Identification of Predictive Markers for Response to Neoadjuvant Chemoradiation in Rectal Carcinomas by Proteomic Isotope Coded Protein Label (ICPL) Analysis
Abstract
:1. Introduction
2. Results
2.1. Tumor Samples and Laser Capture Microdissection
2.2. Proteome Analysis by Isotope Coded Protein Label (ICPL)
| Accession Number | Protein Name | Protein Expression ICPL in nCRT I (Dworak 1 + 2/Dworak 3 + 4) | Protein Expression ICPL in nCRT II (Dworak 1+2/Dworak 3 + 4) |
|---|---|---|---|
| Synchronously regulated proteins in nCRT I and II | |||
| IPI00006663 | ALDH2 Aldehyde dehydrogenase, mitochondrial precursor | 0.53 | 0.53 |
| IPI00010290 | FABP1 FABP1 protein (Fragment) | 0.54 | 0.33 |
| IPI00013847 | UQCRC1 Cytochrome b-c1 complex subunit 1, mitochondrial precursor | 0.63 | 0.52 |
| IPI00014898 | PLEC1 Isoform 1 of Plectin-1 | 0.48 | 0.43 |
| IPI00026185 | CAPZB Isoform 1 of F-actin-capping protein subunit beta | 0.64 | 0.58 |
| IPI00027444 | SERPINB1 Leukocyte elastase inhibitor | 0.30 | 0.49 |
| IPI00216256 | WDR1 Isoform 2 of WD repeat-containing protein 1 | 0.40 | 0.56 |
| IPI00289334 | FLNB Isoform 1 of Filamin-B | 0.36 | 0.39 |
| IPI00298860 | LTF Growth-inhibiting protein 12 | 0.56 | 0.41 |
| IPI00337335 | MYH14 Isoform 1 of Myosin-14 | 0.41 | 0.57 |
| IPI00426051 | IGHG2, Putative uncharacterized protein DKFZp686C15213 | 2.36 | 2.35 |
| IPI00479186 | PKM2 Isoform M2 of Pyruvate kinase isozymes M1/M2 | 0.43 | 0.43 |
| IPI00643920 | TKT Transketolase | 0.38 | 0.47 |
| IPI00793199 | ANXA4 annexin IV | 0.41 | 0.58 |
| Up regulated proteins in good responders (Dworak 3 + 4) nCRT I | |||
| IPI00000105 | MVP Major vault protein | 0.48 | n.r. |
| IPI00000874 | PRDX1 Peroxiredoxin-1 | 0.59 | n.r. |
| IPI00004657 | HLA-B major histocompatibility complex, class I, B | 0.45 | n.r. |
| IPI00007750 | TUBA4A Tubulin α-4A chain | 0.37 | n.r. |
| IPI00007752 | TUBB2C Tubulin beta-2C chain | 0.40 | n.r. |
| IPI00007797 | FABP5;FABP5L7 Fatty acid-binding protein, epidermal | 0.31 | n.r. |
| IPI00008274 | CAP1 Adenylyl cyclase-associated protein | 0.55 | n.r. |
| IPI00010133 | CORO1A Coronin-1A | 0.57 | n.r. |
| IPI00010154 | GDI1 Rab GDP dissociation inhibitor α | 0.53 | n.r. |
| IPI00011654 | TUBB Tubulin beta chain | 0.51 | n.r. |
