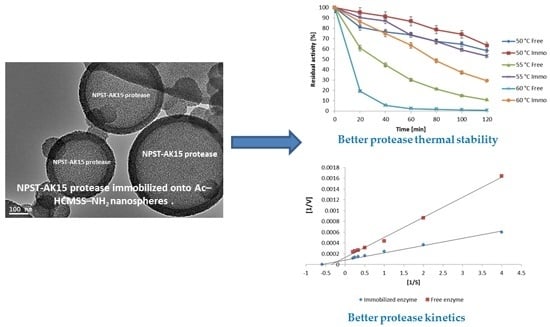
Enhancement of Alkaline Protease Activity and Stability via Covalent Immobilization onto Hollow Core-Mesoporous Shell Silica Nanospheres
Abstract
:1. Introduction
2. Results and Discussion
2.1. Fabrication and Characterization of Hollow Core-Mesoporous Shell Silica Nanospheres
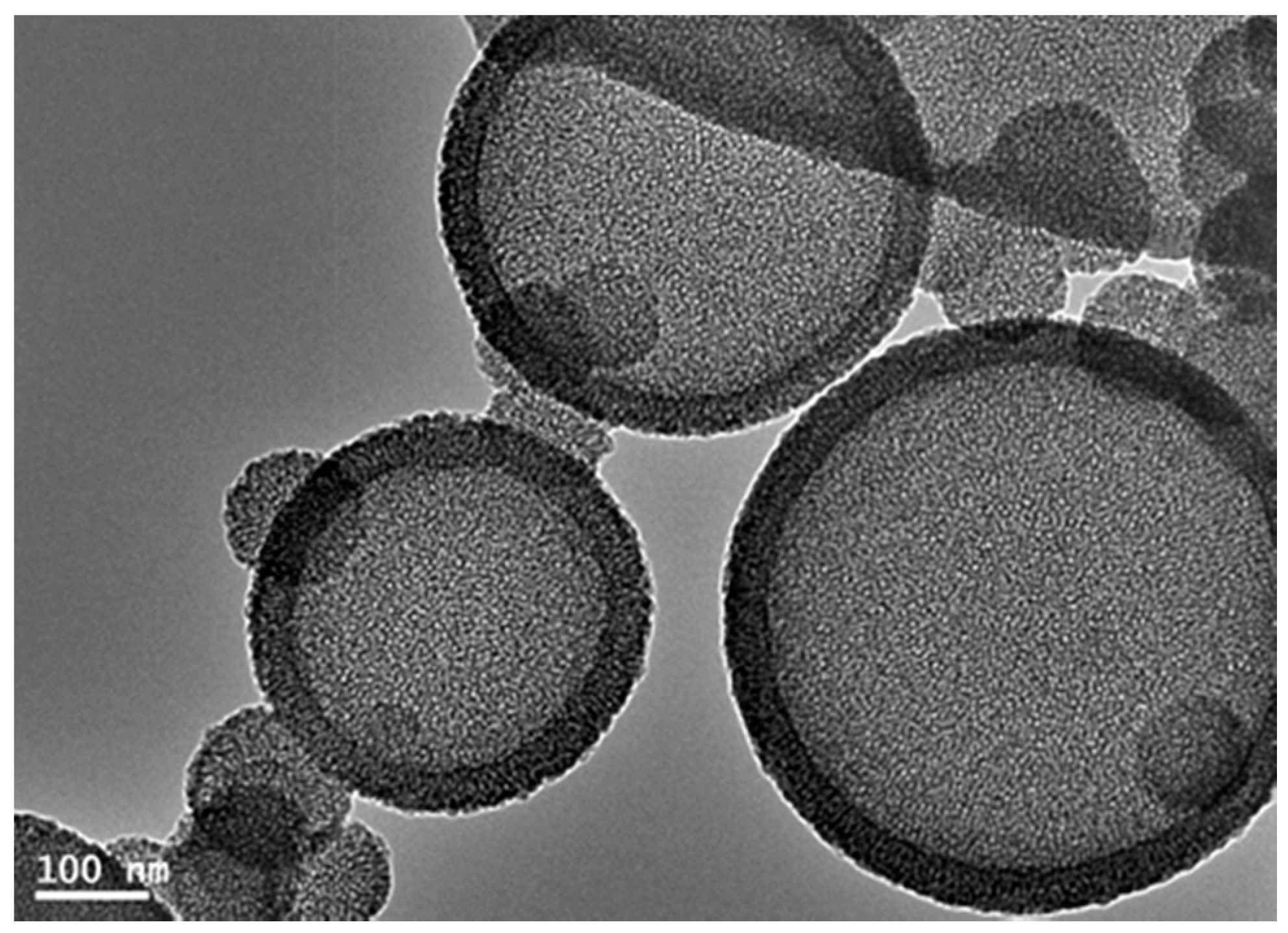

2.2. Protease Immobilization
| Matrix | Immobilization Method | Immobilization Yield * (%) | Activity Yield ** (%) | Loading Efficiency *** (%) |
|---|---|---|---|---|
| HCMSS-non | Physical adsorption | 44.6 | 40.0 | 41.2 |
| HCMSS–NH2 | Physical adsorption | 47.3 | 42.3 | 44.7 |
| HCMSS–C2H5 | Physical adsorption | 19.3 | 16.3 | 18.0 |
| HCMSS–NH2 | Covalent attachment | 75.6 | 72.2 | 73.6 |
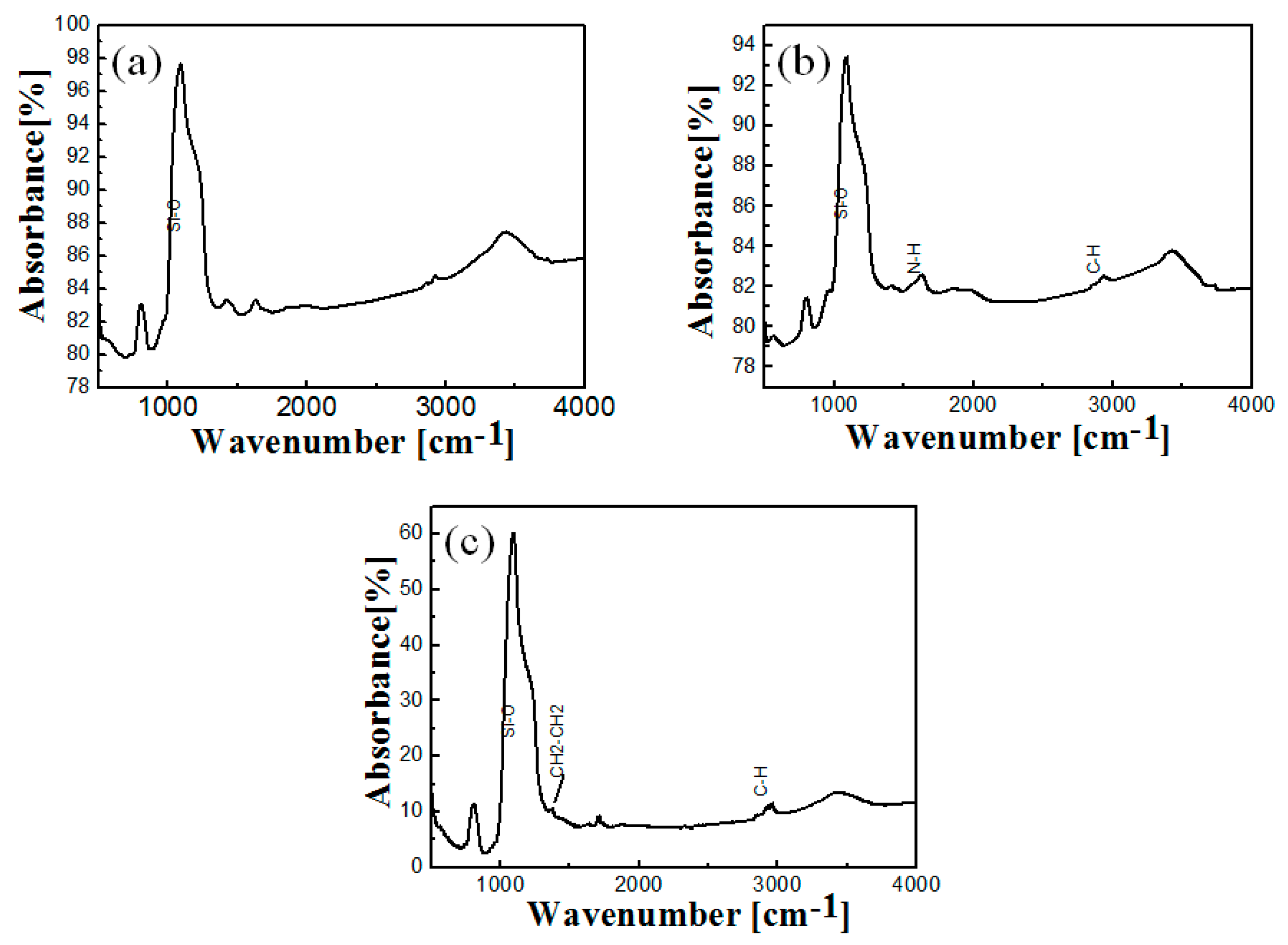
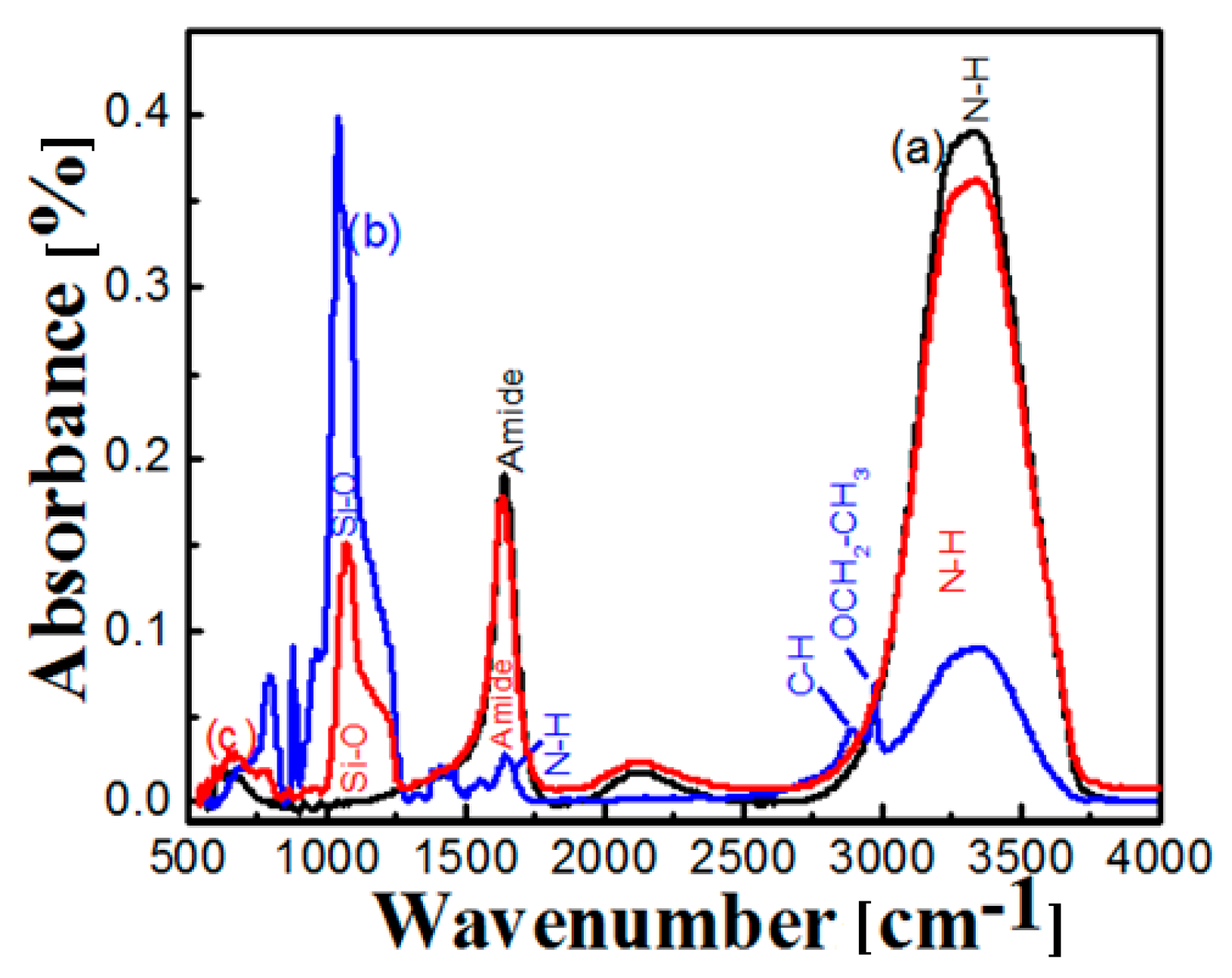
2.3. FT-IR of Free and Immobilized Protease
2.4. Loading Capacity
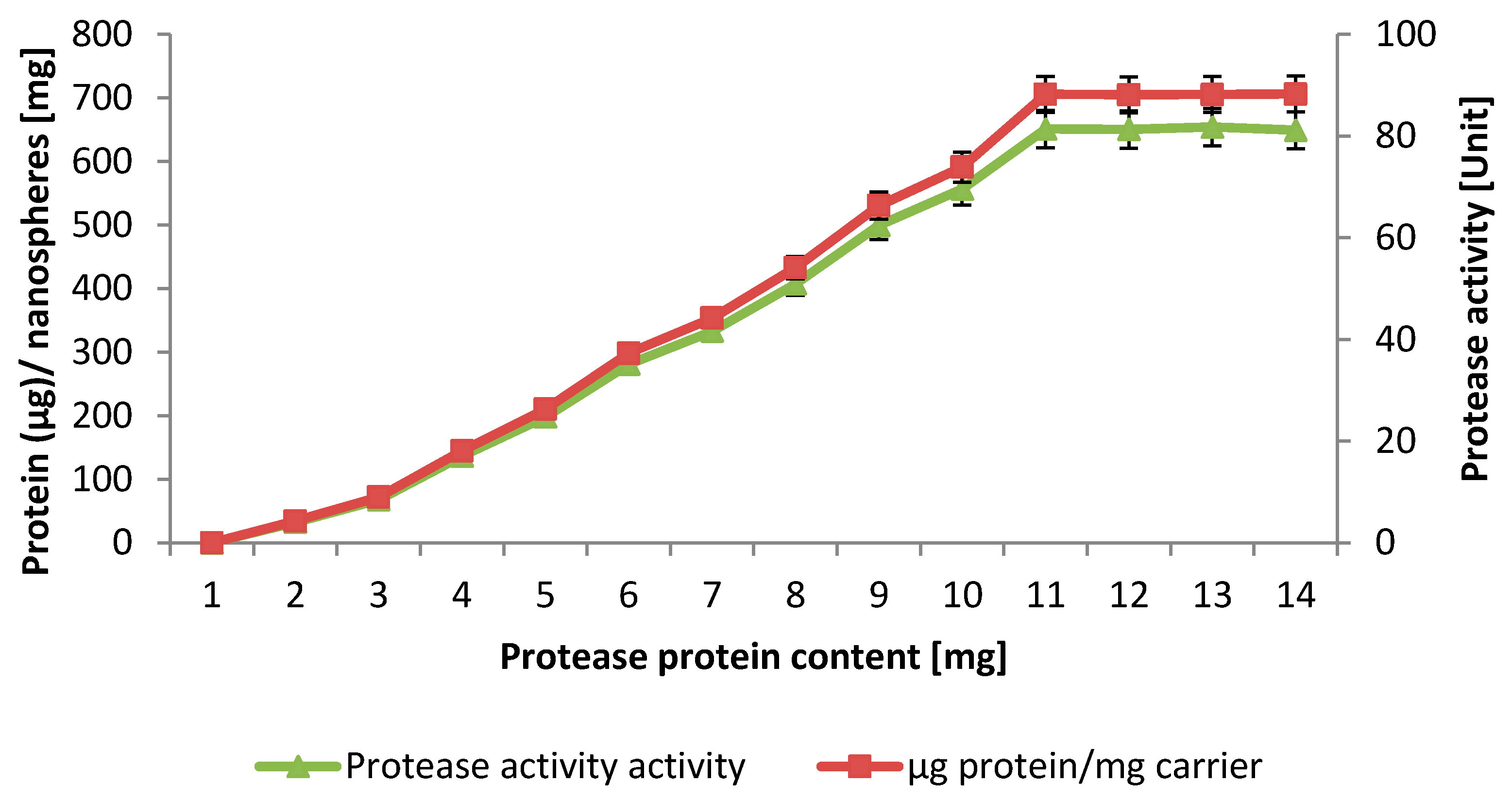
2.5. Properties of the Immobilized NPST-AK15 Protease
2.5.1. Influence of pH and Temperature

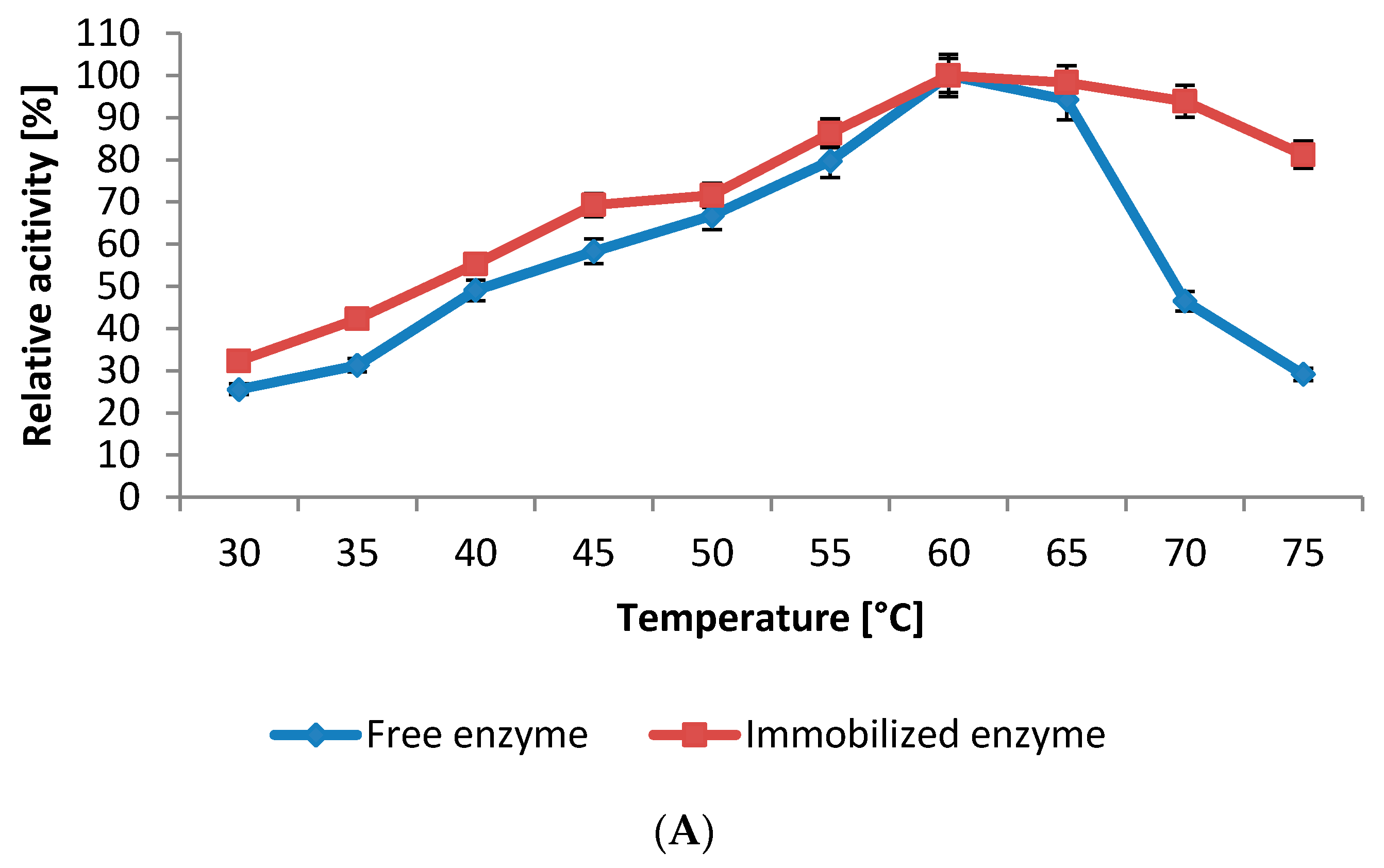

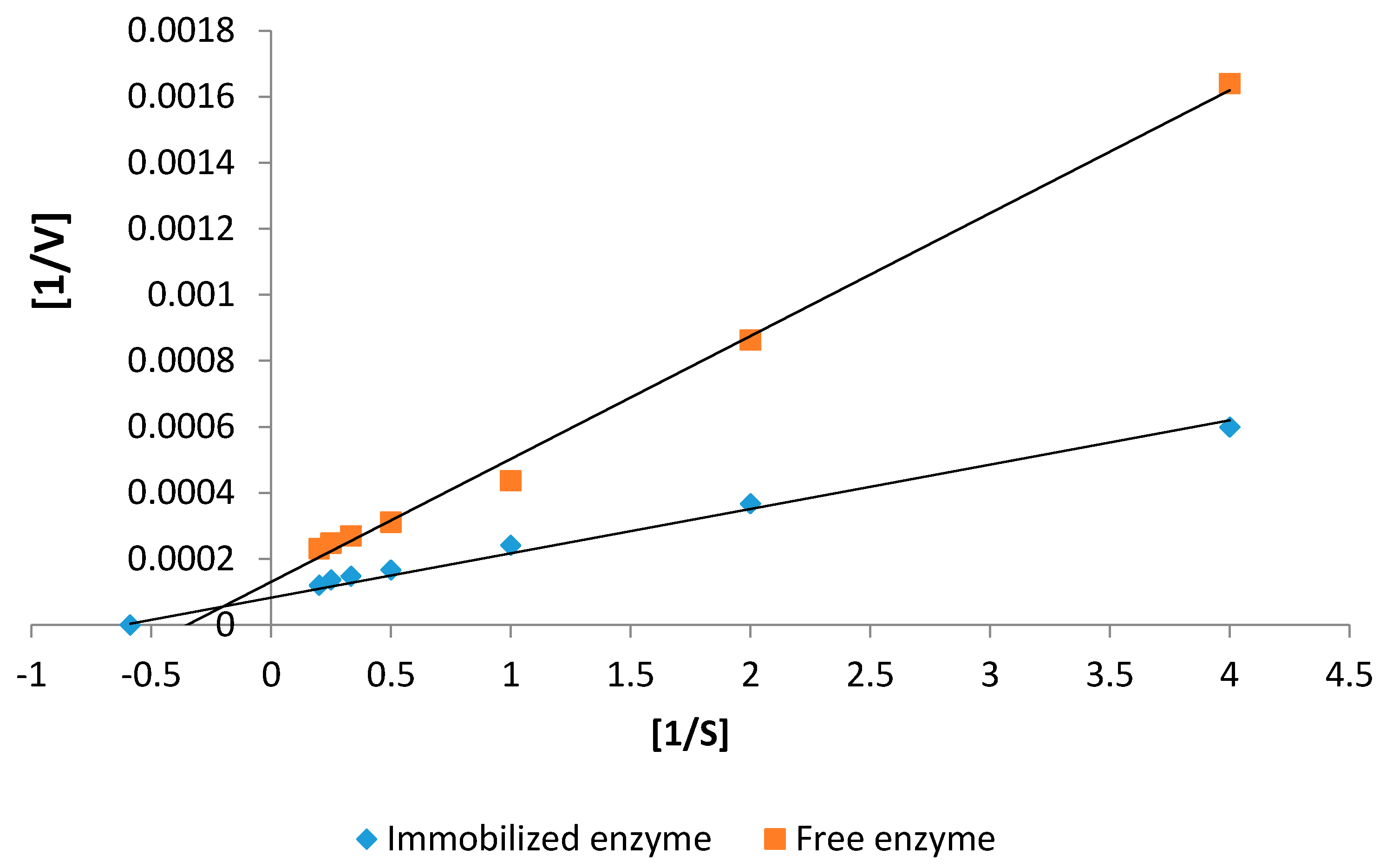
2.5.2. Kinetics Studies
| Kinetic Parameters | Free Protease | Immobilized Protease |
|---|---|---|
| Km (mM) | 0.109 | 0.074 |
| Vmax (µM·min−1·mg−1) | 41.2 | 66.1 |
| kcat (s−1) | 1156.5 | 1855.4 |
| kcat/Km (mM−1·s−1) | 10.6 × 103 | 25.1 × 103 |
2.5.3. Effect of Inhibitors and Organic Solvents
| Inhibitor | Concentration | Free Enzyme | Immobilized Enzyme |
|---|---|---|---|
| None | 100 | 100 | |
| β-mercaptoethanol | 1 | 94.3 ± 2.4 | 94.0 |
| 5 | 88.0 ± 2.2 | 89.0 | |
| PMSF | 1 | 15.5 ± 0.16 | 92.9 |
| 5 | 0 | 86.3 | |
| DTT | 1 | 90.2 ± 1.9 | 101.8 |
| 5 | 22.60 ± 0.8 | 97.5 | |
