Role of miR-222-3p in c-Src-Mediated Regulation of Osteoclastogenesis
Abstract
:1. Introduction
2. Results
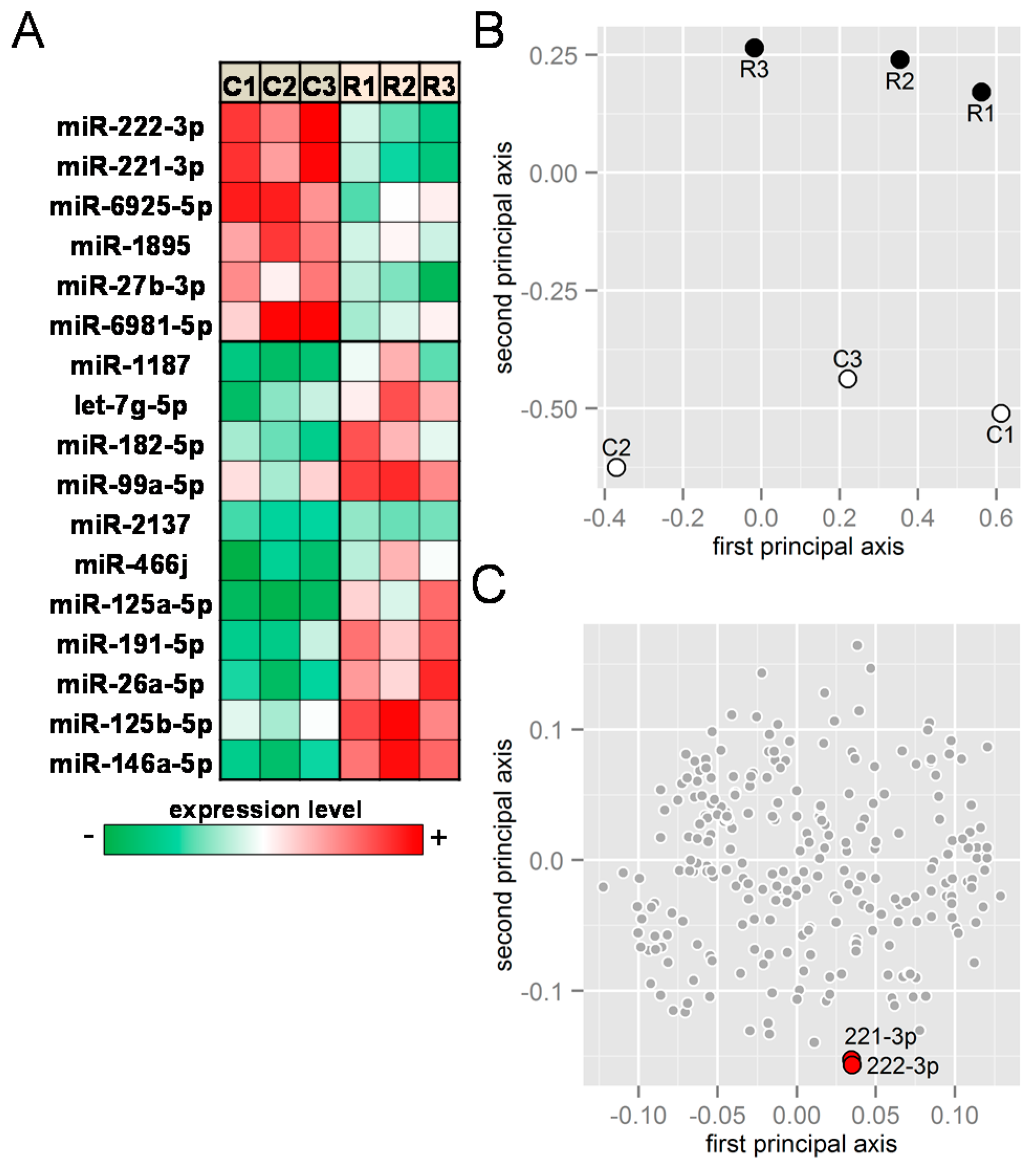
2.1. Receptor Activator of Nuclear Factor κ-B Ligand (RANKL) Responsive miRNAs
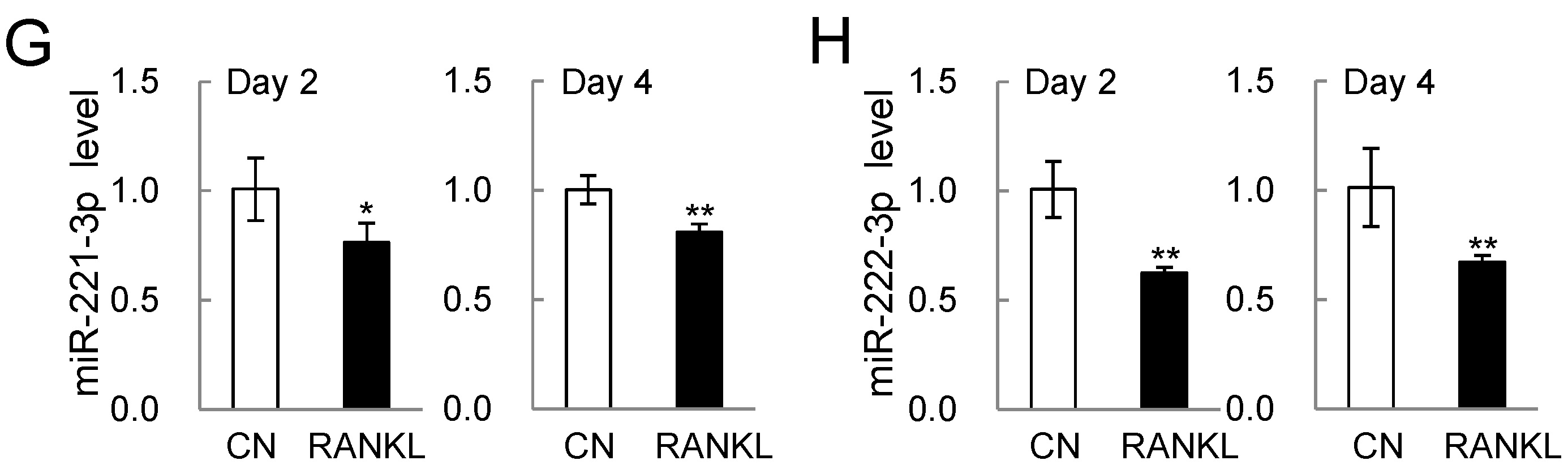
2.2. PCR-Based mRNA and miRNA Expression Levels
2.3. Effects of Inhibitor and Mimic miR221-3p and miR222-3p

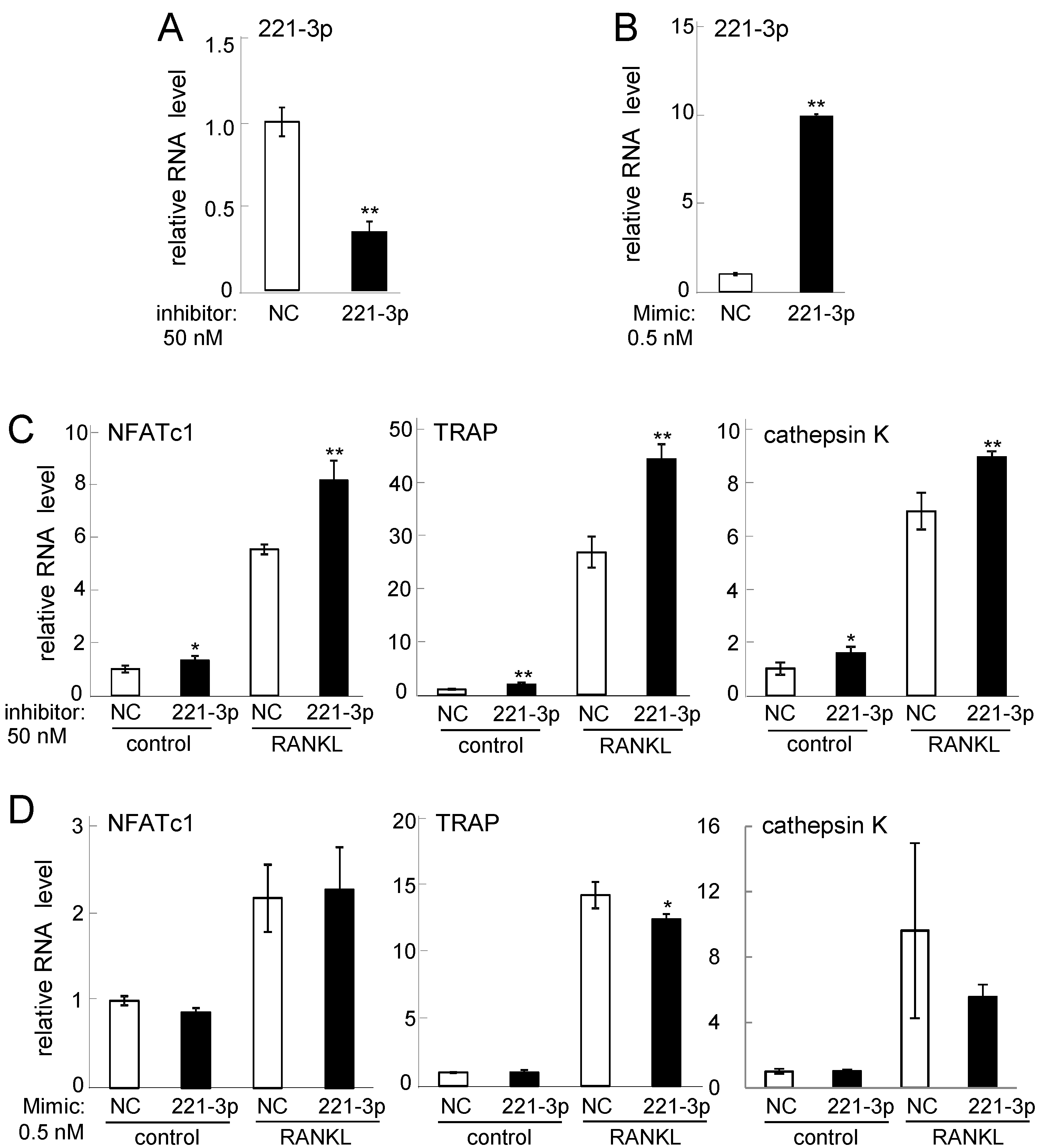

2.4. Western Blot Analysis of Nuclear Factor of Activated T-Cells, Cytoplasmic 1 (NFATc1), Tartrate-Resistant Acid Phosphatase (TRAP), and Cathepsin K
2.5. RANKL Responsive Genes and the Prediction of miRNA Responsive Genes


2.6. c-Src Expression and Activity in Response to the Inhibitor of miR-222-3p

2.7. Effects of Silencing c-Src in RANKL-Driven Osteoclastogenesis


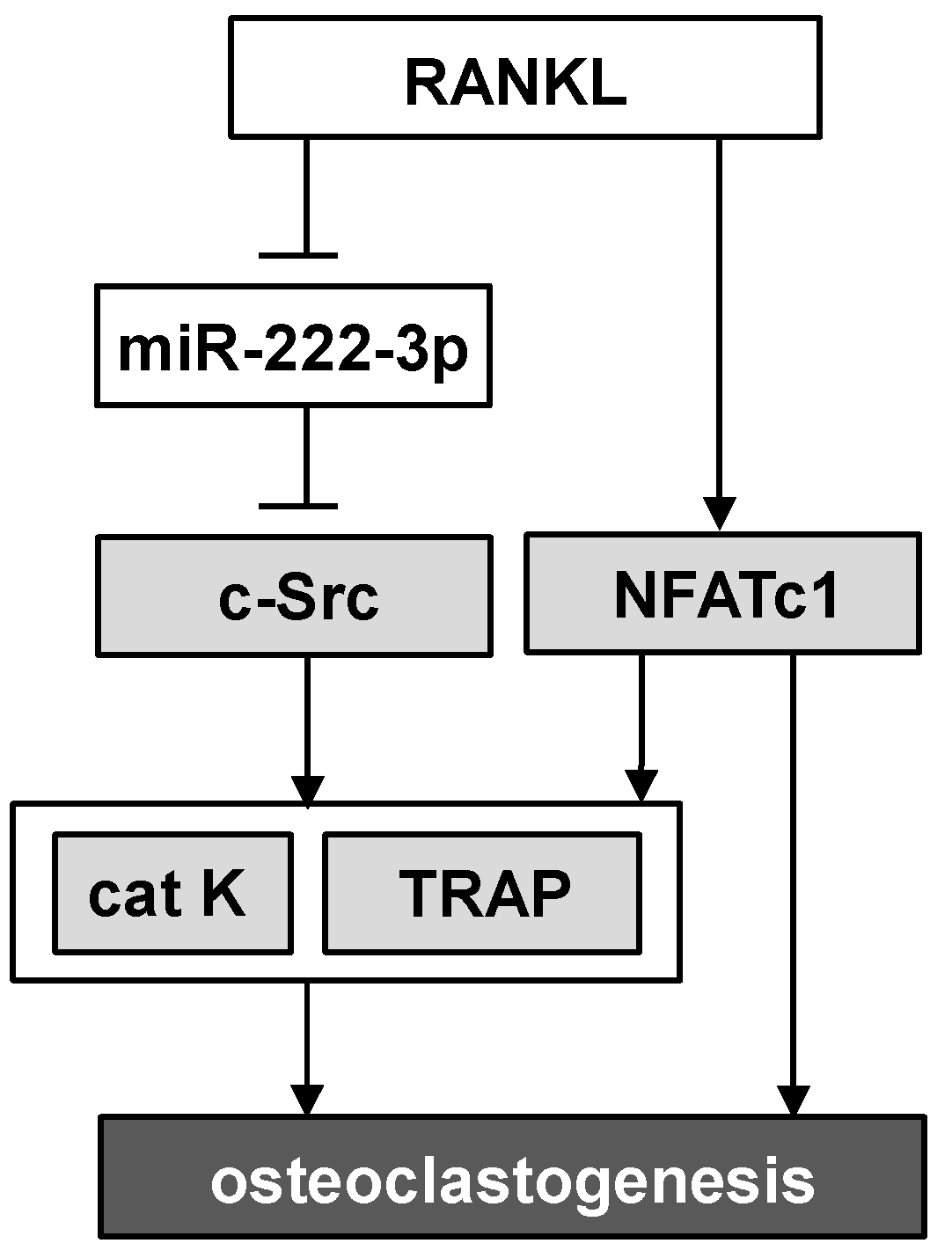
3. Discussion
4. Experimental Section
4.1. Cell Culture
4.2. Microarray Experiments for Genome-Wide miRNAs and mRNAs
4.3. Principal Component Analysis
4.4. In Silico Prediction of miRNA Target Genes
4.5. Transfection of the Inhibitor and Mimic of miR-221-3p, miR-222-3p and c-Src siRNA
4.6. Quantitative Real-Time PCR
| miRNA | Targeted miRNA Sequences |
|---|---|
| 27b-3p | 5′-UUCACAGUGGCUAAGUUCUGC-3′ |
| 125b-5p | 5′-UCCCUGAGACCCUAACUUGUGA-3′ |
| 146a-5p | 5′-UGAGAACUGAAUUCCAUGGGUU-3′ |
| 182-5p | 5′-UUUGGCAAUGGUAGAACUCACACCG-3′ |
| 221-3p | 5′-AGCUACAUUGUCUGCUGGGUUUC-3′ |
| 222-3p | 5′-AGCUACAUCUGGCUACUGGGU-3′ |
| SnoRNA202 | 5′-GCTGTACTGACTTGATGAAAGTACTTTTGAACCCTTTTCCATCTGATG-3′ |
| Gene | Forward Primer | Backward Primer |
|---|---|---|
| c-Src | 5′-TCCTTTGGGATTCTGCTGAC-3′ | 5′-TGTGGCTCAGTGGACGTAAA-3′ |
| cat K | 5′-CAGCTTCCCCAAGATGTGAT-3′ | 5′-AGCACCAACGAGAGGAGAAA-3′ |