| IPI00011937 | PRDX4 Peroxiredoxin-4 | 0.11 | n.r. |
| IPI00013683 | TUBB3 Tubulin beta-3 chain | 0.50 | n.r. |
| IPI00013881 | HNRPH1 Heterogeneous nuclear ribonucleoprotein H | 0.59 | n.r. |
| IPI00013890 | SFN Isoform 1 of 14-3-3 protein sigma | 0.44 | n.r. |
| IPI00024095 | ANXA3 Annexin A3 | 0.51 | n.r. |
| IPI00025252 | PDIA3 Protein disulfide-isomerase A3 precursor | 0.49 | n.r. |
| IPI00025874 | RPN1 Dolichyl-diphosphooligosaccharide--protein glycosyltransferase 67 kDa subunit precursor | 0.50 | n.r. |
| IPI00027463 | S100A6 Protein S100-A6 | 0.49 | n.r. |
| IPI00028931 | DSG2 Desmoglein-2 precursor | 0.49 | n.r. |
| IPI00031461 | GDI2 Rab GDP dissociation inhibitor beta | 0.56 | n.r. |
| IPI00169383 | PGK1 Phosphoglycerate kinase 1 | 0.42 | n.r. |
| IPI00171903 | HNRPM Isoform 1 of Heterogeneous nuclear ribonucleoprotein M | 0.65 | n.r. |
| IPI00180675 | TUBA1A Tubulin α-1A chain | 0.31 | n.r. |
| IPI00216049 | HNRPK Isoform 1 of Heterogeneous nuclear ribonucleoprotein K | 0.50 | n.r. |
| IPI00218782 | CAPZB Capping protein | 0.64 | n.r. |
| IPI00218852 | VIL1 Villin-1 | 0.50 | n.r. |
| IPI00219153 | RPL22 60S ribosomal protein L22 | 0.53 | n.r. |
| IPI00220644 | PKM2 Isoform M1 of Pyruvate kinase isozymes M1/M2 | 0.53 | n.r. |
| IPI00220739 | PGRMC1 Membrane-associated progesterone receptor component 1 | 0.56 | n.r. |
| IPI00297779 | CCT2 T-complex protein 1 subunit beta | 0.46 | n.r. |
| IPI00299000 | PA2G4 Proliferation-associated protein 2G4 | 0.46 | n.r. |
| IPI00401264 | TXNDC4 Thioredoxin domain-containing protein 4 precursor | 0.48 | n.r. |
| IPI00410693 | SERBP1 Isoform 1 of Plasminogen activator inhibitor 1 RNA-binding protein | 0.51 | n.r. |
| IPI00419585 | PPIA;PPIAL3;LOC654188 Peptidyl-prolyl cis-trans isomerase A | 0.59 | n.r. |
| IPI00419880 | RPS3A 40S ribosomal protein S3a | 0.39 | n.r. |
| IPI00465431 | LGALS3 Galectin-3 | 0.46 | n.r. |
| IPI00465439 | ALDOA Fructose-bisphosphate aldolase A | 0.47 | n.r. |
| IPI00472855 | HLA-A HLA class I histocompatibility antigen, A-30 α chain precursor | 0.45 | n.r. |
| Down regulated proteins in good responders (Dworak 3 + 4) nCRT I | |||
| IPI00010790 | BGN Biglycan precursor | 2.46 | n.r. |