| EDTA | 2 | 62.2 ± 2.1 | 95.0 |
| 5 | 30.1 ± 1.5 | 81.5 |
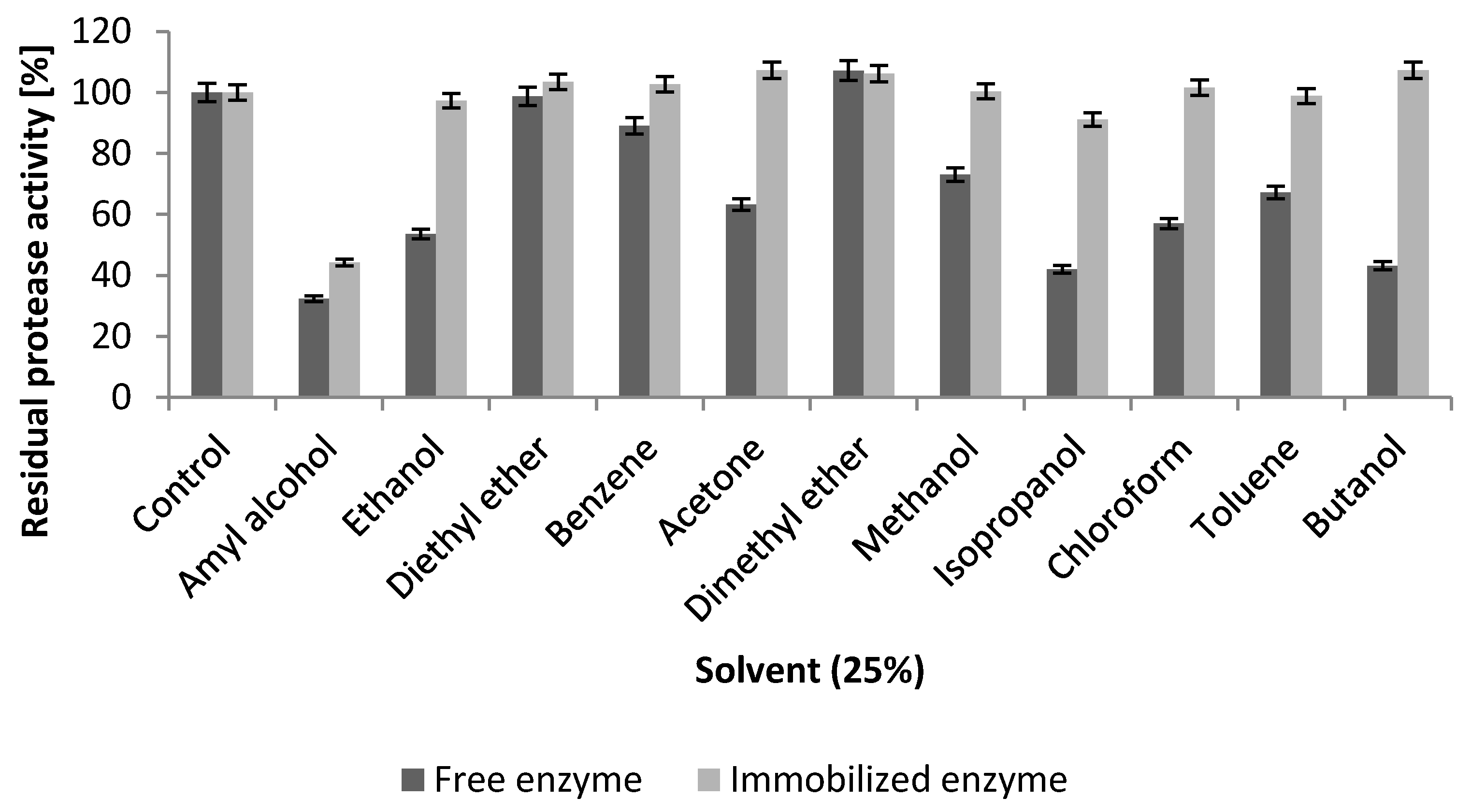
2.5.4. Influence of Surfactants and Commercial Laundry Detergents

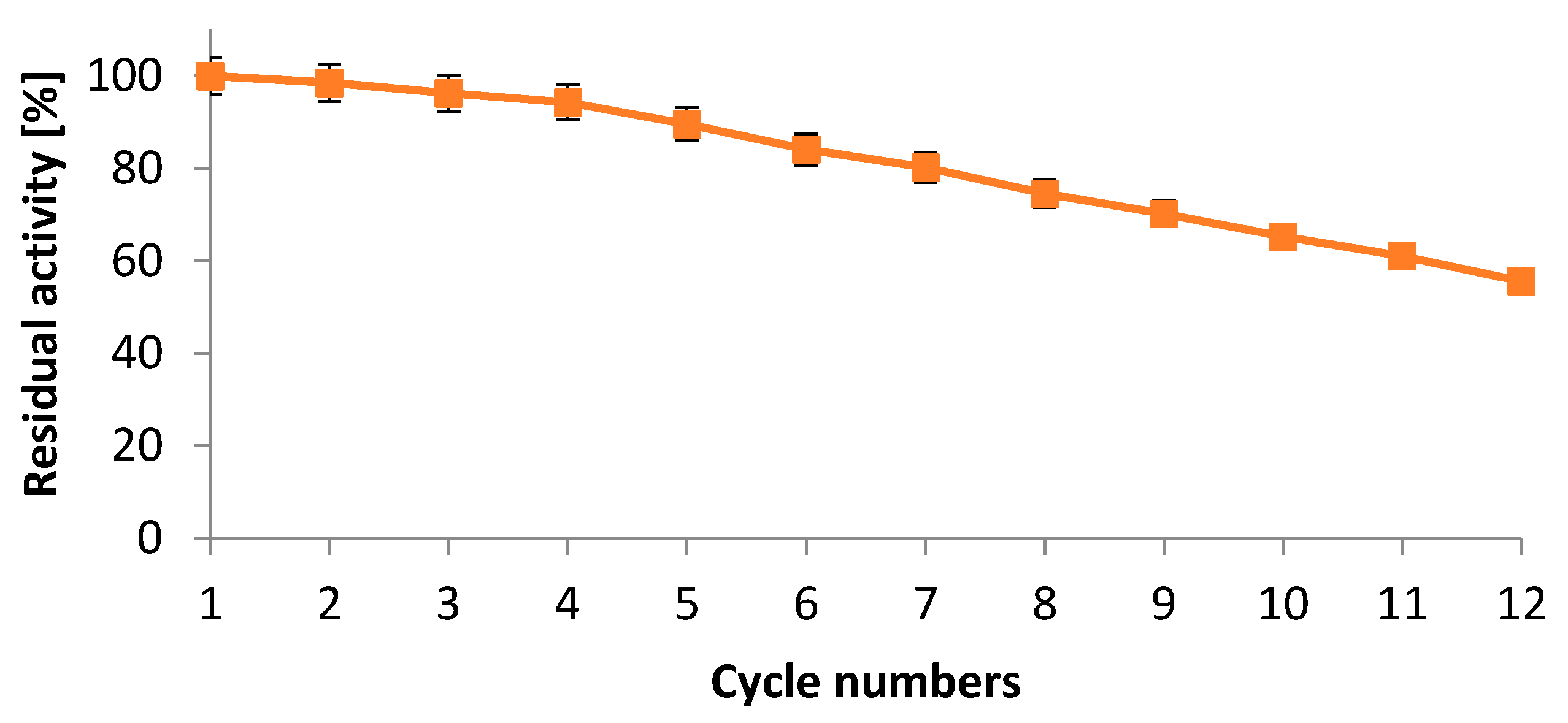
2.5.5. Operational Stability of Immobilized Protease
3. Experimental Section
3.1. Alkaline Protease Production and Purification
3.2. Fabrication of Hollow Core-Mesoporous Shell Silica Nanospheres
3.2.1. Amino-Functionalized Hollow Core-Mesoporous Shell Silica Nanospheres
3.2.2. Hydrophobic Ethane-Functionalized Hollow Core-Mesoporous Shell Silica Nanospheres
3.3. Characterization of the Synthesized Mesoporous Silica Based Nanospheres
3.4. Alkaline Protease Immobilization
3.4.1. Physical Adsorption
3.4.2. Covalent Attachment
3.5. Assay of Alkaline Protease Activity
3.6. Properties of Immobilized Protease
3.6.1. Influence of Temperature on Protease Activity and Stability
3.6.2. Influence of pH on Protease Activity
3.6.3. Influence of Solvents and Inhibitors
3.6.4. Influence of Surfactants and Commercial Detergents
3.6.5. Determination of Kinetic Parameters
3.6.6. Reusability of the Immobilized NPST-AK15 Protease
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jain, D.; Pancha, I.; Mishra, S.K.; Shrivastav, A.; Mishra, S. Purification and characterization of haloalkaline thermoactive, solvent stable and SDS-induced protease from Bacillus sp.: A potential additive for laundry detergents. Bioresour. Technol. 2012, 115, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Soozanipour, A.; Taheri-Kafrani, A.; Isfahani, A.L. Covalent attachment of xylanase on functionalized magnetic nanoparticles and determination of its activity and stability. Chem. Eng. J. 2015, 270, 235–243. [Google Scholar] [CrossRef]