| Dcstamp | 5′-AAAACCCTTGGGCTGTTCTT-3′ | 5′-AATCATGGACGACTCCTTGG-3′ |
| NFATc1 | 5′-GGTGCTGTCTGGCCATAACT-3′ | 5′-GCGGAAAGGTGGTATCTCAA-3′ |
| TRAP | 5′-TCCTGGCTCAAAAAGCAGTT-3′ | 5′-ACATAGCCCACACCGTTCTC-3′ |
| GAPDH | 5′-TGCACCACCAACTGCTTAG-3′ | 5′-GGATGCAGGGATGATGTTC-3′ |
4.7. Western Immunoblotting
4.8. Live Cell Imaging for c-Src Activity Using Fluorescence Resonance Energy Transfer (FRET)
4.9. TRAP Staining and Bond Resorption Assay
4.10. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Calin, G.A.; Croce, C.M. MicroRNA signatures in human cancers. Nat. Rev. Cancer 2006, 6, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Li, P.; Chen, X.; Zong, L.; Jiang, Z.; Nan, L.; Lei, J.; Duan, W.; Zhang, D.; Li, X.; et al. miR-221/222 induces pancreatic cancer progression through the regulation of matrix metalloproteinases. Oncotarget 2015, 6, 14153–14164. [Google Scholar] [CrossRef] [PubMed]
- Corsten, M.F.; Dennert, R.; Jochems, S.; Kuznetsova, T.; Devaux, Y.; Hofstra, L.; Wagner, D.R.; Staessen, J.A.; Heymans, S.; Schroen, B.; et al. Circulating microRNA-208b and microRNA-499 reflect myocardial damage in cardiovascular disease. Circ. Cardiovasc. Genet. 2010, 3, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Bak, M.; Silahtaroglu, A.; Møller, M.; Christensen, M.; Rath, M.F.; Skryabin, B.; Tommerup, N.; Kauppinen, S. MicroRNA expression in the adult mouse central nervous system. RNA 2008, 14, 432–444. [Google Scholar] [CrossRef] [PubMed]
- Rottiers, V.; Näär, A.M. MicroRNAs in metabolism and metabolic disorders. Nat. Rev. Mol. Cell Biol. 2012, 13, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Hatfield, S.D.; Shcherbata, H.R.; Fischer, K.A.; Nakahara, K.; Carthew, R.W.; Ruohola-Baker, H. Stem cell division is regulated by the microRNA pathway. Nature 2005, 435, 974–978. [Google Scholar] [CrossRef] [PubMed]