| IPI00014516 | CALD1 Isoform 1 of Caldesmon | 3.16 | n.r. |
| IPI00020986 | LUM Lumican precursor | 2.22 | n.r. |
| IPI00021885 | FGA Isoform 1 of Fibrinogen α chain precursor | 4.12 | n.r. |
| IPI00021891 | FGG Isoform Gamma-B of Fibrinogen gamma chain precursor | 3.04 | n.r. |
| IPI00022391 | APCS Serum amyloid P-component precursor | 2.41 | n.r. |
| IPI00022395 | C9 Complement component C9 precursor | 1.74 | n.r. |
| IPI00022418 | FN1 Isoform 1 of Fibronectin precursor | 2.26 | n.r. |
| IPI00029717 | FGA Isoform 2 of Fibrinogen α chain precursor | 1.90 | n.r. |
| IPI00031008 | TNC Isoform 1 of Tenascin precursor | 1.89 | n.r. |
| IPI00168728 | IGHM FLJ00385 protein (Fragment) | 1.82 | n.r. |
| IPI00215983 | CA1 Carbonic anhydrase 1 | 1.79 | n.r. |
| IPI00216134 | TPM1 tropomyosin 1 α chain isoform 7 | 3.04 | n.r. |
| IPI00218695 | CALD1 Isoform 3 of Caldesmon | 2.55 | n.r. |
| IPI00219713 | FGG Isoform Gamma-A of Fibrinogen gamma chain precursor | 3.05 | n.r. |
| IPI00298497 | FGB Fibrinogen beta chain precursor | 2.64 | n.r. |
| IPI00399007 | IGHG2 Putative uncharacterized protein DKFZp686I04196 (Fragment) | 1.83 | n.r. |
| IPI00472961 | IGKC IGKC protein | 2.08 | n.r. |
| IPI00550640 | IGHG4 IGHG4 protein | 2.30 | n.r. |
| IPI00553153 | ATPIF1 Putative uncharacterized protein DKFZp564G0422 | 1.86 | n.r. |
| Up regulated proteins in good responders (Dworak 3 + 4) nCRT II | |||
| IPI00001539 | ACAA2 3-ketoacyl-CoA thiolase, mitochondrial | n.r. | 0.48 |
| IPI00003269 | Beta-actin-like protein 2_DKFZp686D0972 hypothetical protein LOC345651 | n.r. | 0.52 |
| IPI00008603 | ACTA2 Actin, aortic smooth muscle | n.r. | 0.58 |
| IPI00011107 | IDH2 Isocitrate dehydrogenase [NADP], mitochondrial precursor | n.r. | 0.23 |
| IPI00013508 | ACTN1 α-actinin-1 | n.r. | 0.52 |
| IPI00019502 | MYH9 Myosin-9 | n.r. | 0.40 |
| IPI00021428 | ACTA1 Actin, α skeletal muscle | n.r. | 0.19 |
| IPI00021439 | ACTB Actin, cytoplasmic 1 | n.r. | 0.57 |
| IPI00021440 | Actin, cytoplasmic 2 | n.r. | 0.45 |
| IPI00023006 | ACTC1 Actin, α cardiac muscle 1 | n.r. | 0.29 |
| IPI00024145 | VDAC2 Voltage-dependent anion-selective channel protein 2 | n.r. | 0.46 |
| IPI00024870 | MYH11 smooth muscle myosin heavy chain 11 isoform SM2A | n.r. | 0.50 |