- Bouacem, K.; Bouanane-Darenfed, A.; Laribi-Habchi, H.; ben Elhoul, M.; Hmida-Sayari, A.; Hacene, H.; Ollivier, B.; Fardeau, M.L.; Jaouadi, B.; Bejar, S. Biochemical characterization of a detergent stable serine alkalineprotease from Caldicoprobacter guelmensis. Int. J. Biol. Macromol. 2015, 81, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Ben Elhoul, M.; Jaouadi, N.Z.; Rekik, H.; Bejar, W.; Touioui, S.B.; Hmidi, M.; Badis, A.; Bejar, S.; Jaouadi, B. A novel detergent-stable solvent-tolerant serine thiol alkaline protease from Streptomyces koyangensis TN650. Int. J. Biol. Macromol. 2015, 79, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Patil, U.; Chaudhari, A. Purification and characterizationofsolvent-tolerant, thermostable, alkaline metalloprotease from alkalophilic Pseudomonas aeruginosa MTCC 7926. J. Chem. Technol. Biotechnol. 2009, 84, 1255–1262. [Google Scholar] [CrossRef]
- Mokashe, N.; Chaudhari, A.; Patil, U. Optimal production and characterization of alkaline protease from newly isolated halotolerant Jeotgalicoccus sp. Biocatal. Agric. Biotechnol. 2015, 4, 235–243. [Google Scholar] [CrossRef]
- Gupta, A.; Khare, S.K. Enhanced production and characterization of a solvent stable protease from solvent tolerant Pseudomonas aeruginosa PseA. Enzym. Microb. Technol. 2007, 42, 11–16. [Google Scholar] [CrossRef]
- Alqueres, S.C.; Almeida, R.V.; Clementino, M.M.; Vieira, R.P.; Almeida, W.I.; Cardoso, A.M.; Martins, O.B. Exploring the biotechnological applications in the archaeal domain. Braz. J. Microbiol. 2007, 38, 398–405. [Google Scholar] [CrossRef]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzym. Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Kumar, A.G.; Swarnalatha, S.; Kamatchia, P.; Sekaran, G. Immobilization of high catalytic acid protease on functionalized mesoporous activated carbon particles. Biochem. Eng. J. 2009, 43, 185–190. [Google Scholar] [CrossRef]