- Takayanagi, H.; Kim, S.; Koga, T.; Nishina, H.; Isshiki, M.; Yoshida, H.; Saiura, A.; Isobe, M.; Yokochi, T.; Inoue, J.; et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev. Cell 2002, 3, 889–901. [Google Scholar] [CrossRef]
- Franceschetti, T.; Dole, N.S.; Kessler, C.B.; Lee, S.K.; Delany, A.M. Pathway analysis of microRNA expression profile during murine osteoclastogenesis. PLoS ONE 2014, 9, e107262. [Google Scholar] [CrossRef] [PubMed]
- Sugatani, T.; Hruska, K.A. MicroRNA-223 is a key factor in osteoclast differentiation. J. Cell. Biochem. 2007, 101, 996–999. [Google Scholar] [CrossRef] [PubMed]
- Ell, B.; Mercatali, L.; Ibrahim, T.; Campbell, N.; Schwarzenbach, H.; Pantel, K.; Amadori, D.; Kang, Y. Tumor-induced osteoclast miRNA changes as regulators and biomarkers of osteolytic bone metastasis. Cancer Cell 2013, 24, 542–556. [Google Scholar] [CrossRef] [PubMed]
- Krzeszinski, J.Y.; Wei, W.; Huynh, H.; Jin, Z.; Wang, X.; Chang, T.C.; Xie, X.J.; He, L.; Mangala, L.S.; Lopez-Berestein, G.; et al. miR-34a blocks osteoporosis and bone metastasis by inhibiting osteoclastogenesis and Tgif2. Nature 2014, 512, 431–435. [Google Scholar] [CrossRef] [PubMed]
- M’Baya-Moutoula, E.; Louvet, L.; Metzinger-Le Meuth, V.; Massy, Z.A.; Metzinger, L. High inorganic phosphate concentration inhibits osteoclastogenesis by modulating miR-223. Biochim. Biophys. Acta 2015, 1852, 2202–2212. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.J.; Shen, J. Circular RNA participates in the carcinogenesis and the malignant behavior of cancer. RNA Biol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Dweep, H.; Sticht, C.; Pandey, P.; Gretz, N. miRWalk—Database: Prediction of possible miRNA binding sites by “walking” the genes of three genomes. J. Biomed. Inform. 