| IPI00024919 | PRDX3 Thioredoxin-dependent peroxide reductase, mitochondrial precursor | n.r. | 0.53 |
| IPI00031522 | HADHA Trifunctional enzyme subunit α, mitochondrial precursor | n.r. | 0.38 |
| IPI00103467 | ALDH1B1 Aldehyde dehydrogenase X, mitochondrial precursor | n.r. | 0.27 |
| IPI00216308 | VDAC1 Voltage-dependent anion-selective channel prote | n.r. | 0.45 |
| IPI00217975 | LMNB1 Lamin-B1 | n.r. | 0.47 |
| IPI00291006 | MDH2 Malate dehydrogenase, mitochondrial precursor | n.r. | 0.46 |
| IPI00302592 | FLNA filamin A, α isoform 1 | n.r. | 0.22 |
| IPI00418169 | ANXA2 annexin A2 isoform 1 | n.r. | 0.50 |
| IPI00555733 | Actin-like protein (Fragment) | n.r. | 0.61 |
| Down regulated proteins in good responders (Dworak 3 + 4) nCRT II | |||
| IPI00002535 | FKBP2 FK506-binding protein 2 precursor_Peptidyl-prolyl cis-trans isomerase FKBP2 | n.r. | 1.70 |
| IPI00014263 | EIF4H;LOC653994 Isoform Long of Eukaryotic translation initiation factor 4H | n.r. | 1.85 |
| IPI00021841 | APOA1 Apolipoprotein A-I precursor | n.r. | 1.78 |
| IPI00032313 | S100A4 Protein S100-A4 | n.r. | 0.23 |
| IPI00102821 | MGC29506 hypothetical protein LOC51237 | n.r. | 2.58 |
| IPI00217468 | HIST1H1B Histone H1.5 | n.r. | 2.16 |
| IPI00304612 | RPL13A 60S ribosomal protein L13a | n.r. | 1.83 |
| IPI00412714 | SERBP1 Isoform 4 of Plasminogen activator inhibitor 1 RNA-binding protein | n.r. | 1.68. |
| IPI00470498 | SERBP1 Isoform 3 of Plasminogen activator inhibitor 1 RNA-binding protein | n.r. | 1.71 |
| IPI00479997 | STMN1 Stathmin | n.r. | 1.65 |
| Reciprocal regulated proteins in nCRT I and II | |||
| IPI00000230 | TPM1 tropomyosin 1 α chain isoform 2 | 2.58 | 0.47 |
| IPI00003881 | HNRPF Heterogeneous nuclear ribonucleoprotein F | 0.31 | 2.26 |
| IPI00003949 | UBE2N Ubiquitin-conjugating enzyme E2 N | 0.52 | 1.51 |
| IPI00004573 | PIGR Polymeric immunoglobulin receptor precursor | 0.56 | 1.78 |
| IPI00008176 | SHROOM4 Isoform 1 of Protein Shroom4 | 2.26 | 0.53 |
| IPI00022792 | MFAP4 Microfibril-associated glycoprotein 4 precursor | 4.81 | 0.09 |
| IPI00096066 | SUCLG2 Succinyl-CoA ligase (GDP-forming) beta-chain, mitochondrial precursor | 2.13 | 0.46 |
| IPI00010779 | TPM4 Isoform 1 of Tropomyosin α-4 chain | 2.44 | 0.30 |