- Wang, P. Nanoscale biocatalyst systems. Curr. Opin. Biotechnol. 2006, 17, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Lin, T.S.; Mou, C.Y. Mesoporous materials for encapsulating enzymes. Nano Today 2009, 4, 165–179. [Google Scholar] [CrossRef]
- Fan, J.; Lei, J.; Wang, L.M.; Yu, C.Z.; Tu, B.; Zhao, D.Y. Rapid and highcapacity immobilization of enzymes based on mesoporous silicas with controlled morphologies. Chem. Commun. 2003, 17, 2140–2141. [Google Scholar] [CrossRef]
- Blasi, L.; Longo, L.; Vasapollo, G.; Cingolani, R.; Rinaldi, R.; Rizzello, T.; Acierno, R.; Maffia, M. Characterization of glutamate dehydrogenase immobilization on silica surface by atomic force microscopy and kinetic analyses. Enzym. Microb. Technol. 2005, 36, 818–823. [Google Scholar] [CrossRef]
- Xiao, Q.G.; Tao, X.; Zoub, H.K.; Chen, J.F. Comparative study of solid silica nanoparticles and hollow silica nanoparticles for the immobilization of lysozyme. Chem. Eng. J. 2008, 137, 38–44. [Google Scholar] [CrossRef]
- Ibrahim, A.S.; Al-Salamah, A.A.; El-Badawi, Y.B.; El-Tayeb, M.A.; Ibrahim, S.S. NPST-AK15 isolated from hyper saline soda lakes. Electron. J. Biotechnol. 2015, 18, 236–243. [Google Scholar] [CrossRef]
- Ibrahim, A.S.; Al-Salamah, A.A.; El-Badawi, Y.B.; El-Tayeb, M.A.; Antranikian, G. Detergent-, solvent- and salt-compatible thermoactive alkaline serine protease from halotolerant alkaliphilic Bacillus sp. NPST-AK15: Purification and characterization. Extremophiles 2015, 19, 961–971. [Google Scholar] [CrossRef] [PubMed]