2011, 44, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Rehmsmeier, M.; Steffen, P.; Hochsmann, M.; Giegerich, R. Fast and effective prediction of microRNA/target duplexes. RNA 2004, 10, 1507–1517. [Google Scholar] [CrossRef] [PubMed]
- Boyce, B.F.; Yoneda, T.; Lowe, C.; Soriano, P.; Mundy, G.R. Requirement of pp60c-src expression for osteoclasts to form ruffled borders and resorb bone in mice. J. Clin. Investig. 1992, 90, 1622–1627. [Google Scholar] [CrossRef] [PubMed]
- Schwartzberg, P.L.; Xing, L.; Hoffmann, O.; Lowell, C.A.; Garrett, L.; Boyce, B.F.; Varmus, H.E. Rescue of osteoclast function by transgenic expression of kinase-deficient Src in src−/− mutant mice. Genes Dev. 1997, 11, 2835–2844. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Sanjay, A.; Neff, L.; Tanaka, S.; Horne, W.C.; Baron, R. Src kinase activity is essential for osteoclast function. J. Biol. Chem. 2004, 279, 17660–17666. [Google Scholar] [CrossRef] [PubMed]
- Yagi, M.; Miyamoto, T.; Sawatani, Y.; Iwamoto, K.; Hosogane, N.; Fujita, N.; Morita, K.; Ninomiya, K.; Suzuki, T.; Miyamoto, K.; et al. DC-STAMP is essential for cell-cell fusion in osteoclasts and foreign body giant cells. J. Exp. Med. 2005, 202, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Peterson, S.M.; Thompson, J.A.; Ufkin, M.L.; Sathyanarayana, P.; Liaw, L.; Congdon, C.B. Common features of microRNA target prediction tools. Front. Genet. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Witkos, T.M.; Koscianska, E.; Krzyzosiak, W.J. Practical aspects of microRNA target prediction. Curr. Mol. Med. 2011, 11, 93–109. [Google Scholar] [CrossRef] [PubMed]