| IPI00016801 | GLUD1 Glutamate dehydrogenase 1, mitochondrial precursor | 1.18 | 0.50 |
| IPI00018853 | TPM1 Tropomyosin isoform | 1.87 | 0.54 |
| IPI00020501 | MYH11 Myosin-11 | 3.09 | 0.43 |
| IPI00218820 | TPM2 Isoform 3 of Tropomyosin beta chain | 2.09 | 0.52 |
| IPI00218693 | APRT Adenine phosphoribosyltransferase | 0.53 | 2.02 |
| IPI00219757 | GSTP1 Glutathione S-transferase P | 043 | 1.85 |
| IPI00025512 | HSPB1 Heat shock protein beta-1 | 1.55 | 0.41 |
| IPI00027350 | PRDX2 Peroxiredoxin-2 | 2.18 | 0.49 |
| IPI00183968 | TPM3 tropomyosin 3 isoform 1 | 2.00 | 0.30 |
| IPI00216135 | TPM1 Isoform 3 of Tropomyosin α-1 chain | 2.28 | 0.51 |
| IPI00216138 | TAGLN Transgelin | 4.03 | 0.28 |
| IPI00218319 | TPM3 Isoform 2 of Tropomyosin α-3 chain | 2.44 | 0.56 |
| IPI00218343 | TUBA1C Tubulin α-1C chain | 0.36 | 2.10 |
| IPI00220362 | HSPE1 10 kDa heat shock protein, mitochondrial | 1.78 | 0.49 |
| IPI00220709 | TPM2 Isoform 2 of Tropomyosin beta chain | 2.52 | 0.51 |
| IPI00299547 | LCN2 Neutrophil gelatinase-associated lipocalin precursor | 0.46 | 1.90 |
| IPI00333771 | CALD1 Isoform 5 of Caldesmon | 3.16 | 0.55 |
| IPI00335168 | MYL6 Isoform Non-muscle of Myosin light polypeptide 6 | 2.18 | 0.49 |
| IPI00382606 | F7 Factor VII active site mutant immunoconjugate | 2.55 | 0.53 |
| IPI00549291 | IGHM IGHM protein | 2.29 | 0.24 |
| IPI00604537 | TPM1 tropomyosin 1 α chain isoform 3 | 2.55 | 0.47 |
| IPI00736885 | LOC440786 Ig kappa chain V-II region TEW | 3.38 | 0.34 |
| IPI00743194 | Kappa light chain variable region (Fragment) | 3.38 | 0.34 |
| IPI00746963 | IGKC IGKC protein | 2.03 | 0.43 |
| IPI00815926 | IGHG1 IGHG1 protein | 2.16 | 0.47 |
| IPI00843757 | TPM2 Uncharacterized protein TPM2 (Fragment) | 1.96 | 0.19 |
| IPI00876888 | IGHV4-31 immunoglobulin heavy variable 4-31- cDNA FLJ78387 | 2.37 | 0.20 |
2.3. Immunohistochemistry
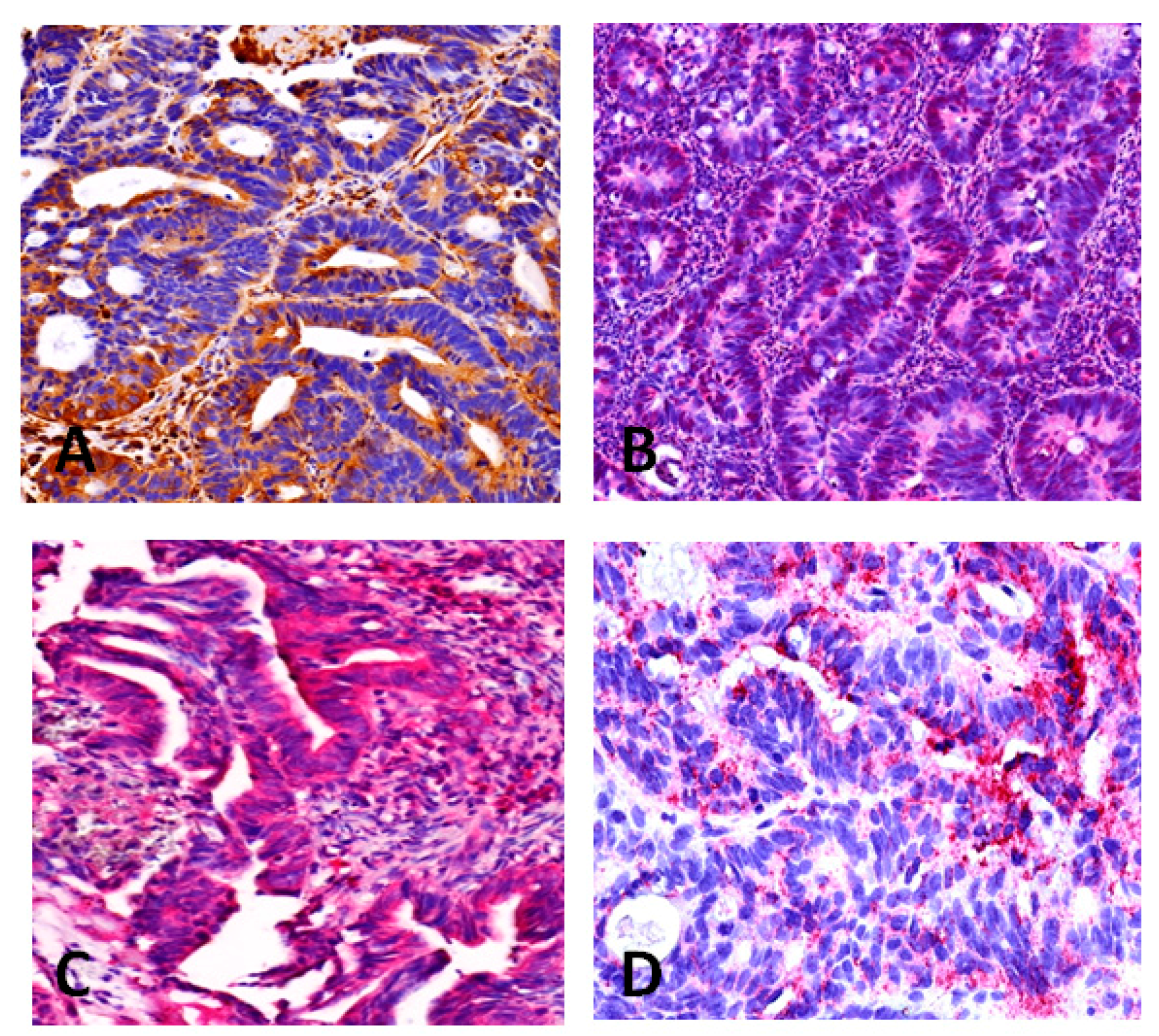
3. Discussion
4. Material and Methods
4.1. Patients and Tumor Biopsies
| Patients | nCRT I | nCRT II |
|---|---|---|
| n | 10 | 10 |
| Male | 9 | 7 |
| Female | 2 | 3 |
| ypT-category | ||
| yT0 | 1 | 3 |
| yT1 | 0 | 0 |
| yT2 | 3 | 1 |
| yT3 | 5 | 6 |
| yT4 | 1 | 0 |
| ypN-category | ||
| yN0 | 4 | 7 |
| yN1 | 5 | 3 |
| yN2 | 1 | 0 |