- Nikolić, M.P.; Giannakopoulos, K.P.; Bokorov, M.; Srdić, V.V. Effect of surface functionalization on synthesis of mesoporous silica core/shell particles. Microporous Mesoporous Mater. 2012, 155, 8–13. [Google Scholar] [CrossRef]
- Yamaura, M.; Camilo, R.L.; Sampaio, L.C.; Macedo, M.A.; Nakamura, M.; Toma, H.E. Preparation and characterization of (3-aminopropyl)triethoxysilane-coated magnetite nanoparticles. J. Magn. Magn. Mater. 2004, 279, 210–217. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, J.; Yang, J.; Yang, Q.; Li, C. Direct synthesis of highly ordered amine functionalized mesoporous ethane-silicas. Microporous Mesoporous Mater. 2008, 109, 172–183. [Google Scholar] [CrossRef]
- Sulek, F.; Knez, Z.; Habulin, M. Immobilization of cholesterol oxidase to finely dispersed silica coated maghemite nanoparticles based magnetic fluid. Appl. Surf. Sci. 2010, 256, 4596–4600. [Google Scholar] [CrossRef]
- Lei, C.; Soares, T.A.; Shin, Y.; Liu, J.; Ackerman, E.J. Enzyme specific activity in functionalized nanoporous supports. Nanotechnology 2008, 19, 125102–125111. [Google Scholar] [PubMed]
- Jin, X.; Li, J.; Huang, P.; Dong, X.; Guo, L.; Yang, L.; Cao, Y.; Wei, F.; Zhao, Y.; Chen, H. Immobilized protease on the magnetic nanoparticles used for the hydrolysis of rapeseed meals. J. Magn. Magn. Mater. 2010, 322, 2031–2037. [Google Scholar] [CrossRef]
- Kharrat, N.; Ben-Ali, Y.; Marzouk, S.; Gargouria, Y.T.; Karra-Chaabounia, M. Immobilization of Rhizopus oryzae lipase on silica aerogels by adsorption: Comparison with the free enzyme. Process Biochem. 2011, 46, 1083–1089. [Google Scholar] [CrossRef]