- Ohtsuka, M.; Ling, H.; Doki, Y.; Mori, M.; Calin, G.A. MicroRNA processing and human cancer. J. Clin. Med. 2015, 4, 1651–1667. [Google Scholar] [CrossRef] [PubMed]
- Saiselet, M.; Gacquer, D.; Spinette, A.; Craciun, L.; Decaussin-Petrucci, M.; Andry, G.; Detours, V.; Maenhaut, C. New global analysis of the microRNA transcriptome of primary tumors and lymph node metastases of papillary thyroid cancer. BMC Genom. 2015, 16, 828. [Google Scholar] [CrossRef] [PubMed]
- Kara, M.; Yumrutas, O.; Ozcan, O.; Celik, O.; Bozgeyik, E.; Bozgeyik, I.; Tasdemir, S. Differential expressions of cancer associated genes and their regulatory miRNA in colorectal carcinoma. Gene 2015, 567, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Chen, H.; Wang, T.; Zhang, W.; Ru, G.; Lang, J. Expression profile analysis of microRNAs in prostate cancer by next-generation sequencing. Prostate 2015, 75, 500–516. [Google Scholar] [CrossRef] [PubMed]
- Goto, Y.; Kojima, S.; Nishikawa, R.; Kurozumi, A.; Kato, M.; Enokida, H.; Matsushita, R.; Yamazaki, K.; Ishida, Y.; Nakagawa, M.; et al. MicroRNA expression signature of castration-resistant prostate cancer: The microRNA-221/222 cluster functions as a tumour suppressor and disease progression marker. Br. J. Cancer 2015, 113, 1055–1065. [Google Scholar] [CrossRef] [PubMed]
- Dentelli, P.; Rosso, A.; Orso, F.; Olgasi, C.; Taverna, D.; Brizzi, M.F. microRNA-222 controls neovascularization by regulating signal transducer and activator of transcription 5A expression. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1562–1568. [Google Scholar] [CrossRef] [PubMed]
