.; Woo, D.G.; Lee, D.R.; Yoon, T.K.; Park, K.H. Chondrogenic potential of stem cells derived from amniotic fluid, adipose tissue, or bone marrow encapsulated in fibrin gels containing TGF-β3. Biomaterials 2011, 32, 8139–8149. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.J.; Cho, K.S.; Park, H.Y.; Roh, H.J. Adipose tissue-derived stem cells for cell therapy of airway allergic diseases in mouse. Acta Histochem. 2011, 113, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.M.; Harn, H.J.; Lin, H.P.; Chiu, S.C.; Lin, P.C.; Wang, H.I.; Ho, L.I.; Chuu, C.P.; Chiou, T.W.; Hsieh, A.C.; et al. The use of ADSCs as a treatment for chronic stroke. Cell Transplant. 2014, 23, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.G.; Tang, X.F. New advances in the mesenchymal stem cells therapy against skin flaps necrosis. World J. Stem Cells 2014, 6, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Wang, Y.; Zhao, C.; Guo, S.; Liu, S.; Jia, W.; Tuan, R.S.; Zhang, C. Comparative evaluation of MSCs from bone marrow and adipose tissue seeded in PRP-derived scaffold for cartilage regeneration. Biomaterials 2012, 33, 7008–7018. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Han, C.; Song, Y.; Yan, X.; Li, D.; Chai, Z.; Feng, Z.; Dong, Y.; Li, L.; Xie, X.; et al. The performance of bone marrow mesenchymal stem cell-implant complexes prepared by cell sheet engineering techniques. Biomaterials 2010, 31, 3212–3221. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Li, L.; Zhao, J.-H.; Wang, Z.-F.; Tan, X.-Y.; Xu, H.-Y.; Au, R.; Liu, Y.-P. Platelet-rich fibrin and adipose-derived stem cells improve the efficacy of fat transplantation and soft tissue repair. J. Biomater. Tissue Eng. 2015, 5, 275–282. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, F.; Ho, S.T.; Woodruff, M.A.; Lim, T.M.; Hutmacher, D.W. Combined marrow stromal cell-sheet techniques and high-strength biodegradable composite scaffolds for engineered functional bone grafts. Biomaterials 2007, 28, 814–824. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Li, Z.; Dai, T.; Zong, C.; Liu, Y.; Liu, B. Addition of Adipose-Derived Stem Cells to Mesenchymal Stem Cell Sheets Improves Bone Formation at an Ectopic Site. Int. J. Mol. Sci. 2016, 17, 70. https://doi.org/10.3390/ijms17020070
Wang Z, Li Z, Dai T, Zong C, Liu Y, Liu B. Addition of Adipose-Derived Stem Cells to Mesenchymal Stem Cell Sheets Improves Bone Formation at an Ectopic Site. International Journal of Molecular Sciences. 2016; 17(2):70. https://doi.org/10.3390/ijms17020070
Chicago/Turabian StyleWang, Zhifa, Zhijin Li, Taiqiang Dai, Chunlin Zong, Yanpu Liu, and Bin Liu. 2016. "Addition of Adipose-Derived Stem Cells to Mesenchymal Stem Cell Sheets Improves Bone Formation at an Ectopic Site" International Journal of Molecular Sciences 17, no. 2: 70. https://doi.org/10.3390/ijms17020070