| Distant metastasis | ||
| M0 | 9 | 9 |
| M1 | 1 | 1 |
| Grading | ||
| G1/2 | 6 | 6 |
| G3/4 | 3 | 2 |
| GX | 1 | 2 |
| Regression Grading (Dworak) | ||
| Dworak 1 | 2 | 0 |
| Dworak 2 | 3 | 5 |
| Dworak 3 | 4 | 2 |
| Dworak 4 | 1 | 3 |
4.2. Study Protocol
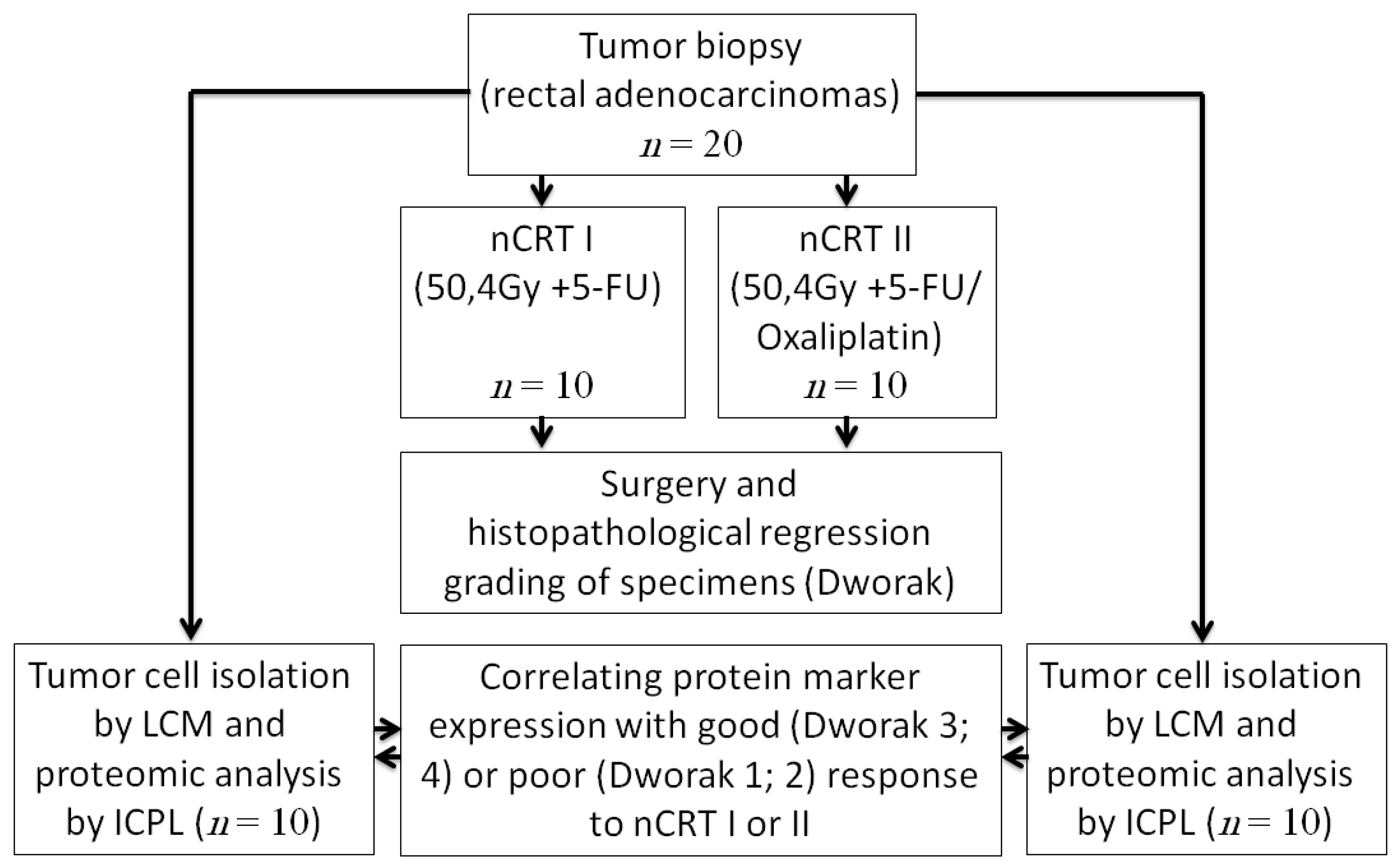
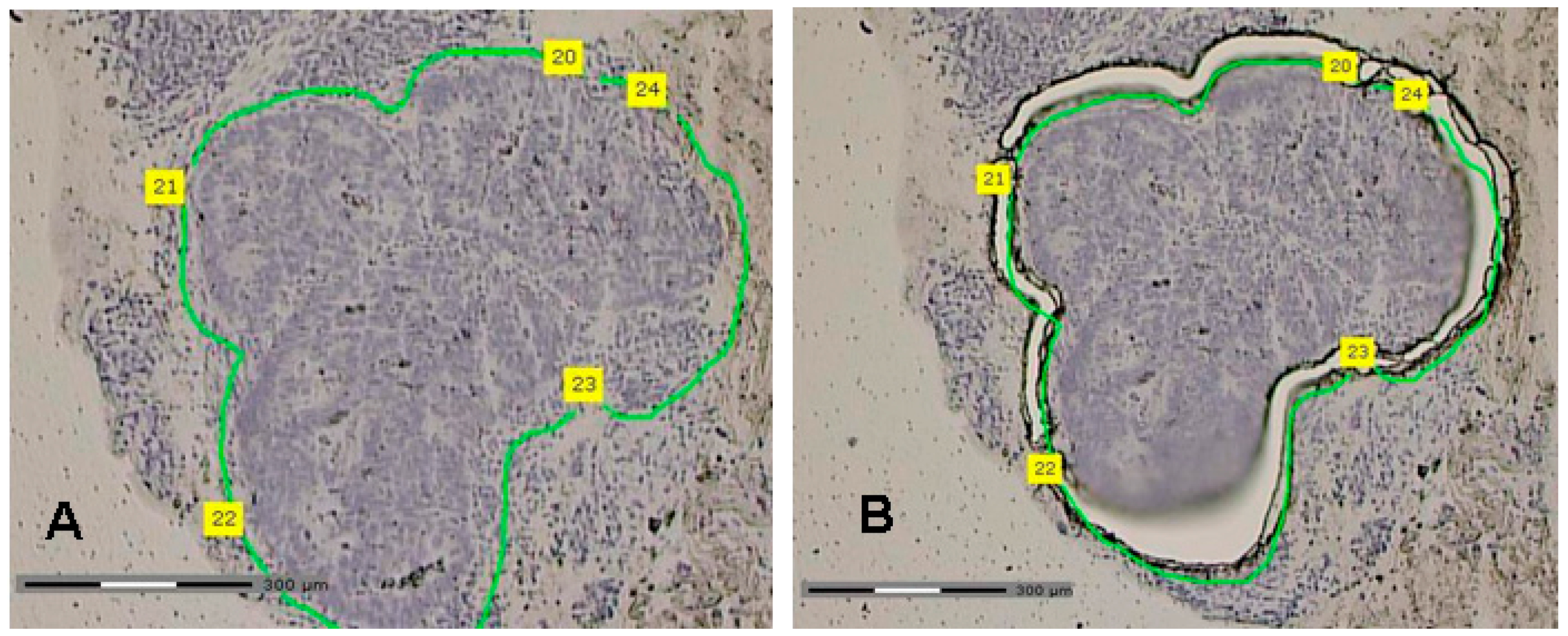
4.3. Laser Capture Microdissection (LCM)
4.4. Isotope Coded Protein Labeling (ICPL)
4.5. Immunohistochemistry
4.6. Statistics
4.7. Quantification and Identification of Potential Protein Signatures
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sauer, R.; Becker, H.; Hohenberger, W.; Rodel, C.; Wittekind, C.; Fietkau, R.; Martus, P.; Tschmelitsch, J.; Hager, E.; Hess, C.F.; et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N. Engl. J. Med. 2004, 351, 1731–1740. [Google Scholar] [CrossRef] [PubMed]
- Sauer, R.; Fietkau, R.; Wittekind, C.; Martus, P.; Rodel, C.; Hohenberger, W.; Jatzko, G.; Sabitzer, H.; Karstens, J.H.; Becker, H.; et al. Adjuvant versus neoadjuvant radiochemotherapy for locally advanced rectal cancer. A progress report of a phase-III randomized trial (protocol CAO/ARO/AIO-94). Strahlenther. Onkol. 2001, 177, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Sauer, R.; Fietkau, R.; Wittekind, C.; Rodel, C.; Martus, P.; Hohenberger, W.; Tschmelitsch, J.; Sabitzer, H.; Karstens, J.H.; Becker, H.; et al. Adjuvant vs. neoadjuvant radiochemotherapy for locally advanced rectal cancer: The German trial CAO/ARO/AIO-94. Colorectal Dis. 2003, 5, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Sauer, R.; Liersch, T.; Merkel, S.; Fietkau, R.; Hohenberger, W.; Hess, C.; Becker, H.; Raab, H.R.; Villanueva, M.T.; Witzigmann, H.; et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: Results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J. Clin. Oncol. 2012, 30, 1926–1933. [Google Scholar] [CrossRef] [PubMed]
- Schmiegel, W.; Pox, C.; Reinacher-Schick, A.; Adler, G.; Arnold, D.; Fleig, W.; Folsch, U.R.; Fruhmorgen, P.; Graeven, U.; Heinemann, V.; et al. S3 guidelines for colorectal carcinoma: Results of an evidence-based consensus conference on 6/7 February 2004 and 8/9 June 2007 (for the topics IV, VI and VII). Z. Gastroenterol. 2010, 48, 65–136. [Google Scholar] [CrossRef] [PubMed]