- Huang, J.; Liu, H.; Zhang, P.; Zhang, P.; Li, M.; Ding, L. Immobilization of cholesterol oxidase on magnetic fluorescent core-shell-structured nanoparticles. Mater. Sci. Eng. C 2015, 57, 31–37. [Google Scholar]
- Kim, K.D.; Kim, S.S.; Choa, Y.H.; Kim, H.T. Formation and surfacemodification of Fe3O4 nanoparticles by co-precipitation and sol–gel method. J. Ind. Eng. Chem. 2007, 13, 1137–1141. [Google Scholar]
- Cui, Y.; Li, Y.; Yang, Y.; Liu, X.; Lei, L.; Zhou, L. Facile synthesis of aminosilane modified superparamagnetic Fe3O4 nanoparticles and application for lipase immobilization. J. Biotechnol. 2010, 150, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Su, R.; Shi, P.; Zhu, M.; Hong, F.; Li, D. Studies on the properties of graphene oxide-alkaline protease bio-composites. Bioresour. Technol. 2012, 115, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Ranjbakhsh, E.; Bordbar, A.K.; Abbasi, M.; Khosropour, A.R.; Shams, E. Enhancement of stability and catalytic activity of immobilized lipase on silica-coated modified magnetite nanoparticles. Chem. Eng. J. 2012, 179, 272–276. [Google Scholar] [CrossRef]
- Ansari, S.A.; Husain, Q. Potential applications of enzymes immobilized on/in nano materials: A review. Biotechnol. Adv. 2011, 30, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, M.; Kuhad, R.C. Immobilization of xylanase from Bacillus pumilus strain MK001 and its application in production of xylo-oligosaccharides. Appl. Biochem. Biotechnol. 2007, 142, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Jellouli, K.; Ghorbel-Bellaaj, O.; Ayed, H.; Manni, L.; Agrebi, R.; Nasri, M. Alkaline-protease from Bacillus licheniformis MP1: Purification, characterization and potential application as a detergents additive and for shrimp waste deproteinization. Process Biochem. 2011, 46, 1248–1256. [Google Scholar] [CrossRef]