- Lopes, F.C.; Ferreira, R.; Albuquerque, D.M.; Silveira, A.A.; Costa, R.; Soares, R.; Costa, F.F.; Conran, N. In vitro and in vivo anti-angiogenic effects of hydroxyurea. Microvasc. Res. 2014, 94, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Perry, M.M.; Baker, J.E.; Gibeon, D.S.; Adcock, I.M.; Chung, K.F. Airway smooth muscle hyperproliferation is regulated by microRNA-221 in severe asthma. Am. J. Respir. Cell Mol. Biol. 2014, 50, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Hamamura, K.; Chen, A.; Tanjung, N.; Takigawa, S.; Sudo, A.; Yokota, H. In vitro and in silico analysis of an inhibitory mechanism of osteoclastogenesis by salubrinal and guanabenz. Cell Signal. 2015, 27, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.; Domrachev, M.; Lash, A.E. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002, 30, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Hamamura, K.; Chen, A.; Nishimura, A.; Tanjung, N.; Sudo, A.; Yokota, H. Predicting and validating the pathway of Wnt3a-driven suppression of osteoclastogenesis. Cell Signal. 2014, 26, 2358–2369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maragkakis, M.; Vergoulis, T.; Alexiou, P.; Reczko, M.; Plomaritou, K.; Gousis, M.; Kourtis, K.; Koziris, N.; Dalamagas, T.; Hatzigeorgiou, A.G. DIANA-microT Web server upgrade supports Fly and Worm miRNA target prediction and bibliographic miRNA to disease association. Nucleic Acids Res. 2011, 39, W145–W148. [Google Scholar] [CrossRef] [PubMed]
- Betel, D.; Koppal, A.; Agius, P.; Sander, C.; Leslie, C. Comprehensive modeling of microRNA targets predicts functional non-conserved and non-canonical sites. Genome Biol. 2010, 11, R90. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; EI Naqa, I.M. Prediction of both conserved and nonconserved microRNA targets in animals. Bioinformatics 2008, 24, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Vejnar, C.E.; Blum, M.; Zdobnov, E.M. miRmap web: Comprehensive microRNA target prediction online. Nucleic Acids Res. 2013, 41, W165–W168. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.D.; Chu, C.H.; Tsou, A.P.; Chen, S.J.; Chen, H.C.; Hsu, P.W.; Wong, Y.H.; Chen, Y.H.; Chen, G.H.; Huang, H.D. miRNAMap 2.0: Genomic maps of microRNAs in metazoan genomes. Nucleic Acids Res. 2008, 36, D165–D169. [Google Scholar] [CrossRef] [PubMed]
- Kertesz, M.; Iovino, N.; Unnerstall, U.; Gaul, U.; Segal, E. The role of site accessibility in microRNA target recognition. Nat. Genet. 2007, 39, 1278–1284. [Google Scholar] [CrossRef] [PubMed]
- Loher, P.; Rigoutsos, I. Interactive exploration of RNA22 microRNA target predictions. Bioinformatics 2012, 28, 3322–3323. [Google Scholar] [CrossRef] [PubMed]
- Friedman, R.C.; Farh, K.K.; Burge, C.B.; Bartel, D.P. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009, 19, 92–105. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takigawa, S.; Chen, A.; Wan, Q.; Na, S.; Sudo, A.; Yokota, H.; Hamamura, K. Role of miR-222-3p in c-Src-Mediated Regulation of Osteoclastogenesis. Int. J. Mol. Sci. 2016, 17, 240. https://doi.org/10.3390/ijms17020240
Takigawa S, Chen A, Wan Q, Na S, Sudo A, Yokota H, Hamamura K. Role of miR-222-3p in c-Src-Mediated Regulation of Osteoclastogenesis. International Journal of Molecular Sciences. 2016; 17(2):240. https://doi.org/10.3390/ijms17020240
Chicago/Turabian StyleTakigawa, Shinya, Andy Chen, Qiaoqiao Wan, Sungsoo Na, Akihiro Sudo, Hiroki Yokota, and Kazunori Hamamura. 2016. "Role of miR-222-3p in c-Src-Mediated Regulation of Osteoclastogenesis" International Journal of Molecular Sciences 17, no. 2: 240. https://doi.org/10.3390/ijms17020240