- Sauer, R.; Rodel, C. Adjuvant and neoadjuvant radiochemotherapy of rectal carcinoma. Praxis 2002, 91, 1476–1484. [Google Scholar] [CrossRef] [PubMed]
- Dworak, O.; Keilholz, L.; Hoffmann, A. Pathological features of rectal cancer after preoperative radiochemotherapy. Int. J. Colorectal Dis. 1997, 12, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Hermanek, P.; Merkel, S.; Hohenberger, W. Prognosis of rectal carcinoma after multimodal treatment: ypTNM classification and tumor regression grading are essential. Anticancer Res. 2013, 33, 559–566. [Google Scholar] [PubMed]
- Rodel, C.; Grabenbauer, G.G.; Papadopoulos, T.; Bigalke, M.; Gunther, K.; Schick, C.; Peters, A.; Sauer, R.; Rodel, F. Apoptosis as a cellular predictor for histopathologic response to neoadjuvant radiochemotherapy in patients with rectal cancer. Int. J. Radiat. Oncol. Biol. Phys. 2002, 52, 294–303. [Google Scholar] [CrossRef]
- Kellermann, J. ICPL--isotope-coded protein label. Methods Mol. Biol. 2008, 424, 113–123. [Google Scholar] [PubMed]
- Lottspeich, F.; Kellermann, J. ICPL labeling strategies for proteome research. Methods Mol. Biol. 2011, 753, 55–64. [Google Scholar] [PubMed]
- Rodel, C.; Graeven, U.; Fietkau, R.; Hohenberger, W.; Hothorn, T.; Arnold, D.; Hofheinz, R.D.; Ghadimi, M.; Wolff, H.A.; Lang-Welzenbach, M.; et al. Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): Final results of the multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2015, 16, 979–989. [Google Scholar] [CrossRef]
- Gines, A.; Bystrup, S.; Ruiz de Porras, V.; Guardia, C.; Musulen, E.; Martinez-Cardus, A.; Manzano, J.L.; Layos, L.; Abad, A.; Martinez-Balibrea, E. PKM2 subcellular localization is involved in oxaliplatin resistance acquisition in HT29 human colorectal cancer cell lines. PLoS ONE 2015, 10, e0123830. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.F.; Li, X.B.; Sun, H.; Zhang, B.; Han, Y.S.; Jiang, Y.; Zhuang, Q.L.; Fang, J.; Wu, G.H. Pyruvate kinase type M2 is upregulated in colorectal cancer and promotes proliferation and migration of colon cancer cells. IUBMB Life 2012, 64, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Foldi, M.; Stickeler, E.; Bau, L.; Kretz, O.; Watermann, D.; Gitsch, G.; Kayser, G.; Zur Hausen, A.; Coy, J.F. Transketolase protein TKTL1 overexpression: A potential biomarker and therapeutic target in breast cancer. Oncol. Rep. 2007, 17, 841–845. [Google Scholar] [CrossRef] [PubMed]