- Corici, L.; Frissen, A.; Zoelen, D.; Eggen, I.; Peter, F.; Davidescu, C.; Boeriu, C. Sol–gel immobilization of Alcalase from Bacillus licheniformis for application in the synthesis of C-terminal peptide amides. J. Mol. Catal. B Enzym. 2011, 73, 90–97. [Google Scholar] [CrossRef]
- Zheng, H.; Gao, C.; Che, S. Amino and quaternary ammonium group functionalized mesoporous silica: An efficient ion-exchange method to remove anionic surfactant from AMS. Microporous Mesoporous Mater. 2008, 116, 299–307. [Google Scholar] [CrossRef]
- El-Toni, A.M.; Habila, M.A.; Ibrahim, M.A.; Labis, J.P.; al Othman, Z.A. Simple and facile synthesis of amino functionalized hollow core-mesoporous shell silica spheres using anionic surfactant for Pb(II), Cd(II), and Zn(II) adsorption and recovery. Chem. Eng. J. 2014, 251, 441–451. [Google Scholar] [CrossRef]
- Brunel, D. Functionalized micelle-templated silicas (MTS) and their use as catalysts for fine chemicals. Microporous Mesoporous Mater. 1999, 27, 329–344. [Google Scholar] [CrossRef]
- Ibrahim, A.S.; Al-Salamah, A.A.; El-Toni, A.M.; El-Tayeb, M.A.; Elbadawi, Y.B. Immobilization of cyclodextrin glucanotransferase on aminopropyl-functionalized silica-coated superparamagnetic nanoparticles. Electron. J. Biotechnol. 2013, 16, 1–8. [Google Scholar] [CrossRef]
- Shi, B.; Wang, Y.; Ren, J.; Liu, X.; Zhang, Y.; Guo, Y. Superparamagnetic aminopropyl functionalized silica core–shell microspheres as magnetically separable carriers for immobilization of penicillin G acylase. J. Mol. Catal. B Enzym. 2010, 63, 50–56. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Mathews, C.K.; Holde, K.E.; Holde, K.F. Dynamic of Life: Catalysis and Control of Biochemical Reactions. In Biochemistry, 4th ed.; Prentice Hall: New Jersey, NJ, USA, 2012. [Google Scholar]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, A.S.S.; Al-Salamah, A.A.; El-Toni, A.M.; Almaary, K.S.; El-Tayeb, M.A.; Elbadawi, Y.B.; Antranikian, G. Enhancement of Alkaline Protease Activity and Stability via Covalent Immobilization onto Hollow Core-Mesoporous Shell Silica Nanospheres. Int. J. Mol. Sci. 2016, 17, 184. https://doi.org/10.3390/ijms17020184
Ibrahim ASS, Al-Salamah AA, El-Toni AM, Almaary KS, El-Tayeb MA, Elbadawi YB, Antranikian G. Enhancement of Alkaline Protease Activity and Stability via Covalent Immobilization onto Hollow Core-Mesoporous Shell Silica Nanospheres. International Journal of Molecular Sciences. 2016; 17(2):184. https://doi.org/10.3390/ijms17020184
Chicago/Turabian StyleIbrahim, Abdelnasser Salah Shebl, Ali A. Al-Salamah, Ahmed M. El-Toni, Khalid S. Almaary, Mohamed A. El-Tayeb, Yahya B. Elbadawi, and Garabed Antranikian. 2016. "Enhancement of Alkaline Protease Activity and Stability via Covalent Immobilization onto Hollow Core-Mesoporous Shell Silica Nanospheres" International Journal of Molecular Sciences 17, no. 2: 184. https://doi.org/10.3390/ijms17020184
APA StyleIbrahim, A. S. S., Al-Salamah, A. A., El-Toni, A. M., Almaary, K. S., El-Tayeb, M. A., Elbadawi, Y. B., & Antranikian, G. (2016). Enhancement of Alkaline Protease Activity and Stability via Covalent Immobilization onto Hollow Core-Mesoporous Shell Silica Nanospheres. International Journal of Molecular Sciences, 17(2), 184. https://doi.org/10.3390/ijms17020184