- Dworak, O.; Knoch, M. Surgery versus radiotherapy for advanced rectal cancer. Lancet 1997, 349, 877; author reply 877–878. [Google Scholar] [CrossRef]
- Espina, V.; Milia, J.; Wu, G.; Cowherd, S.; Liotta, L.A. Laser capture microdissection. Methods Mol. Biol. 2006, 319, 213–229. [Google Scholar] [PubMed]
- Biringer, R.G.; Amato, H.; Harrington, M.G.; Fonteh, A.N.; Riggins, J.N.; Huhmer, A.F. Enhanced sequence coverage of proteins in human cerebrospinal fluid using multiple enzymatic digestion and linear ion trap LC-MS/MS. Brief. Funct. Genom. Proteom. 2006, 5, 144–153. [Google Scholar]
- Brunner, A.; Keidel, E.M.; Dosch, D.; Kellermann, J.; Lottspeich, F. ICPLQuant-A software for non-isobaric isotopic labeling proteomics. Proteomics 2010, 10, 315–326. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Croner, R.S.; Sevim, M.; Metodiev, M.V.; Jo, P.; Ghadimi, M.; Schellerer, V.; Brunner, M.; Geppert, C.; Rau, T.; Stürzl, M.; et al. Identification of Predictive Markers for Response to Neoadjuvant Chemoradiation in Rectal Carcinomas by Proteomic Isotope Coded Protein Label (ICPL) Analysis. Int. J. Mol. Sci. 2016, 17, 209. https://doi.org/10.3390/ijms17020209
Croner RS, Sevim M, Metodiev MV, Jo P, Ghadimi M, Schellerer V, Brunner M, Geppert C, Rau T, Stürzl M, et al. Identification of Predictive Markers for Response to Neoadjuvant Chemoradiation in Rectal Carcinomas by Proteomic Isotope Coded Protein Label (ICPL) Analysis. International Journal of Molecular Sciences. 2016; 17(2):209. https://doi.org/10.3390/ijms17020209
Chicago/Turabian StyleCroner, Roland S., Müzeyyen Sevim, Metodi V. Metodiev, Peter Jo, Michael Ghadimi, Vera Schellerer, Maximillian Brunner, Carol Geppert, Tilman Rau, Michael Stürzl, and et al. 2016. "Identification of Predictive Markers for Response to Neoadjuvant Chemoradiation in Rectal Carcinomas by Proteomic Isotope Coded Protein Label (ICPL) Analysis" International Journal of Molecular Sciences 17, no. 2: 209. https://doi.org/10.3390/ijms17020209








